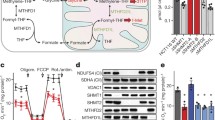
Abstract
Mammalian cells require activated folates to generate nucleotides for growth and division. The most abundant circulating folate species is 5-methyl tetrahydrofolate (5-methyl-THF), which is used to synthesize methionine from homocysteine via the cobalamin-dependent enzyme methionine synthase (MTR). Cobalamin deficiency traps folates as 5-methyl-THF. Here, we show using isotope tracing that MTR is only a minor source of methionine in cell culture, tissues or xenografted tumours. Instead, MTR is required for cells to avoid folate trapping and assimilate 5-methyl-THF into other folate species. Under conditions of physiological extracellular folates, genetic MTR knockout in tumour cells leads to folate trapping, purine synthesis stalling, nucleotide depletion and impaired growth in cell culture and as xenografts. These defects are rescued by free folate but not one-carbon unit supplementation. Thus, MTR plays a crucial role in liberating THF for use in one-carbon metabolism.




Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on request.
Code availability
The ‘Accucor’ package for natural isotope correction is publicly available through GitHub (https://github.com/lparsons/accucor).
References
Voet, D., Voet, J. G. & Pratt, C. W. Fundamentals of Biochemistry: Life at the Molecular Level 5th edn (Wiley, 2016).
Tibbetts, A. S. & Appling, D. R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev. Nutr. 30, 57–81 (2010).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Fox, J. T. & Stover, P. J. in Vitamins & Hormones (ed. Litwack, G.) Ch. 1, 1–44 (Academic Press, 2008).
Dudman, N. P., Slowiaczek, P. & Tattersall, M. H. Methotrexate rescue by 5-methyltetrahydrofolate or 5-formyltetrahydrofolate in lymphoblast cell lines. Cancer Res. 42, 502–507 (1982).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Labuschagne, C. F. et al. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014).
Wright, A. J. et al. Differential kinetic behavior and distribution for pteroylglutamic acid and reduced folates: a revised hypothesis of the primary site of PteGlu metabolism in humans. J. Nutr. 135, 619–623 (2005).
Patanwala, I. et al. Folic acid handling by the human gut: implications for food fortification and supplementation. Am. J. Clin. Nutr. 100, 593–599 (2014).
Običan, S. G. et al. Folic acid in early pregnancy: a public health success story. FASEB J. 24, 4167–4174 (2010).
Finkelstein, J. D. Methionine metabolism in mammals. J. Nutr. Biochem. 1, 228–237 (1990).
Rose, W. C., Johnson, J. E. & Haines, W. J. The amino acid requirements of man. 1. Role of valine methionine. J. Biol. Chem. 182, 541–556 (1950).
Sowers, J. E., Stockland, W. L. & Meade, R. J. l-Methionine and l-cystine requirements of the growing rat. J. Anim. Sci. 35, 782–788 (1972).
Lu, S. C. S-adenosylmethionine. Int. J. Biochem. Cell Biol. 32, 391–395 (2000).
Finkelstein, J. D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 45, 1694–1699 (2007).
Zheng, Y. et al. Regulation of folate and methionine metabolism by multisite phosphorylation of human methylenetetrahydrofolate reductase. Sci. Rep. 9, 4190 (2019).
Baker, D. H. & Czarnecki, G. L. Transmethylation of homocysteine to methionine: efficiency in the rat and chick. J. Nutr. 115, 1291–1299 (1985).
Bennett, M. A. Utilization of homocystine for growth in presence of vitamin B12 and folic acid. J. Biol. Chem. 187, 751–756 (1950).
du Vigneaud, V., Ressler, C. & Rachele, J. R. The biological synthesis of ‘labile methyl groups’. Science 112, 267–271 (1950).
Banerjee, R. V. & Matthews, R. G. Cobalamin‐dependent methionine synthase. FASEB J. 4, 1450–1459 (1990).
Watkins, D. & Rosenblatt, D. S. Inborn errors of cobalamin absorption and metabolism. Am. J. Med. Genet. Part C 157, 33–44 (2011).
Watkins, D. et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am. J. Hum. Genet. 71, 143–153 (2002).
Swanson, D. A. et al. Targeted disruption of the methionine synthase gene in mice. Mol. Cell. Biol. 21, 1058–1065 (2001).
Geiger, T. et al. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol. Cell. Proteom. 12, 1709–1722 (2013).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Pajares, M. A. & Pérez-Sala, D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 63, 2792–2803 (2006).
Zhang, W. et al. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 65, 1554–1560 (2005).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 (2017).
Hoffbrand, A. V. & Waters, A. H. Observations on the biochemical basis of megaloblastic anaemia. Br. J. Haematol. 23, 109–118 (1972).
Shane, B. & Stokstad, E. R. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 5, 115–141 (1985).
Kondo, H. et al. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. J. Clin. Investig. 67, 1270–1283 (1981).
Horne, D. W. & Briggs, W. T. Effect of dietary and nitrous oxide-induced vitamin B-12 deficiency on uptake of 5-methyltetrahydrofolate by isolated rat hepatocytes. J. Nutr. 110, 223–230 (1980).
Matthews, R. G. & Drummond, J. T. Providing one-carbon units for biological methylations: mechanistic studies on serine hydroxymethyltransferase, methylenetetrahydrofolate reductase, and methyltetrahydrofolate-homocysteine methyltransferase. Chem. Rev. 90, 1275–1290 (1990).
Palmer, A. M. et al. Folate rescues vitamin B12 depletion-induced inhibition of nuclear thymidylate biosynthesis and genome instability. Proc. Natl Acad. Sci. USA 114, E4095–E4102 (2017).
Herbert, V. & Zalusky, R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J. Clin. Investig. 41, 1263–1276 (1962).
Noronha, J. On folic acid, vitamin B12, methionine and formiminoglutamic acid metabolism. In Proc. Second European Symposium on Vitamin B12 and Intrinsic Factor (ed. Heinrich, H. C.) (Ferdinand Enke, 1962).
Fazili, Z., Pfeiffer, C. M. & Zhang, M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and Bio-Rad radioassay. Clin. Chem. 53, 781–784 (2007).
Fazili, Z. et al. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal. Bioanal. Chem. 405, 4549–4560 (2013).
Maddocks, O. D. et al. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell 61, 210–221 (2016).
Yang, L. et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 31, 809–821.e6 (2020).
Sunden, S. L. et al. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 345, 171–174 (1997).
Pellanda, H. et al. A splicing variant leads to complete loss of function of betaine–homocysteine methyltransferase (BHMT) gene in hepatocellular carcinoma. Int. J. Biochem. Cell Biol. 44, 385–392 (2012).
Golani, L. K. et al. Tumor targeting with novel 6-substituted pyrrolo [2,3-d] pyrimidine antifolates with heteroatom bridge substitutions via cellular uptake by folate receptor α and the proton-coupled folate transporter and inhibition of de novo purine nucleotide biosynthesis. J. Med. Chem. 59, 7856–7876 (2016).
García-Cañaveras, J. C. et al. SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia. Leukemia 35, 377–388 (2021).
Motoshima, H. et al. AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 574, 63–71 (2006).
Sullivan, M. R. et al. Methionine synthase is essential for cancer cell proliferation in physiological folate environments. https://doi.org/10.1038/s42255-021-00486-5 (in the press).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017).
Vande Voorde, J. et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 5, eaau7314 (2019).
Kwon, Y. K. et al. A domino effect in antifolate drug action in Escherichia coli. Nat. Chem. Biol. 4, 602–608 (2008).
Allegra, C. J. et al. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc. Natl Acad. Sci. USA 82, 4881–4885 (1985).
Allegra, C. J. et al. Evidence for direct inhibition of de novo purine synthesis in human MCF-7 breast cells as a principal mode of metabolic inhibition by methotrexate. J. Biol. Chem. 262, 13520–13526 (1987).
Stover, P. & Schirch, V. 5-Formyltetrahydrofolate polyglutamates are slow tight binding inhibitors of serine hydroxymethyltransferase. J. Biol. Chem. 266, 1543–1550 (1991).
Matthews, R. G., Drummond, J. T. & Webb, H. K. Cobalamin-dependent methionine synthase and serine hydroxymethyltransferase: targets for chemotherapeutic intervention? Adv. Enzym. Regul. 38, 377–392 (1998).
Walling, J. From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Invest. N. Drugs 24, 37–77 (2006).
Gonen, N. & Assaraf, Y. G. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist. Upd. 15, 183–210 (2012).
Zhang, Z. et al. Mechanism-based design, synthesis and biological studies of N5-substituted tetrahydrofolate analogs as inhibitors of cobalamin-dependent methionine synthase and potential anticancer agents. Eur. J. Med. Chem. 58, 228–236 (2012).
Tang, C. et al. Two newly synthesized 5-methyltetrahydrofolate-like compounds inhibit methionine synthase activity accompanied by cell cycle arrest in G1/S phase and apoptosis in vitro. Anticancer Drug. 19, 697–704 (2008).
Banks, E. C. et al. Inhibition of cobalamin-dependent methionine synthase by substituted benzo-fused heterocycles. FEBS J. 274, 287–299 (2007).
Chen, L. et al. An LC–MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal. Bioanal. Chem. 409, 5955–5964 (2017).
Wang, L. et al. Peak annotation and verification engine for untargeted LC–MS metabolomics. Anal. Chem. 91, 1838–1846 (2019).
Lu, W. et al. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 82, 3212–3221 (2010).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Acknowledgements
We thank W. Lu and other members of the Rabinowitz laboratory for helpful comments and suggestions. LentiCRISPR v.2 was a gift from F. Zhang (Addgene plasmid no. 52961). This work was supported by National Institutes of Health grant nos. 1DP1DK113643 and R01 CA163591 to J.D.R.
Author information
Authors and Affiliations
Contributions
J.M.G. conceived the study. J.M.G., X.X., J.Z.W. and J.D.R. designed the experiments. J.Z.W., X.X., J.M.G., L.Y., R.-P.R. and L.W. conducted the experiments. J.Z.W., X.X., J.M.G. and J.D.R. wrote the paper with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
J.D.R. is a paid adviser and stockholder in Kadmon Pharmaceuticals, L.E.A.F. Pharmaceuticals and Rafael Pharmaceuticals; a paid consultant of Pfizer; a founder, director, and stockholder of Farber Partners and Serien Therapeutics. J.D.R. and J.M.G. are inventors of patents in the area of folate metabolism held by Princeton University. The other authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Kivanc Birsoy, Jason Locasale and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 MTR is a minor source of methionine in vitro and in vivo.
(a) Schematic of methionine labeling from [U-13C]methionine. Red circles indicate 13C atoms. MTR = methionine synthase. (b) Schematic of methionine labeling from [U-13C] or [3-13C]serine. Blue circles indicate 13C atoms. MTHFR = methylenetetrahydrofolate reductase, SHMT = serine hydroxymethyltransferase. (c) Methionine labeling in cell lines after culturing for 4 h in media containing [U-13C]serine (for 293 T) or [3-13C]serine (for HCT116 and HepG2) (mean ± SD, n = 2). (d) Methionine M + 1 fraction from 4 h [3-13C]serine tracing in HCT116 cultured in media containing indicated methionine and folate concentrations (mean ± SD, n = 3 for each condition). Labeling of (e) serine and (f) methionine in serum, PDAC tumors, and normal tissues of male C57BL/6 mice after [U-13C]serine infusion for 2.5 h. (mean ± SD, n = 3 mice; two technical replicates were included for each tumour). (g) Schematic of methionine labeling from [13C5,15N]betaine. Orange circles indicate 13C atoms, green circles indicate 15N atoms. BHMT = betaine-homocysteine S-methyltransferase, DMG = dimethylglycine.
Extended Data Fig. 2 MTR is important for cell growth in physiological folates.
(a) Expression of MTR in the HCT116, 8988 T, and HepG2 cell lines as reported in the Cancer Cell Line Encyclopedia.63 (b) Cell growth curves in the media containing indicated folate sources (mean ± SD, n = 2). (c) Cell growth curves in media containing indicated folate and methionine concentrations (mean ± SD, n = 3). (d) Individual tumor volumes for HCT116 xenografts in female CD-1 nude mice (n = 10 mice). (e) Terminal tumor mass of HCT116 xenografts in female CD-1 nude mice (mean ± SEM, n = 10 mice). P values were determined by a two-sided paired Student’s t-test comparing ΔMTR-1 to wild-type, and ΔMTR-2 to CRISPR control (control-1). (f) Growth of subcutaneous HCT116 xenografts in male CD-1 nude mice on a standard folate (4ppm) or low folate diet (mean ± SEM, n = 10 mice). (g) Western blot analysis of MTR and eGFP in HCT116 wild-type (WT), CRISPR control-1 or ΔMTR-1 which was also engineered to express a vector containing either eGFP or MTR cDNA. Loading control (COXIV) was analyzed on a separate gel from parallel experiments. Results are representative of 2 independent biological replicates with similar results. SI = small intestine, SM = skeletal muscle.
Extended Data Fig. 3 Loss of MTR disrupts nucleotide synthesis.
(a) Water-soluble metabolite levels from HCT116 control and MTR knockout cells cultured in indicated media conditions. Each box reflects one independent biological measurement, normalized to the average of control cells cultured in folic acid. (b) Relative nucleotide mono- and diphosphate abundances in HCT116 control and MTR knockout cells in indicated media. Intensities are normalized to the average of control-1 cells in folic acid (mean ± SD, n = 3). (c) Relative thymidylate species abundances in HCT116 control and knockout cells in indicated media. Intensities are normalized to the average of control-1 cells in folic acid (mean ± SD, n = 3). (d) Cell growth dose response curves for HCT116 WT and MTR knockout cells treated with SHMT1/2 inhibitor SHIN2 under different folate conditions (mean ± SD, n = 5). For (B) and (C), P values were determined by a one-way ANOVA comparing control to MTR knockout in the same medium followed by Dunnett’s post hoc analysis.
Extended Data Fig. 4 Metabolomics of MTR knockout tumors.
(a) Water-soluble metabolites levels from individual HCT116 control and MTR knockout subcutaneous tumors (normalized to wild-type tumor average). (b) Relative abundance of an S-ribosylhomocysteine isomer in HCT116 control and MTR knockout subcutaneous tumors (mean ± SD, n = 10 tumors for control-1, and n = 9 tumors for each other group). Mice were fed standard chow. (c) MS/MS spectrum of m/z 268.0848 peak in positive-ion mode. Fragmentation pattern suggests an S-ribosylhomocysteine (SRH) isomer. WT = wild-type.
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
About this article
Cite this article
Ghergurovich, J.M., Xu, X., Wang, J.Z. et al. Methionine synthase supports tumour tetrahydrofolate pools. Nat Metab 3, 1512–1520 (2021). https://doi.org/10.1038/s42255-021-00465-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00465-w
- Springer Nature Limited
This article is cited by
-
Glycolytic enzymes in non-glycolytic web: functional analysis of the key players
Cell Biochemistry and Biophysics (2024)
-
Rethinking our approach to cancer metabolism to deliver patient benefit
British Journal of Cancer (2023)
-
Multimodal metabolomics pinpoint new metabolic vulnerability in colorectal cancer
Nature Metabolism (2023)
-
Thorough research and modification of one-carbon units cycle for improving L-methionine production in Escherichia coli
3 Biotech (2023)
-
Metabolic profiling stratifies colorectal cancer and reveals adenosylhomocysteinase as a therapeutic target
Nature Metabolism (2023)