Abstract
The mechanism of silver-catalyzed hydroamidation of siloxy-alkynes reaction remains controversial. Using density functional theory (DFT), we revealed that the reaction takes place through a silylium ion migration mediated hydroamination (SMH) pathway. The SMH pathway goes through two steps, the first step is Ag+ promoted proton and silylium ion exchange between siloxy-alkynes and amide, leading to ketene and silyl-imines, the second step is Ag+ catalyzed nucleophilic addition between ketene and silyl-imines, following with a silylium ion migration afford the final product. In this reaction, Ag+ activates the siloxy-alkyne into silylium ion (TIPS+) and silver-ketene through the p–π conjugate effect, the silylium ion then catalyzes the reaction. According to our calculation, the scopes of alkynes in this reaction may be extended to silyl-substituted ynamines or silyl-substituted ynamides. The scopes of amide may be extended into the p–π conjugate system such as diazoles, diazepines, etc. Our calculations also reveal a concise way to construct enamides through Ag+ catalyzed nucleophilic addition between substituted-ketenes and silyl-substituted p–π conjugate system.
Similar content being viewed by others
Introduction
Hydroamidations have attracted tremendous attention as it is an atomic-economy and substrate’s easily accessible way to construct enamides1,2,3,4,5,6,7,8,9. Hydroamidations are not spontaneous mainly due to the relatively high barrier of the nucleophilic process10. Significant progress has been made in catalyzed hydroamidation, and high levels of chemo-, regio-, and stereo-selectivity have been reported4,11,12,13. However, currently reported examples are mainly centered on hydroamidation14,15,16,17 of terminal alkynes9,18.
To date, the catalytic variant of hydroamidation remains rather limited, especially for hydroamidation of internal alkynes. For example, a recent work reported by Chang’s group shows a NiH catalyzed hydroamidations with both terminal and internal alkynes as suitable alkyne-substrates13. Although Chang’s work has made important breakthroughs, this reaction is not atomic-economic, and the scopes of amide is also very limited as the reaction requires active dioxazolones as amide source and dimethoxymethylsilane as its sacrifice reagent. So far, there were only two examples with internal alkynes as substrates that satisfy atomic-economic character19,20. Indisputably, making clear the mechanisms of such reactions can provide direct insights to development of new and better catalytic reactions.
In 2016, Cui’ group reported hydroamidation of phenyl-substituted alkyne with Ru(II) as catalyst19. The reaction proceeds under relatively high temperature (90 °C) and the substrate’s scope of amides is very limited, only the N1-benzyl-N2, N2-diisopropyloxalamide, and its analogs are suitable substrates for this reaction.
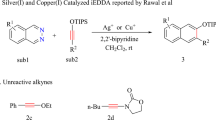
In 2006, Kozmin’s group reported the first example of hydroamidation of internal alkynes20. They found that AgNTf2 can promote the addition of second-amides or carbamates to the triple-bond of siloxy-alkyne successfully at 20 °C within 30 min (Fig. 1a). In this reaction, the E-configured Markovnikov product is obtained exclusively.
The authors also found that the commonly used ynamides and internal alkynes are inactive in the same condition. The author proposed a silver-induced nucleophilic addition mechanism (SNU mechanism, Fig. 1b) based on the kinetic studies and deuterium label experiment. In this mechanism, the AgNTf2 coordinates with siloxy-alkyne 1 to form π-complex INTA, the amide 2 then nucleophilic attack the siloxy-alkyne 1 to form Z-INTB. Proton transfer of Z-INTB led to the final product Z-3. The nucleophilic attack step is thought to be the rate-determining step (RDS).
The SNU mechanism complies with some experimental observations but does not explain why electron-richer ynamides are non-reactive under the same condition. It should be more reactive if this reaction proceeds through SNU pathway21,22,23. Further, the transition metal-induced nucleophilic attack favors takes place at the trans-side of the metal atom due to the β-effect24, which implies that cis-addition product may be disfavored if the reaction go through SNU pathway.
Based on DFT computations, we propose a silylium ion migration dominated hydroamidation (SMH) mechanism (Fig. 2). According to SMH, the reaction takes place through relatively two independent steps. In the first step, silver(I) activates siloxy-alkyne into silylium ion INT2B and silver-ketene INT2A, following with a proton transfer afford silver(I) coordinated ketene INT3A and silyl-imine INT3B. In the second step, silver(I) promotes nucleophilic addition between INT3A and INT3B afford INT4A, which undergo a silylium ion migration afford final product Z-3. The SMH pathway comply with all experimental observations. The SMH pathway indicate a general approach to obtaining substituted enamides through silver(I)-catalyzed nucleophilic addition between ketenes (INT3A and its similarities) and silyl-imines INT3B. Our calculations show that the silyl-substituted ynamines or silyl-substituted ynamides, and the p–π conjugate system such as diazoles, diazepines, etc may also be suitable substrates for this reaction.
Results and discussion
Nucleophilic attack at carbon atom (SNU mechanism)
At the beginning of the SNU pathway (Fig. 3), the coordination of 1a with the AgNTf2 is exergonic by 10.1 kcal mol−1 (Fig. 3a). AgNTf2 coordinates with the triple-bond of 1a, making 1a susceptible to nucleophilic attack. Kozmin’s earlier report25 also showed strong interaction between the siloxy-alkynes and AgNTf2 in the same condition. The 1a is vulnerable to nucleophilic attack at three points, which are α-C, β-C, and the silicon atom (Fig. 3a). As for the nucleophilic attack on the two sp-hybridized carbon atoms, nucleophilic at the α-C position is much more facile due to the p–π conjugate effect22. So, we only consider the nucleophilic attack against the α-C position. There are two nucleophilic attack directions at the α-C position, if the nucleophilic attack takes place at the AgNTf2 side, cis-addition product Z-3a will be obtained; otherwise, trans-addition product E-3a will be formed. Our calculations indicate that the nucleophilic attack of 2a prefers to occur in the opposite direction of AgNTf2 to generate trans-conformation product E-3a. The main cause of the trans-selectivity is the trans-β effect24. The C–N formation leading to Z-3a is energetically highly disfavored with a barrier (Z-tsa) of 35.8 kcal mol−1, which implies that the SNU pathway is disfavored.
We also calculated the pathway that the isomer of 2a act as nucleophile (see details in Fig. S1). Our calculations show that the barrier of nucleophilic addition step as high as 39.5 kcal mol−1, indicating this pathway is energetically highly disfavored.
Silylium ion migration dominated hydroamidation (SMH) mechanism
The nucleophilic attack at the silicon atom of siloxy-alkyne is often overlooked for the steric effect of the TIPS group. Against our chemical intuition, this process can take place quite easily in some cases22. Enlighted by our previous work22. we proposed a silylium ion migration dominated hydroamidation (SMH) mechanism (Fig. 2).
The key feature of the SMH mechanism is that Ag+ strongly interacts with the C≡C bond of siloxy-alkynes and activates the R3Si group into silylium ion (SiR3+) via the p–π conjugate effect. We have discussed the SMH mechanism in two parts for convenience, the first part (part I) is Ag+ induced SiR3+ and proton exchange between siloxy-alkyne 1 and amide 2, which generates AgNTf2 coordinated ketene int3A and silyl-imine int3B; the second part (part II) is nucleophilic addition between int3A and int3B to form int4A, which then undergo a silylium migration to afford final product Z-3 (Fig. 2). By its nature, the SMH pathway is dominated by the exchange between Ag+ and SiR3+, which has emerged as a useful catalytic method, especially for unique transformations hardly accessible by Lewis acid22,23.
Formation of ketene and silyl-imines
Complexation of AgNTf2 with 1a makes silicon atom of TIPS group susceptible to SN2 nucleophilic attack by carbamate 2a. The barrier of this process (ts1) is only 18.3 kcal mol−1 (Fig. 4). Unlike common SN2 nucleophilic additions in organocarbon chemistry, this process leads to five coordinated silicon complex int1, which then dissociate into silver-ketene int2a and TIPS+ coordinated carbamate int2b. The relatively larger size and the d-orbital of the silicon atom facilitated the SN2 process26,27. The strong interaction between Ag+ and π-bond makes silver-ketene int2a a very good leaving group, which also contribute to SN2 process22.
Formation of int2a converts initial siloxy-alkynes 1a into a strong nucleophile28, the nucleophile N-index of 1a is 3.2, while the nucleophile N-index of 2a is 7.2. Polarity inversion of 1a takes place in this process. The electron transfer from the hydrogen atom of carbamate 2a into the C–Ag bond of the int2a. NBO charge distributions show that the natural charge of C–Ag changed from 044e to −0.16e from inta to int2a, and the natural charge of hydrogen in the carbamate 2a changed from 0.16e to 0.46e. The hydrogen atom of amid-group in the int2b has been activated into the proton by TIPS+.
Our previous work has shown that Ag+ can activate the TIPS group into TIPS+ through p–π conjugation. In this reaction, Ag+ activates TIPS into TIPS+, TIPS+ then activates the hydrogen atom into a proton through p–π conjugation, the net effect is Ag+ activates the hydrogen atom into a proton, which can be regarded as π-acid and σ-acid exchange process.
The proton transfer between the int2b and C–Ag bond of int2a is energetically highly feasible with a barrier (ts2) of only 0.9 kcal mol−1, leading to silyl-imine int3b and AgNTf2 coordinated ketene int3a. In this process, the electron transfer from the electron-rich C–Ag bond into the proton. NBO charge distribution shows that the natural charge of C–Ag changed from −0.16e to 0.11e, and the natural charge of the hydrogen atom changed from 0.46e to 0.30e in this process. The net effect of this process is the AgNTf2 promoted proton and TIPS+ exchange between siloxy-alkyne 1a and carbamate 2a to form ketene int3a-1 and silyl-imine int3b. Ketene int3a-1 and silyl-imine int3b are energetically 2.8 kcal mol−1 more stable than siloxy-alkyne 1a and carbamate 2a.
Recent work by Sun’s group29 reported that Ag+ can promote proton and TIPS+ exchange between siloxy-alkynes and alcohols to generate ketene species. In their report, the alcohols act as sacrificial agents. Their work may provide an experimental support for our proposed mechanism.
In the SMH pathway, silylium ion migration takes place before proton transfer, the reverse of the two steps is energetically disfavored. The most active intermediate is 31.5 kcal mol−1 unstable than reference point (see details in Fig. S2).
Silylium ion migration leads to the final product
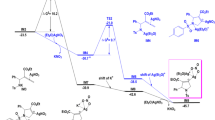
Hydroamination or hydroamidation of allenes is quite easy for the high activity of the two cumulated π-bond1,12,21. Ketenes are analog of allenes, it’s a very important intermediate in the organic chemistry30,31,32,33. Nucleophilic attack favors takes place at the intersection point of the two cumulated π-bond of ketenes. Formation of int4a is quite easy with a barrier of only 8.8 kcal mol−1. The electron is transferred from Si–O to Ag–C in this process. NBO charge distribution shows that the national charge of C–Ag changed from 0.01e to −0.14e, and the natural charge of Si–O changed from 1.22e to 1.32e. The bond length of Si–O changed from 1.72 Å to 1.79 Å (Fig. 5b), which implies the bond between silicon atom and oxygen atom is weakened. The migration of TIPS group generates AgNTf2 coordinated product Z-int5, and the barrier (Z-ts4) of this process is only 14.5 kcal mol−1. In the Z-ts4, the two-carbonyl group is almost in the same plane, and the five p-orbitals constitute a large π-delocalized system, which lowers the TIPS transfer barrier. After the formation of Z-int5, electrons are transferred from C–Ag to Si–O. NBO charge distributions show that the natural charge of C–Ag changes from −0.14e to 0.04e and Si–O changes from 1.32e to 1.26e, respectively. The TIPS migration leads to trans-hydroamidation product E-3a is relatively energetically disfavored, the barrier (E-ts4) of this process is 5.8 kcal mol−1 higher than Z-ts4, which is mainly caused by the steric effect between methyl group (–CH3) and methylene group (–CH2–, Fig. 5b). The transition states (Z-ts4 or E-ts4) of the TIPS migration step favor planar configuration and form a big delocalized π-system, which is very sensitive to the repulsion between methyl (–CH3) and methylene (–CH2–). The averaged distance of the closest hydrogen atoms between methyl (–CH3) and methylene (–CH2–) is 2.29 Å in E-ts4, and 2.49 Å in Z-ts4, respectively (Fig. 5b), which indicate a larger repulsion in the E-ts4.
a Gibbs free-energy profile. Gibbs energies are in kcal mol−1. b Optimized geometries. the hydrogen atom of Z-ts4 and E-TS4 was set to transparent, and the hydrogen atoms of the other geometries were omitted, NTf2- anion, and the isopropyl of the triisopropylsilyl (TIPS) group was set into transparent for clarity.
Our calculations are in accordance with all experimental observations. Kozmin and colleagues claimed that the ynamides and simple internal alkynes are unreactive in the optimized conditions, which indicate an indispensable role of the silyl-group in this reaction. Ynamides are electron richer than siloxy-alkyne, the ynamides should be more reactive if this reaction takes place through the SNU pathway22,23. According to the experiment, when using deuterated amide 2a as substrate, no primary deuterium isotope effect (kH/kD = 1.03) was observed, which means that hydrogen migration is not the rate-determining step. Our calculations show that the barrier of proton transfer (ts2) is only 0.9 kcal mol−1, which is much lower than the barrier of rate-determining step (ts1, 18.3 kcal mol−1).
Kozmin and colleagues carried out a series of kinetic studies and found that the reaction was first-order with respect to both carbamate 2a and the silver catalyst AgNTf2, and zero-order with respect to siloxy-alkyne 1a. According our calculations, when the siloxy-alkyne 1a is at high concentrations, AgNTf2 is saturated with 1a to form inta, and the reaction is zero-order with respect 1a. The siloxy-alkyne activation is followed by the rate-determining step (ts1), which entails 2a as nucleophile. So, the reaction is first-order with respect 2a.
A general way to synthesize substituted enamides
This reaction goes through relatively independent two steps. In the first step, AgNTf2 promote transformation of siloxy-alkyne 1 and amide 2 into ketene int3A-1 and silyl-imine int3B (Fig. 2). In the second step, AgNTf2 promotes nucleophilic addition between ketene int3A-1 and silyl-imine int3B, followed by a silylium ion migration to afford the final product.
Synthesizing substituted ketenes has been extensively studied30,31,32, and silyl-imines can be easily obtained (one of the feasible approaches is the reaction between chlorotriisopropylsilane (TIPSCl) and amide with the base as a catalyst in low temperature). If the hydrogen atom of ketene int3A-1 was changed into other groups (such as halogens and other groups), substituted enamides may be generated, and the regio-selective is steric-controlled (Fig. 6a). So, our calculation provides a general approach to obtaining substituted enamides (Fig. 6). Note that the barrier of int3b tautomerize into int3b-1 through intramolecular TIPS migration is only 22.8 kcal mol−1 (ts3a-1, Fig. 5a, with int4a as the reference point), once the thermodynamically more stable int3b-1 is formed, the reaction will be quenched. So, the smallest substituent R7 of ketene A-INT3A-1 should not be too large to ensure the formation of Z-A-3 is kinetically more favored (Fig. 6a).
This method could also be used to obtain the two amino (or acylamino) groups substituted alkynes Z-B-3 with ketene-imines B-INT3A-1 and silyl-imine INT3B as substrates (Fig. 6b). The regio-selectivity gets rather complicated. In this case, the steric effect of R7 and R2, and the steric effect of R6 and R4 together determine the ΔG of cis-configuration transition state Z-B-TS4. Similarly, the steric effect between R6 and R2, and the steric effect between R7 and R4 together determine the ΔG of the trans-configuration transition state E-B-TS4. Our calculations reveal that when N-acetyl-substituted ketene-imines B-int3a-1 and TMS-substituted imine TMS-int3b are used as a model substrate, the cis-configuration product may be favored (cis-selectivity vs trans-selectivity = 9.0 kcal mol−1 vs. 11.4 kcal mol−1, see details in Fig. S3).
In the reactions we have studied in Fig. 6, the silyl-group of INT3B was activated into silylium ion through the p–π conjugate effect. So, the substrates containing such p–π conjugation segment may be also suitable for this reaction (Fig. 6c). We choose silyl-1,2-diazole C-INT3B as an example to verify this speculation. Our calculations show that the barrier of the rate-determining step of this reaction is only 10.6 kcal mol−1 (see details in Fig. S4).
Substrate scopes of SMH pathway
According to our proposed SMH mechanism, there are rooms to expand the scopes of alkynes. For example, if we change siloxy-alkyne into N-silyl protected ynamide (or ynamine) B-1, this reaction may also take place (Fig. 7a). We use carbamate 2a and N-TMS-substituted ynamide B-TMS-1 as a model to clarify this point of view (see details in Fig. S5), our calculations show that the barrier of the rate-determining step of this reaction is only 18.0 kcal mol−1.
Hydroamidation of B-1 is very sensitive to the volume of the silyl-group, when TIPS-substituted ynamide B-TIPS-1 is used as substrate, this reaction does not take place for the relatively high barrier of silylium ion migration step (25.8 kcal mol−1, Fig. 7a), which is mainly caused by steric effect between TIPS and -acetyl. Owing to the higher barrier of the silylium ion migration step, the isomerization of int3b will occur preferentially (22.8 kcal mol−1 vs. 25.8 kcal mol−1).
In our proposed SMH pathway, the hydrogen atom of amide 2 was activated into silylium ion through the p–π conjugate effect, which implies that some cyclic-p–π conjugation systems, such as diazoles, triazoles, 1H-1,2-diazepine, 1H-1,3-diazepine and so on, may also be suitable substrates for this reaction (see Fig. 7b, c). For example, hydroamination of siloxy-alkyne 1a with 1,2-diazole as substrate is quite easy with a barrier of rate-determining step of only 18.0 kcal mol−1 (see details in Fig. S6). As for 1,3-diazoles, the silylium ion migration of the last step takes place through the SN2 pathway, with a barrier of the rate-determining step only 17.5 kcal mol−1 (see details in Fig. S7). The competing SNU process can be eliminated by reducing the volume of the SiR3 group (Fig. 7c).
Conclusion
Catalyzed hydroamidation of internal alkynes meet limited success yet. Our proposed SMH pathway provides a different perspective for catalyzing hydroamidation of siloxy-alkynes, N-silyl-protected ynamides, or N-silyl-protected ynamines. Our proposed SMH mechanism can be divided into two steps, the first step is Ag+ promoted proton and silylium ion (TIPS+) exchange between siloxy-alkyne and amide, which leads to ketene and silyl-imine, the second step is Ag+ catalyzed nucleophilic addition between ketene and silyl-imine, following with a silylium ion migration lead to the final product. The hydroamidation of N-silyl-protected ynamines or N-silyl-protected ynamides could be completed through a similar pathway. The protocol of synthesis of ketene (or ketene-imines) and silyl-imine have been well documented, which means we can use Ag+ to catalyze ketene (or ketene-imines) and silyl-imine to obtain a variety of substituted enamides. The substrate scope of the N-H source may not only be limited to amides, other p–π conjugated systems such as 1,2-diazoles, 1,3-diazoles, and diazepines may also be suitable for this reaction. In the SMH pathway, the electron extraction between Ag+ and silylium ion (TIPS+) triggers every elementary reaction and leads to the final product. This π-acid and silylium ion exchange scheme may be a concise way to generate silylium ion in situ, which is an important supplement to the silylium ion chemistry.
Methods
Model reaction
Methyl-substituted siloxy-alkyne 1a and carbamate 2a were chosen as model reaction, and AgNTf2 was chosen as the catalyst. Dichloromethane (DCM, ε = 8.93) was chosen as solvent.
Computational details
Density function calculations (DFT) were performed with the Gaussian 16 program package34. The reference point is 1a, 2a, and AgNTf2.
The geometries of ts1 and int1 were optimized under ωb97xd-SMD/def2-svp35,36 level of theory. We could not load geometries of ts1 and int1 in gas phase due to strong electron static effect22,37. The single-point energy of ts1 and int1 was corrected at ωb97xd-gas/def2tzvpp level of theory, so the Gibbs free energy of ts1 and int1 were evaluated with Eq. 1:
The \({\triangle E}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{gas}}}}\) is evaluated with Eq. 2:
and \(\triangle G\) of ts1 and int1 is evaluated with Eq. 3:
\({G}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{sol}}}}{{\mbox{(}}}0{{\mbox{)}}}\) is the Gibbs free energy of reference point evaluated with Eq. 1.
Except for the ts1 and int1, the remaining geometry optimizations were performed under the ωb97xd-gas/def2svp level of theory. Single-point energy was calculated at ωb97xd-gas/def2tzvpp level of theory. Solvation corrections were carried out at the ωb97xd-SMD/def2svp level of theory. So, in this case, Gibbs free energy were evaluated with Eq. 4:
The \({\triangle E}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{gas}}}}\) in the Eq. 4 is obtained by Eq. 2, and \({\triangle E}_{{{\mbox{def}}}2{{\mbox{svp}}}}^{{{\mbox{sol}}}}\) is obtained by Eq. 5
And the \(\triangle {{\mbox{G}}}\) of is evaluated with Eq. 6
\(\triangle {G}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{corr-sol}}}}{{\mbox{(}}}0{{\mbox{)}}}\) is the Gibbs free energy of reference point evaluated with Eq. 4.
This scheme have been proved well suitable for evaluate the reaction barrier of such a system22,37. Theoretically,\(\,\triangle {G}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{corr-sol}}}}\left({{\mbox{SMD}}}\right)\) is a good approximation of \({\triangle G}_{{{\mbox{def}}}2{{\mbox{tzvpp}}}}^{{{\mbox{sol}}}}\).
Vibrational frequency calculations were carried out at the same level of theory as geometry optimization to verify that the optimized geometries are energy minimums or transition states, and to provide thermal corrections for Gibbs free energies and enthalpies at 298.15 K in 1 atm. IRC calculation was performed for the key transition states to verify that the optimized transition states lead to correct structures38. All the optimized geometries (see details in Supplementary Data 1) were rendered with CYLView39.
The electrophilicity ω-index and nucleophilicity N-index
Global and local reactivity index based on conceptual DFT40,41,42 have emerged as a powerful tool in the study polar character reactions. Parr defined the electrophilicity ω-index42 in 1999, which is very useful to estimate electrophilicity of the reactant or intermediates. The ω-index is defined as Eq. 7:
electronic chemical potential μ is defined as Eq. 8, which measures the feasibility of a system to exchange electron density with the environment in the ground state43:
using finite difference approximation, Koopmans theorem44 and Kohn–Sham formalism45 within the DFT, electronic chemical potential μ can be evaluated with28 Eq. 9:
chemical hardness η is defined as Eq. 10. by Parr43, which can be regard as resistance of a molecule to exchange electron density with the environment28
using finite difference approximation and Kohn–Sham formalism28, chemical hardness η is evaluated with Eq. 11:
So, substitute Eq. 9 and Eq. 11. into the Eq. 7. the electrophilicity ω-index28 are calculated with Eq. 12:
Nucleophilicity of molecular have been evaluated in many ways46. Domingo introduced nucleophilicity N-index for closed-shell molecules based on HOMO energies28,47. N-index within Kohn–Sham scheme is defined by Domingo as Eq. 13:
References
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Goossen, L. J., Blanchot, M., Salih, K. S. M. & Goosen, K. Ruthenium-catalyzed addition of primary amides to alkynes: a stereoselective synthesis of secondary enamides. Synthesis 2009, 2283–2288 (2009).
Goossen, L. J. et al. A practical and effective ruthenium trichloride-based protocol for the regio- and stereoselective catalytic hydroamidation of terminal alkynes. Adv. Synth. Catal. 350, 2701–2707 (2008).
Goossen, L. J., Rauhaus, J. E. & Deng, G. J. Ru-catalyzed anti-markovnikov addition of amides to alkynes: a regio- and stereoselective synthesis of enamides. Angew. Chem. Int. Ed. 44, 4042–4045 (2005).
Rodriguez, A. L., Koradin, C., Dohle, W. & Knochel, P. Versatile indole synthesis by a 5-endo-dig cyclization mediated by potassium or cesium bases. Angew. Chem. Int. Ed. 39, 2488–2490 (2000).
Rieder, C. L. & Cole, R. Chromatid cohesion during mitosis: lessons from meiosis. J. Cell Sci. 112, 2607–2613 (1999).
Tashrifi, Z., Mohammadi Khanaposhtani, M., Biglar, M., Larijani, B. & Mahdavi, M. Recent advances in alkyne hydroamination as a powerful tool for the construction of C-N bonds. Asian J. Org. Chem. 9, 969–991 (2020).
Neto, J. S. S. & Zeni, G. Alkynes and nitrogen compounds: useful substrates for the synthesis of pyrazoles. Chem. Eur. J. 26, 8175–8189 (2020).
Hannedouche, J. & Schulz, E. Hydroamination and hydroaminoalkylation of alkenes by group 3-5 elements: recent developments and comparison with late transition metals. Organometallics 37, 4313–4326 (2018).
Sengupta, M., Das, S., Islam, S. M. & Bordoloi, A. Heterogeneously catalysed hydroamination. ChemCatChem 13, 1089–1104 (2021).
Reznichenko, A. L., Nawara-Hultzsch, A. J. & Hultzsch, K. C. In Stereoselective Formation of Amines Vol. 343 Topics in Current Chemistry-Series (eds. Li, W. & Zhang, X.) 191–260 (Springer, 2014).
Fukumoto, Y. Catalytic hydroamination of C-C multiple bonds. J. Syn. Org. Chem. Jpn. 67, 735–750 (2009).
Lyu, X., Zhang, J., Kim, D., Seo, S. & Chang, S. Merging NiH catalysis and inner-sphere metal-nitrenoid transfer for hydroamidation of alkynes. J. Am. Chem. Soc. 143, 5867–5877 (2021).
Serrano-Becerra, J. M. et al. Mono- vs. dinuclear gold-catalyzed intermolecular hydroamidation. Eur. J. Org. Chem. 2014, 4515–4522 (2014).
Wong, V. H. L., Hor, T. S. A. & Hii, K. K. Silver-catalysed intramolecular hydroamination of alkynes with trichloroacetimidates. Chem. Commun. 49, 9272–9274 (2013).
Zhang, X. et al. Silver-catalyzed intramolecular hydroamination of alkynes in aqueous media: efficient and regioselective synthesis for fused benzimidazoles. Green. Chem. 13, 397–405 (2011).
Monge, D., Jensen, K. L., Franke, P. T., Lykke, L. & Jorgensen, K. A. Asymmetric one-pot sequential organo- and gold catalysis for the enantioselective synthesis of dihydropyrrole derivatives. Chem. Eur. J. 16, 9478–9484 (2010).
Kociecka, P., Czelusniak, I. & Szymanska-Buzar, T. Efficient and selective synthesis of e-vinylamines via tungsten(0)-catalyzed hydroamination of terminal alkynes. Adv. Synth. Catal. 356, 3319–3324 (2014).
Kuai, C. et al. Efficient and selective synthesis of (E)-enamides via Ru(II)-catalyzed hydromidation of internal alkynes. Acs Catal. 6, 186–190 (2016).
Sun, J. & Kozmin, S. A. Silver-catalyzed hydroamination of siloxy alkynes. Angew. Chem. Int. Ed. 45, 4991–4993 (2006).
Fang, G. & Bi, X. Silver-catalysed reactions of alkynes: recent advances. Chem. Soc. Rev. 44, 8124–8173 (2015).
Wang, H.-D. & Fan, H.-J. Silylium ion mediated 2+2 cycloaddition leads to 4+2 Diels-Alder reaction products. Commun. Chem. 3, 126–135 (2020).
Turkmen, Y. E., Montavon, T. J., Kozmin, S. A. & Rawal, V. H. Silver-catalyzed formal inverse electron-demand Diels-Alder reaction of 1,2-diazines and siloxy alkynes. J. Am. Chem. Soc. 134, 9062–9065 (2012).
Haines, B. E., Sarpong, R. & Musaev, D. G. Generality and strength of transition metal beta-effects. J. Am. Chem. Soc. 140, 10612–10618 (2018).
Sweis, R. F., Schramm, M. P. & Kozmin, S. A. Silver-catalyzed 2+2 cycloadditions of siloxy alkynes. J. Am. Chem. Soc. 126, 7442–7443 (2004).
Lee, V. Y. Tricoordinate silyl cations (silylium ions). Russ. Chem. Rev. 88, 351–369 (2019).
Mueller, T. In Functional Molecular Silicon Compounds I: Regular Oxidation States: Vol. 155 (Structure and Bonding) (ed. Scheschkewitz, D.) 107–162 (Springer, 2014).
Domingo, L. R., Rios-Gutierrez, M. & Perez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21, 748–769 (2016).
Wu, A., Feng, Q., Sung, H. H. Y., Williams, I. D. & Sun, J. Synthesis of eight-membered lactams through formal 6 + 2 cyclization of siloxy alkynes and vinylazetidines. Angew. Chem. Int. Ed. 58, 6776–6780 (2019).
Huang, L., Wu, J., Hu, J., Bi, Y. & Huang, D. Ketene dithioacetals in organic synthesis. Tetrahedron Lett. 61, 151363–151377 (2020).
Allen, A. D. & Tidwell, T. T. Recent advances in ketene chemistry. ARKIVOC 415–490 (2016).
Allen, A. D. & Tidwell, T. T. In Advances in Physical Organic Chemistry. Vol. 48 (eds. Williams, I. H. & Williams, N. H.) 229–324 (2014).
Minnihan, E. C., Colletti, S. L., Toste, F. D. & Shen, H. C. Gold(I)-catalyzed regioselective cyclizations of silyl ketene amides and carbamates with alkynes. J. Org. Chem. 72, 6287–6289 (2007).
Gaussian 16 Rev. C.01 v. Revision C.01 (Gaussian, Inc., Wallingford, CT, 2016).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Wang, H.-D., Jiang, L., Liang, H.-Y. & Fan, H.-J. Mechanism of silver-catalyzed [2 + 2] cycloaddition between siloxy-alkynes and carbonyl compound: a silylium ion migration approach. Chin. J. Org. Chem. 41, 4327–4337 (2021).
Fukui, K. The path of chemical reactions—the IRC approach. Acc. Chem. Res. 14, 363–368 (1981).
CYLview, 1.0b (Université de Sherbrooke, 2009).
Ayers, P. W. & Levy, M. Perspective on “Density functional approach to the frontier-electron theory of chemical reactivity”. Theor. Chem. Acc. 103, 353–360 (2000).
Geerlings, P., De Proft, F. & Langenaeker, W. Conceptual density functional theory. Chem. Rev. 103, 1793–1874 (2003).
Parr, R. G., Szentpály, L. V. & Liu, S. Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999).
Parr, R. G. & Pearson, R. G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983).
Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1, 104–113 (1934).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Jaramillo, P., Pérez, P., Contreras, R., Tiznado, W. & Fuentealba, P. Definition of a nucleophilicity scale. J. Phys. Chem. A 110, 8181–8187 (2006).
Domingo, L. R., Chamorro, E. & Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 73, 4615–4624 (2008).
Acknowledgements
Financial support was obtained from National Natural Science Foundation of China (21873096,92061114); Chinese Academy of Sciences (XDB17010200).
Author information
Authors and Affiliations
Contributions
H.-D.W. conceived and designed the study, performed calculations, and wrote the manuscript. L.J. and H.-J.F. contributed to design the study. H.-J.F. supervised the project, contributed to write, and review the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, HD., Jiang, L. & Fan, HJ. Silylium ion migration dominated hydroamidation of siloxy-alkynes. Commun Chem 5, 133 (2022). https://doi.org/10.1038/s42004-022-00751-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00751-y
- Springer Nature Limited