Abstract
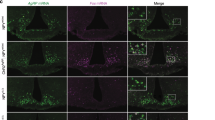
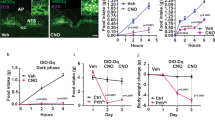
Hypothalamic AgRP/NPY neurons are key players in the control of feeding behaviour. Ghrelin, a major orexigenic hormone, activates AgRP/NPY neurons to stimulate food intake and adiposity. However, cell-autonomous ghrelin-dependent signalling mechanisms in AgRP/NPY neurons remain poorly defined. Here we show that calcium/calmodulin-dependent protein kinase ID (CaMK1D), a genetic hot spot in type 2 diabetes, is activated upon ghrelin stimulation and acts in AgRP/NPY neurons to mediate ghrelin-dependent food intake. Global Camk1d-knockout male mice are resistant to ghrelin, gain less body weight and are protected against high-fat-diet-induced obesity. Deletion of Camk1d in AgRP/NPY, but not in POMC, neurons is sufficient to recapitulate above phenotypes. In response to ghrelin, lack of CaMK1D attenuates phosphorylation of CREB and CREB-dependent expression of the orexigenic neuropeptides AgRP/NPY in fibre projections to the paraventricular nucleus (PVN). Hence, CaMK1D links ghrelin action to transcriptional control of orexigenic neuropeptide availability in AgRP neurons.






Similar content being viewed by others
Data availability
All raw data related to the studies shown in figures and Extended Data Figures. Source data are provided with this paper.
References
Kim, K.-S., Seeley, R. J. & Sandoval, D. A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 19, 185–196 (2018).
Jais, A. & Brüning, J. C. Arcuate nucleus-dependent regulation of metabolism — pathways to obesity and diabetes mellitus. Endocr. Rev. 9, 314–328 (2021).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Timper, K. & Brüning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model Mech. 10, 679–689 (2017).
Chen, S.-R. et al. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 142, 512–520 (2017).
Zigman, J. M., Bouret, S. G. & Andrews, Z. B. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol. Metab. 27, 54–63 (2016).
Bonnefond, A. & Froguel, P. Rare and common genetic events in type 2 diabetes: what should biologists know? Cell Metab. 21, 357–368 (2015).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Kooner, J. S. et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat. Genet. 43, 984–989 (2011).
Shu, X. O. et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 6, e1001127 (2010).
Zeggini, E. et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 40, 638–645 (2008).
Morris, A. P. et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990 (2012).
Thurner, M. et al. Integration of human pancreatic islet genomic data refines regulatory mechanisms at Type 2 Diabetes susceptibility loci. eLife 7, e31977 (2018).
Xue, A. et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 9, 2941 (2018).
Simonis-Bik, A. M. et al. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic beta-cell function. Diabetes 59, 293–301 (2010).
Haney, S. et al. RNAi screening in primary human hepatocytes of genes implicated in genome-wide association studies for roles in type 2 diabetes identifies roles for CAMK1D and CDKAL1, among others, in hepatic glucose regulation. PLoS ONE 8, e64946 (2013).
Buchser, W. J., Slepak, T. I., Gutierrez-Arenas, O., Bixby, J. L. & Lemmon, V. P. Kinase/phosphatase overexpression reveals pathways regulating hippocampal neuron morphology. Mol. Syst. Biol. 6, 391 (2010).
Wayman, G. A. et al. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 24, 3786–3794 (2004).
Takemoto-Kimura, S. et al. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIγ. Neuron 54, 755–770 (2007).
Schmitt, J. M., Guire, E. S., Saneyoshi, T. & Soderling, T. R. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 25, 1281–1290 (2005).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Gu, G., Dubauskaite, J. & Melton, D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Hatting, M., Tavares, C. D. J., Sharabi, K., Rines, A. K. & Puigserver, P. Insulin regulation of gluconeogenesis. Ann. NY Acad. Sci. 1411, 21–35 (2018).
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Müller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Andrews, Z. B. et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851 (2008).
Hoffman, G. E., Smith, M. S. & Verbalis, J. G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 14, 173–213 (1993).
van den Pol, A. N. et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J. Neurosci. 29, 4622–4639 (2009).
Haribabu, B. et al. Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. EMBO J. 14, 3679–3686 (1995).
Andersson, U. et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279, 12005–12008 (2004).
Sakkou, M. et al. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 5, 450–463 (2007).
Sheng, M., Thompson, M. A. & Greenberg, M. E. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252, 1427–1430 (1991).
Flavell, S. W. & Greenberg, M. E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev. Neurosci. 31, 563–590 (2008).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Chen, H. Y. et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145, 2607–2612 (2004).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Wu, C.-S. et al. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int. J. Mol. Sci. 18, 832 (2017).
Wang, Q. et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 3, 64–72 (2014).
Anderson, K. A. et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008).
López, M. et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 7, 389–399 (2008).
García, A., Alvarez, C. V., Smith, R. G. & Diéguez, C. Regulation of Pit-1 expression by ghrelin and GHRP-6 through the GH secretagogue receptor. Mol. Endocrinol. 15, 1484–1495 (2001).
Gonzalez, G. A. & Montminy, M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–680 (1989).
Kohno, D., Gao, H.-Z., Muroya, S., Kikuyama, S. & Yada, T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52, 948–956 (2003).
Martins, L. et al. Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS ONE 7, e46923 (2012).
Stevanovic, D. et al. Ghrelin-induced food intake and adiposity depend on central mTORC1/S6K1 signaling. Mol. Cell. Endocrinol. 381, 280–290 (2013).
Albert, V., Cornu, M. & Hall, M. N. mTORC1 signaling in Agrp neurons mediates circadian expression of Agrp and NPY but is dispensable for regulation of feeding behavior. Biochem. Biophys. Res. Commun. 464, 480–486 (2015).
Codner, G. F. et al. Universal southern blot protocol with cold or radioactive probes for the validation of alleles obtained by homologous recombination. Methods 191, 59–67 (2021).
Lundbaek, K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br. Med J. 1, 1507–1513 (1962).
Even, P. C. & Nadkarni, N. A. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R459–R476 (2012).
Müller, T. D., Klingenspor, M. & Tschöp, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Ye, J. H., Zhang, J., Xiao, C. & Kong, J.-Q. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J. Neurosci. Methods 158, 251–259 (2006).
Dodt, H. U. & Zieglgänsberger, W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 537, 333–336 (1990).
Horn, R. & Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 92, 145–159 (1988).
Akaike, N. & Harata, N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn. J. Physiol. 44, 433–473 (1994).
Acknowledgements
We thank T. Alquier at University of Montreal for helpful discussions and D. Dembele at the IGBMC for ANCOVA analysis. We thank the Imaging Center of the IGBMC (ICI), the IGBMC molecular biology and virus core facility, the Institut Clinique de la souris (ICS) core facilities and other core facilities for their support on this research. This work was supported by the Agence Nationale de la Recherche (ANR) (AAPG 2021 HypoCaMK)), by the Fondation de Recherche Médicale (FRM) – Program: Equipe FRM (EQU201903007859, Prix Roger PROPICE pour la recherche sur le cancer du pancréas), by the FHU-OMAGE of region Grand-Est, from the European Foundation for the Study of Diabetes (EFSD)/Novo Nordisk Diabetes Research Programme and by the ANR-10-LABX-0030-INRT grant as well as the ANR-11-INBS-0009-INGESTEM grant, both French State funds managed by the ANR under the frame program Investissements d’Avenir. K.V. was supported by an Individual Fellowship (798961 INSULYSOSOME) in the framework of the Marie-Sklodovska Curie actions of the European Commission and a research grant from Société Francophone du Diabète. G.Y. received financial doctoral support from DFG-233886668/RTG1960. M. Quiñones was funded by a research contract Miguel Servet (CP21/00108) from the ISCIII co-funded by the European Union.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.R., R.N, P.K and S.L. Software: E.G. Methodology: K.V., E.P., Z.Z, Ma.Q, G.Y., E.E., E.C.–C. and M. Qu. Validation: K.V. Formal Analysis: K.V., C.M. Investigation: K.V., G.M., E.P., Z.Z., M. Quiñones., E.E., G.Y., M. Qu., A.S., M.F, C.M. Resources: E.E., F.B. Writing — Original Draft: R.R. and K.V., Supervision: K.V., S.L., P.K., R.N., A.C., I.S. and R.R, Funding Acquisition: R.R. and S.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Timo D. Müller, Dominic Withers and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Deletion of CaMK1D in mice does not affect body size and CaMK1D knockout mice are born without any gross abnormalities.
a, Schematic screen of targeting of the CaMK1D locus using Knockout-first targeting strategy and generation of floxed and global knockout mice. For further details see material and methods. b, Validation of targeted ES clone by southern blot. Genomic DNA was digested using BspHI, BstEII, ApalI or EcoRI. The table shows the expected size of bands corresponding to the restriction enzyme used. A probe recognizing the neoR cassette was used. c, Gender distribution of mice and d, Mendelian ratios of born mice with indicated genetoypes. e, Body length and f, Tibia Length measurements of 7-week-old mice with indicated genotypes (CaMK1D+/+ n = 5; CaMK1D−/− n = 7). Data are presented as mean values +/− SEM.
Extended Data Fig. 2 CaMK1D signaling is redundant in pancreatic beta cell and liver function.
a, Blood insulin levels during IPGTTs in mice on a High fat diet (HFD). (n = 11/group). b, Glucose-stimulated insulin secretion (GSIS) in pancreatic islets isolated from mice. (CaMK1D+/+ n = 6; CaMK1D−/− n = 5). c, Expression of CaMK1D protein in pancreas and brain from mice with indicated genotypes. d, Body weight gain of mice on a Chow diet (CD) or on a High Fat Diet (HFD). (CD - CaMK1Dflox/flox n = 13; CD - AgRPCre/+; CaMK1Dflox/flox n = 13; HFD - CaMK1Dflox/flox n = 12; CD - AgRPCre/+; CaMK1Dflox/flox n = 10). e-f, Blood glucose levels during IPGTTs in mice on a CD (e), or on a HFD (f). (CD - CaMK1Dflox/flox n = 7; CD - AgRPCre/+; CaMK1Dflox/flox n = 6; HFD - CaMK1Dflox/flox n = 7; CD - AgRPCre/+; CaMK1Dflox/flox n = 8). g, Expression of CaMK1D mRNA in liver from mice. (CaMK1Dflox/flox n = 3; CD - AlbCre/+; CaMK1Dflox/flox n = 3) h, Body weight gain of mice on a CD or on a HFD. (CD - CaMK1Dflox/flox n = 8; CD - AlbCre/+; CaMK1Dflox/flox n = 9; HFD - CaMK1Dflox/flox n = 8; HFD - AlbCre/+; CaMK1Dflox/flox n = 10). Blood glucose levels during an IPGTT in mice on a CD (i) or on a HFD (j). (CD - CaMK1Dflox/flox n = 6; CD - AlbCre/+; CaMK1Dflox/flox n = 7; HFD - CaMK1Dflox/flox n = 8; HFD - AlbCre/+; CaMK1Dflox/flox n = 5). Blood glucose levels during an ITT in mice on a CD (k) or on a HFD (l). (CD - CaMK1Dflox/flox n = 5; CD - AlbCre/+; CaMK1Dflox/flox n = 6; HFD - CaMK1Dflox/flox n = 12; HFD - AlbCre/+; CaMK1Dflox/flox n = 14). Data are presented as mean values +/− SEM.
Extended Data Fig. 3 Deletion of CaMK1D does not result in anxiety-like behavior and stress-induced anhedonia.
a,Distance travelled each 5 min, and (b) in total. (c) Rear number each 5 min, and (d) in total. e, Number of entries in the center or periphery of the arena. f, Time spent in the center or periphery of the arena. g, Average speed during an open field test. (n = 8/group). h, Amount of sucrose and water consumed over 3 day in while sucrose and water were both available in the same time. (CaMK1D+/+ n = 8; CaMK1D−/− n = 7). Data are presented as mean values +/− SEM.
Extended Data Fig. 4 Deletion of CaMK1D in the Nervous Tissue reproduces phenotypes in whole-body knockout mice.
a, Expression of CaMK1D protein in mice. b, Body weight gain on a Chow diet (CD) or on a High Fat Diet (HFD). (CD - CaMK1Dflox/flox n = 12; CD - NestinCre/+ n = 11 and CD - NestinCre/+; CaMK1Dflox/flox n = 10; HFD - CaMK1Dflox/flox n = 12; HFD - NestinCre/+ n = 12 HFD - NestinCre/+; CaMK1Dflox/flox n = 11). Cumulative food intake on (c) CD or on (d) HFD. (CD - CaMK1Dflox/flox n = 12; CD - NestinCre/+ n = 11 and CD - NestinCre/+; CaMK1Dflox/flox n = 10; HFD - CaMK1Dflox/flox n = 12; HFD - NestinCre/+ n = 12 HFD - NestinCre/+; CaMK1Dflox/flox n = 11) (*p ≤ 0.05). (e) Cumulative food intake. (CaMK1Dflox/flox n = 10; NestinCre/+ n = 12 and NestinCre/+; CaMK1Dflox/flox n = 8). (f) Cumulative food intake after ghrelin injection (30 µg / day) on a CD. (CaMK1Dflox/flox n = 7; NestinCre/+ n = 10 and NestinCre/+; CaMK1Dflox/flox n = 9). Data are presented as mean values +/− SEM. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001. Statistical tests included two-way ANOVA bonferroni post hoc test (b,c,d,e,f).
Extended Data Fig. 5 Deletion of CaMK1D in AgRP neurons.
a, Verification of recombination in the CaMK1D locus in hypothalamus (Hyp), cortex (Crtx), liver, tail and white blood cells (WBC) from mice. b, Expression of CaMK1D mRNA in arcuate nucleus, cortex and liver from mice (n = 4 / group).Statistical tests included unpaired t-test (b). Data are presented as mean values +/− SEM.
Extended Data Fig. 6 Deletion of CaMK1D in POMC neurons does not affect energy metabolism in mice.
a, Verification of recombination in the CaMK1D locus in hypothalamus (Hyp), cortex (Crtx), liver, tail and white blood cells (WBC) from mice. b, Body weight gain on a Chow diet (CD) or on a High Fat Diet (HFD). (CD - CaMK1Dflox/flox n = 11; CD -POMCCre/+ n = 8; CD -POMCCre/+; CaMK1Dflox/flox n = 8; HFD - CaMK1Dflox/flox n = 10; HFD -POMCCre/+ n = 10 and HFD -POMCCre/+; CaMK1Dflox/flox n = 13).c, Cumulative food intake on a CD. (CD - CaMK1Dflox/flox n = 10; CD -POMCCre/+ n = 8; CD -POMCCre/+; CaMK1Dflox/flox n = 8). d, Cumulative food intake on a HFD. (HFD - CaMK1Dflox/flox n = 10; HFD -POMCCre/+ n = 8; HFD -POMCCre/+; CaMK1Dflox/flox n = 10). e, Cumulative food intake. Food intake was determined 24 h after fasting. (CaMK1Dflox/flox n = 10; POMCCre/+ n = 8; POMCCre/+; CaMK1Dflox/flox n = 10). f, Cumulative food intake after ghrelin injection. (CaMK1Dflox/flox n = 8; POMCCre/+ n = 7; POMCCre/+; CaMK1Dflox/flox n = 8). Data are presented as mean values +/− SEM. ∗∗p < 0.01 and ∗∗∗p < 0.001. Statistical tests included two-way ANOVA bonferroni post hoc test (f).
Supplementary information
Source data
Source Data Fig. 1
Raw data
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 2
Raw data
Source Data Fig. 3
Raw data
Source Data Fig. 3
Unprocessed microscopy pictures
Source Data Fig. 4
Raw data
Source Data Fig. 5
Raw data
Source Data Fig. 5
Unprocessed microscopy pictures
Source Data Fig. 6
Raw data
Source Data Fig. 6
Unprocessed western blots and microscopy pictures
Source Data Extended Data Fig. 1
Raw data
Source Data Extended Data Fig. 2
Raw data
Source Data Extended Data Fig. 2
Unprocessed western blots
Source Data Extended Data Fig. 3
Raw data
Source Data Extended Data Fig. 4
Raw data
Source Data Extended Data Fig. 4
Unprocessed western blots
Source Data Extended Data Fig. 5
Raw data
Source Data Extended Data Fig. 6
Raw data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vivot, K., Meszaros, G., Pangou, E. et al. CaMK1D signalling in AgRP neurons promotes ghrelin-mediated food intake. Nat Metab 5, 1045–1058 (2023). https://doi.org/10.1038/s42255-023-00814-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00814-x
- Springer Nature Limited
This article is cited by
-
CAMK1D required for ghrelin-mediated food intake
Nature Reviews Endocrinology (2023)