Abstract
Ferroptosis is a recently discovered form of cell death that plays an important role in tumor growth and holds promise as a target for antitumor therapy. However, evidence in the regulation of ferroptosis in lung adenocarcinoma (LUAD) remains elusive. Here, we show that retinoic acid receptor alpha (RARA) is upregulated with the treatment of ferroptosis inducers (FINs). Pharmacological activation of RARA increases the resistance of LUAD to ferroptosis according to cell viability and lipid peroxidation assays, while RARA inhibitor or knockdown (KD) does the opposite. Through transcriptome sequencing in RARA-KD cells and chromatin immunoprecipitation (CHIP)-Seq data, we identify thioredoxin (TXN) and protein phosphatase 1 F (PPM1F) as downstream targets of RARA, both of which inhibit ferroptosis. We confirm that RARA binds to the promotor region of TXN and PPM1F and promotes their transcription by CHIP-qPCR and dual-luciferase assays. Overexpression of TXN and PPM1F reverses the effects of RARA knockdown on ferroptosis in vitro and vivo. Clinically, RARA knockdown or inhibitor increases cells’ sensitivity to pemetrexed and cisplatin (CDDP). Immunohistochemistry (IHC) of LUAD from our cohort shows the same expression tendency of RARA and the downstream targets. Our study uncovers that RARA inhibits ferroptosis in LUAD by promoting TXN and PPM1F, and inhibiting RARA-TXN/PPM1F axis represents a promising strategy for improving the efficacy of FINs or chemotherapy in the treatment of LUAD patients.
Similar content being viewed by others
Introduction
Lung cancer represents the most prevalent malignancy and the primary cause of cancer-related mortality globally1. Non-small cell lung cancer (NSCLC) constitutes 82% of lung cancer cases2, with lung adenocarcinoma (LUAD) being the predominant subtype3. Despite current treatment modalities, the five-year survival rate of LUAD remains suboptimal4,5. Thus, exploring the molecular mechanisms underlying LUAD progression and identifying novel therapeutic targets are urgently needed.
Ferroptosis is a distinct form of regulated cell death that differs from apoptosis and other types of cell death6,7. It is characterized by an imbalance of reactive oxygen species (ROS) production8, which catalyzes the peroxidation of polyunsaturated fatty acids (PUFA) on the biological membrane in the presence of intracellular iron overload via the Fenton reaction9,10. In cancer cells, ROS always accumulated at a high level and was a critical factor in ferroptosis sensitivity11, as well as intracellular iron level12. The glutathione peroxidase 4 (GPX4) system, which relies on glutathione (GSH), is the most crucial intracellular mechanism against ferroptosis13. Commonly used ferroptosis inducers (FINs) such as erastin and RSL3 trigger ferroptosis by targeting the GPX4-dependent GSH system14,15. In recent years, studies based on the application prospect of ferroptosis inducers (FINs)16 in tumor treatment have gradually deepened. FIN could increase the efficacy of conventional therapies such as radiotherapy17, chemotherapy18, and targeted therapy19. When combined with the chemotherapy drug cisplatin (CDDP), erastin is more effective in treating NSCLC than cisplatin alone[18, 20].
Retinoic acid receptor alpha (RARA) is a member of the RAR family and is a transcription factor (TF) regulated by retinoic acid (RA)20. The RARA signaling pathway plays a crucial role in growth and remodeling in tissue homeostasis and participates in the regulation of multiple crucial biological pathways, such as growth, differentiation, reproduction, and tumor progression21. Previous studies have shown that the RARA pathway is associated with the proliferation and metastasis of lung cancer22 and with resistance to all-trans-retinoic acid (ATRA) therapy23,24. Furthermore, RARA signaling is critical in antioxidant function25,26 and is involved in regulating iron metabolism imbalance through transcriptional regulation27,28. Because of the significance of iron and oxidative stress in ferroptosis initiation, it is important to investigate the unclear connection between RARA and ferroptosis to enhance the role of this vital TF in cancer therapy.
Through transcriptome sequencing, we found that RARA expression increased significantly in LUAD cells after FIN treatment, suggesting that RARA may act as an essential mediator in ferroptosis. In this study, we discovered that RARA inhibited LUAD ferroptosis both in vitro and in vivo. With the combination of RNA-Seq and ChIP-seq, we systematically revealed that RARA was a transcription promotor for thioredoxin (TXN) and protein phosphatase 1 F (PPM1F), both of which were critical suppressors of ferroptosis29,30,31. TXN is a protein that participates in numerous redox reactions within cells. Previous research has demonstrated its involvement in regulating inflammation and inhibiting apoptosis32. It has also been reported to inhibit ferroptosis by modulating the activity of GPX4 and GSH levels29. Protein PPM1F is a member of the PP2C family of Ser/Thr protein phosphatases and was demonstrated to dephosphorylate and negatively regulate PAK30,33,34, which promotes the occurrence of ferroptosis by enhancing Yes-associated protein (YAP) activity31. Furthermore, we demonstrated that the knockdown of RARA stimulated the ferroptosis of LUAD and enhanced the efficacy of chemotherapy pemetrexed and CDDP. Our findings suggest that targeting the RARA-TXN/PPM1F pathway may represent a novel therapeutic strategy for treating LUAD.
Results
Transcriptome sequence suggests RARA as a cardinal ferroptosis mediator
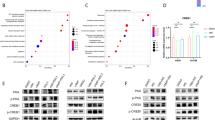
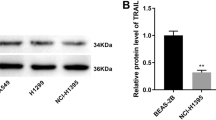
Firstly, we treated A549 cells with the ferroptosis inducers RSL3 (1 μM) and IKE (2.5 μM) for 48 h, and observed a significant upregulation of the seven transcription factors in both FIN-treated groups compared to non-treated cells by RNA sequencing analysis (Fig. 1a, b). Considering the antioxidant and iron-modulating function, we validated the upregulated expression of RARA in mRNA and protein levels in four lung cancer cell lines (Fig. 1c, d). Also, the upregulation of RARA was induced by two other FINs, including erastin (10 μM, 48 h) and FAC (50 μM, 48 h) (Fig. 1e). According to the above results, ferroptosis activates the expression of RARA, suggesting the unexpected relationship between RARA and ferroptosis.
a The volcano plot showed the differentially expressed genes in the FIN-treated group vs. the non-treated group, and RARA was upregulated in the FIN-treated group (A549 cell: RSL3 1 μM and IKE 2.5 μM for 48 h). b Venn plot displayed the ferroptosis-related transcription factors by the intersection of upregulated DEGs in RSL3-treated and IKE-treated groups in A549 cells. c, d qRT-PCR and WB verified the expression of RARA in the FIN-treated group vs. the non-treated group in A549, PC9, H23, and H2122 cells. (n = 3 biologically independent experiments, Student t-test). e qRT-PCR and WB showed the upregulation of RARA by other FINs (erastin 10 μM and FAC 50 μM for 48 h) (n = 3 biologically independent experiments, Student t-test) *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Pharmacological or genetic alteration of RARA changes sensitivity to ferroptosis in LUAD cells
To further understand the role of the RARA pathway in ferroptosis, we pretreated both cell lines with RARA activators and the inhibitor for 24 h and investigated the alteration of cells’ sensitivity to FINs (24 h). Our results demonstrated that specific agonists Ch55 (5 μM) and AM580 (5 μM), as well as the direct agonist ATRA (20 μM), conferred resistance to RSL3 (2 μM) in both A549 (p < 0.01) and PC9 (p < 0.001) cells, with ATRA having the most pronounced effect. Conversely, pre-treatment with the pan-RAR antagonist AGN193109 (5 μM) sensitized cells to RSL3 (2 μM). This effect could be reversed by the ferroptosis inhibitors DFO (100 μM) or ferrostatin-1 (20 μM) (Fig. 2a) but not by the apoptosis inhibitor Z-VAD-FMK (10 μM) or the necroptosis inhibitor necrosulfonamide (1 μM) (Supplementary Fig. 1b). Similar results were obtained upon treatment with IKE (5 μM) (Supplementary Fig. 1a-b). Next, we assessed ferroptosis degrees in different treatment groups. Pre-treatment with agonists decreased the MDA (Fig. 2b) and lipid peroxidation (Fig. 2c) levels in both A549 and PC9 cells, while AGN193109 generated the opposite effect. As for the measurement of mitochondrial membrane potential (MMP, Δψ), RARA activators increased Δψ, indicating mitochondrial hyperpolarization, and AGN193109 decreased it, which showed mitochondrial dysfunction (Fig. 2d). Furthermore, the labile iron pool (LIP) level was measured by the calcein-acetoxymethyl ester (CA-AM) method. Activation of RARA decreased the LIP level, and RARA inhibition enhanced the LIP level (Fig. 2e). The ballooning phenotype was decreased with RARA activators pre-treatment and increased with AGN193109 for 24 h (Fig. 2f).
a CCK8 assays to detect the cell viability of A549 and PC9 cells treated with RSL3 (2 μM), RSL3 (2 μM) plus DFO (100 μM) or fer-1 (20 μM) for 24 h after incubating with DMSO, Ch55 (5 μM), AM580 (5 μM) ATRA (20 μM) or AGN193109 (5 μM) for 24 h. (n = 4 biologically independent experiments, Student t-test). b–e MDA level (b) lipid peroxidation (c), mitochondrial membrane potential (MMP) by TMRE (d), and labile iron pool (LIP) by CA-AM (e) were accessed in A549 and PC9 cells treated with DMSO or RSL3 (1 μM) for 24 h after incubating with DMSO, Ch55 (5 μM), AM580 (5 μM) ATRA (20 μM) or AGN193109 (5 μM) for 24 h. (n = 3 biologically independent experiments, Student t-test). f Light microscopy images showed the degrees of “ballooning phenotype” in A549 and PC9 cells treated with DMSO or RSL3 (1 μM) for 24 h after incubating with DMSO, Ch55 (5 μM), AM580 (5 μM) ATRA (20 μM) or AGN193109 (5 μM) for 24 h. Scale bar:50 μm. The zoomed-in figures, scale bar: 250 μm. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
We further identified the role of RARA in this process by knocking RARA in LUAD cell lines using siRNAs (Supplementary Fig. 1c). Cytotoxicity assays demonstrated that RARA knockdown (KD) sensitized A549 and PC9 cells to RSL3 and IKE (Supplementary Fig. 1d). Next, we designed lentiviruses containing shRNA sequences targeting RARA and transduced them into two cell lines to generate stable RARA-KD clones (Fig. 3a). Similarly, RARA-KD A549 and PC9 cells became more sensitive to ferroptosis induction by RSL3 and IKE (Fig. 3b). The ferroptosis-desensitizing effects, including MDA and lipid peroxidation decrease caused by Ch55, AM580, and ATRA, were abrogated by RARA KD, thus excluding the unspecific RARA-independent effect of these compounds in the regulation of ferroptosis, and the RARA-KD induced ferroptosis more effectively (Fig. 3c–e). Similar results were also verified in the MMP and LIP assays. The Δψ level decreased, and the LIP level enhanced by RARA-KD, indicated more ferroptosis with RARA genetic inhibition. Also, the increase of MMP and the decrease of LIP by RARA activators were impaired by RARA-KD (Fig. 3f, g). The ballooning phenotype verified the effects (Supplementary Fig. 1e).
a qRT-PCR and WB assays confirmed the mRNA and protein expression level of RARA in A549 and PC9 cells after transfection with NC (control shRNA), RARA-SH1, or RARA-SH2 lentivirus. (n = 3 biologically independent experiments, Student t-test) (b) CCK8 assays to detect the cell viability of NC, RARA-SH1, or RARA-SH2 groups in A549 and PC9 cells treated with a gradient dose of RSL3 or IKE for 24 h. (n = 4 biologically independent experiments, two-way ANOVA) (c) CCK8 assays to detect the cell viability of NC, RARA-SH1, or RARA-SH2 groups in A549 and PC9 cells treated with DMSO or RSL3 (2 μM) for 24 h after incubating with DMSO, Ch55 (5 μM), AM580 (5 μM) ATRA (20 μM) or AGN193109 (5 μM) for 24 h. (n = 4 biologically independent experiments, Student t-test). d–g MDA level (d), lipid peroxidation (e), MMP by TMRE (g) and LIP by CA-AM (g) were accessed in NC, RARA-SH1 groups of A549 and PC9 cells treated with DMSO or RSL3 (1 μM) for 24 h after incubating with DMSO, Ch55 (5 μM), AM580 (5 μM) ATRA (20 μM) or AGN193109 (5 μM) for 24 h. (n = 3 biologically independent experiments, Student t-test) ns, not significant *p < 0.05; **p < 0.01; ***p < 0.001;****p < 0.0001.
Knockdown of RARA downregulates the expression of ferroptosis-related genes TXN and PPM1F
As a transcription factor, RARA primarily promotes gene transcription. Thus, to further elucidate the mechanism by which RARA inhibits ferroptosis, we performed RNA-seq analysis to identify the downregulated genes in RARA-KD A549 cells. (Fig. 4a). By intersecting these genes with ferroptosis-associated genes from the FerrDb database (http://www.zhounan.org/)35 and putative RARA targets from the GTRD ChIP-seq dataset (http://www.gtrd.bioumi.org), we identified 4 potential candidate genes, TXN, PPM1F, HMOX1, and GCLC (Fig. 4b). In RARA-KD A549 and PC9 cell lines, the results of qRT-PCR showed that the TXN and PPM1F expression was decreased, while that of HMOX1 and GCLC showed no difference (Fig. 4c). Following treatment of the two cell lines with ATRA (20 μM) and AGN193109 (5 μM) for 24 h, we performed qRT-PCR and observed significant upregulation of TXN and PPM1F in the ATRA group (Fig. 4d), consistent with ATRA-induced changes in gene expression reported in the GEO database36. WB exhibited consistent results with qPCR (Fig. 4e). Furthermore, we validated the expression level of crucial ferroptosis factors including GPX4, ferroptosis suppressor protein 1 (FSP1) and Solute Carrier Family 7 Member 11 (SLC7A11), the qPCR (Fig. 4f) and WB (Fig. 4g) results showed no difference after treatment with ATRA (20 μM) or AGN193109 (5 μM) for 24 h. In summary, our results suggest that the transcription factor RARA upregulates the expression of TXN and PPM1F in LUAD.
a The volcano plot showed the differentially expressed genes in the NC group vs. the RARA-KD group and showed that TXN and PPM1F were down-regulated in the RARA-knockdown group. b Venn plot displayed the predicted targets of RARA by the intersection of DEGs of RNA-Seq, CHIP-Seq, and ferroptosis-related gene set. c qRT-PCR verified the expression of key downstream genes: TXN, PPM1F, HMOX1, and GCLC in the NC group vs. the RARA-KD group in two cell lines. (n = 3 biologically independent experiments, Student t-test). d qRT-PCR revealed the mRNA expression of TXN and PPM1F in A549 and PC9 cells after treatment with ATRA (20 μM) or AGN193109 (5 μM) for 24 h. (n = 3 biologically independent experiments, Student t-test). e WB showed the protein level of TXN and PPM1F in the NC group vs. the RARA-knockdown group in A549 and PC9 cells as well as the two cells after treatment with ATRA (20 μM) or AGN193109 (5 μM) for 24 h. f qRT-PCR showed the expression level of GPX4, FSP1, and SLC7A11 in A549 and PC9 cells. (n = 3 biologically independent experiments, Student t-test). g WB showed the protein level of GPX4, FSP1, and SLC7A11 in A549 and PC9 cells treated with ATRA (20 μM) or AGN193109 (5 μM) for 24 h. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
RARA promotes TXN and PPM1F transcription by binding to promoter regions
Considering that RARA acts as a transcription factor, we consulted the ENCODE database (https://www.encodeproject.org/) and verified the enrichment of RARA in the TXN (Fig. 5a) and PPM1F (Fig. 5d) promoter region close to the transcription start site (TSS). Next, we selected the putative transcription factor binding sites (TFBS) for TXN (Fig. 5b) and PPM1F (Fig. 5e) using the JASPAR network resource (https://jaspar.genereg.net/) and then designed corresponding primers for them(Fig. 5c, f). The results of the ChIP experiment showed that RARA can bind to the BS-1 region of TXN and BS-1 and BS-2 regions of PPM1F promoters (Fig. 5g). These results validate that TXN and PPM1F are the transcriptional targets of RARA.
a Genome-wide data of TXN from the ENCODE database and our CHIP results showed the RARA-binding peak in the promotor region close to the TSS of TXN. b Predicted binding sequences of RARA in TXN showed by JASPAR. c Predicted BS of RARA in TXN and design of primers for ChIP-qPCR. d Genome-wide data of PPM1F from the ENCODE database and our CHIP results showed the RARA-binding peak in the promotor region close to the TSS of PPM1F. e Predicted binding sequences of RARA in PPM1F showed by JASPAR. f Predicted BS of RARA in PPM1F and design of primers for ChIP-qPCR. g ChIP-seq results demonstrated the enrichment of RARA to TXN and PPM1F in A549 and PC9 cells. (n = 3 biologically independent experiments, Student t-test). h Dual-luciferase assays showed the fluorescence intensity of RARA-NC and RARA-KD using luciferase plasmids containing WT or mutated type of the promoter region of TXN and PPM1F. (n = 3 biologically independent experiments, Student t-test) BS binding sites, ChIP chromatin immunoprecipitation, TSS transcription start site, WT wild type. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Next, a dual-luciferase reporter assay was conducted to confirm that RARA directly regulates the transcription of TXN and PPM1F. A firefly luciferase reporter plasmid containing the wild-type RARA promoter region was constructed, along with a corresponding mutant plasmid in which all putative RARA binding sites were randomly altered. In HEK-293T cells transfected with the wild-type plasmid, RARA knockdown significantly reduced firefly luciferase activity. This effect could be abrogated by introducing mutations in the TXN and PPM1F promoter regions (Fig. 5h). The above results reveal the mechanism that RARA promotes the transcription of TXN and PPM1F by binding to their promotors.
TXN and PPM1F reverse the ferroptosis sensitizing effect rendered by RARA knockdown
To validate that RARA inhibits ferroptosis in LUAD by promoting TXN and PPM1F, we generated A549 and PC9 cells overexpressing TXN and PPM1F, respectively (Fig. 6a). Cytotoxicity assays, MDA analysis, lipid peroxidation, and LIP assays demonstrated that respective overexpression of TXN or PPM1F made cells resistant to ferroptosis and partly reversed the ferroptosis-sensitizing effect rendered by RARA knockdown (Fig. 6b–e). The resistant effect was further enhanced when TXN and PPM1F were simultaneously overexpressed. Interestingly, our data revealed that overexpression of TXN confers greater resistance to ferroptosis than PPM1F, potentially due to the downstream action of PPM1F via kinases. We also assessed the sensitivity of cells co-overexpressing TXN and PPM1F to ferroptosis by MMP measurement and transmission electron microscopy. The results showed that mitochondria in RARA-KD A549 cells were significantly wrinkled and exhibited increased mitochondrial membrane density, lower Δψ, indicative of ferroptosis. However, these characteristic changes were not observed in cells co-overexpressing TXN and PPM1F (Fig. 6f, g). The ballooning phenotype was consistent with the above results (Fig. 6h).
a qRT-qPCR and WB showed the TXN and PPM1F expression when transfecting with vector or TXN-overexpression lentivirus or PPM1F-overexpression lentivirus. (n = 3 biologically independent experiments, Student t-test). b Cell toxicity assays demonstrated the rescue effect of TXN and PPM1F on ferroptosis reduced by RSL3 and IKE (24 h) in RARA-KD A549 and PC9 cells. (n = 3 biologically independent experiments, Student t-test). c–f MDA level (c), lipid peroxidation level (d), LIP level (e), and MMP level (f) showed the rescue effect of TXN and PPM1F on ferroptosis induced by RSL3 (1 μM, 24 h) in RARA-KD A549 and PC9 cells. (n = 3 biologically independent experiments, Student t-test). g Significant alterations of mitochondria in NC and RARA-knockdown A549 cells with or without TXN-OE and PPM1F-OE. Scale bar:50 nm. h Light microscopy images showed “ballooning phenotype” degrees in A549 and PC9 cells with or without TXN-OE and PPM1F-OE treated with DMSO or RSL3 (1 μM, 24 h). Scale bar:50 μm. The zoomed-in figures, scale bar: 250 μm. i CCK8 assays showed PPM1F-KD A549 and PC9 cells were more sensitive to RSL3 (2 μM, 24 h), while the PAK phosphorylation inhibitor FRAX486 (0.5 μM, 24 h) had the opposite effect, which can reverse the sensitivity. (n = 4 biologically independent experiments, Student t-test). j–l Lipid peroxidation level (j), MDA level (k), and LIP level (L) in NC and PPM1F-KD A549 and PC9 cells with or without FRAX486 (0.5 μM, 24 h) under the treatment of RSL3 (1 μM, 24 h). (n = 3 biologically independent experiments, Student t-test). m WB showed downregulation of the expression of p-PAK in RARA-knockdown A549 and PC9 cells, while PAK showed no difference. Both PAK and p-PAK showed no difference in NC and TXN-OE cells. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001;****p < 0.0001.
Previous studies indicated that PPM1F inhibited ferroptosis by inhibiting PAK. We constructed PPM1F-KD A549 and PC9 cells (Supplementary Fig. 1f), and demonstrated that p-PAK was decreased in PPM1F-KD cells(Supplementary Fig. 1g). Also, PPM1F-KD was more sensitive to RSL3, and the enhanced effect could be reversed by DFO and fer-1(Fig. 6i). Lipid peroxidation, MDA, and LIP levels also increased in the PPM1F-KD group, which indicated that PPM1F serves as a ferroptosis suppressor (Fig. 6j–l). Furthermore, we used FRAX486 (0.5 μM, 24 h) to inhibit phosphorylation of PAK. As expected, dephosphorylation of PAK conferred resistance to RSL3 in NC and PPM1F-KD cells (Fig. 6i), with decreased lipid peroxidation (Fig. 6j), MDA (Fig. 6k) and LIP(Fig. 6l) levels, indicated that PPM1F inhibits ferroptosis via PAK dephosphorylation. The expression level of PAK showed no difference in NC and RARA-KD cells, but p-PAK was upregulated in RARA-KD A549 and PC9 cells, which demonstrated that RARA stimulated the expression of PPM1F, thereby promoting PAK dephosphorylation and inhibiting ferroptosis. Furthermore, the WB also showed that p-PAK was stable in TXN-OE cells compared to NC cells, which verified that there was no reciprocal regulation between TXN and PPM1F at the protein level (Fig. 6m). The above results demonstrate that RARA inhibits ferroptosis by promoting TXN and PPM1F, respectively.
Pharmacological inhibition and knockdown of RARA enhance the effect of chemotherapy
Our previous study showed that ferroptosis also accounts for the cytotoxicity induced by chemotherapy pemetrexed and CCDP in LUAD37. So we explored the relationship between the effect of chemotherapy and ferroptosis mediated by RARA. Cytotoxicity assays showed that RARA-KD cells enhanced the effect of chemotherapeutic agents, including pemetrexed and CDDP in LUAD, while overexpression of TXN and PPM1F can did the opposite (Fig. 7a). Furthermore, FIN combined with chemotherapeutic agents showed more effect, and genetic or pharmacological inhibition of RARA enhanced the sensitivity (Fig. 7b). We conducted survival analysis in LUAD patients who received chemotherapy in TCGA and found that expression of RARA, as well as TXN and PPM1F, showed resistance to chemotherapy (Supplementary Fig. 1h). All the results above indicated that the RARA signaling pathway could be a potential target in combination therapy.
a Dose-toxicity curves showed relative viability of NC and RARA-SH1 A549 and PC9 cells transfected with or without TXN-OE + PPM1F-OE upon a gradient dose CDDP or pemetrexed treatment for 24 h. (n = 3 biologically independent experiments, two-way ANOVA). b CCK8 assays showed the cell viability in NC, RARA-SH, inhibitor AGN193109 (5 μM, 24 h) groups of A549 and PC9 cells treated with RSL3 (2 μM) or IKE (5 μM), with or without CDDP (2 μM), pemetrexed (1 μM) for 24 h. (n = 3 biologically independent experiments, Student t-test). c Flow chart of the process of subcutaneous tumor formation in male nude mice and the treatment measures. d Representative tumors formed in nude mice by NC, RARA-SH1, NC + TXN-OE + PPM1F-OE, RARA-SH1 + TXN-OE + PPM1F-OE. Tumors were resected after 4 weeks. e Tumor volumes were measured weekly. (n = 6 biologically independent animals, two-way ANOVA). f The tumor weight of each group was weighed after 4 weeks. (n = 6 biologically independent animals, two-way ANOVA). g Representative images of immunohistochemistry staining of mice tumor. Scale bar:100 μm. h Representative images of immunohistochemistry staining of 100 LUAD patient samples. Scale bar:100 μm. i Mechanism diagram for RARA inhibiting ferroptosis through TXN and PPM1F. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
RARA inhibits ferroptosis by promoting TXN and PPM1F in vivo
To further investigate the effects of RARA on ferroptosis in vivo, we carried out a xenograft tumor formation assay in male nude mice. Tumor xenografts were generated with NC, RARA-SH1, NC + TXN-OE + PPM1F-OE, and RARA-SH1 + TXN-OE + PPM1F-OE cell lines. Then mice were treated with either DMSO or IKE (30 mg/kg) or CDDP (6 mg/kg) or IKE (30 mg/kg) plus CDDP (6 mg/kg)via intraperitoneal injection every three days for six doses (Fig. 7c, created with BioRender.com). Treatment with IKE and CDDP significantly affected tumor growth compared to DMSO-treated controls. The combination treatment is more effective in slowing tumor growth compared to monotherapy. Furthermore, in both monotherapy and combination groups, RARA knockdown resulted in reduced tumor volume and weight, an effect that was reversed by overexpression of TXN and PPM1F, which was consistent with vitro experiments (Fig. 7d–f). The IHC staining showed that RARA, TXN, and PPM1F expression were all decreased in RARA knockdown mice tumors, and they showed the same tendency of upregulation upon IKE or CDDP treatment groups (Fig. 7g).
We further conducted IHC staining in 100 LUAD patients from our institution with high or low expression of RARA. The results showed that the expression of TXN and PPM1F showed the same tendency with RARA (Fig. 7h).
Discussion
RARA is a member of the retinoic acid receptor family. Retinoic acid (RA) binds to RARA and plays a crucial role in cell growth and differentiation during embryonic development and adult physiology. Disruption of the RA signaling pathway is implicated in the development and progression of various malignancies21. ATRA is an active metabolite of vitamin A used in the treatment of various tumors, including lung cancer24, due to its effects on cell differentiation, proliferation, and apoptosis38,39. However, resistance to ATRA therapy can occur. Multiple studies have shown a close relationship between RARA and resistance to ATRA. Aberrant RARs phosphorylation and activity are associated with tumor resistance to ATRA40. In acute promyelocytic leukemia (APL), some rare RARA gene rearrangements exhibit resistance to ATRA and arsenic trioxide (ATO), including ZBTB16- RARA and STAT5B- RARA41. The signaling pathways of RARA and PI3K interact to activate the ERK pathway, leading to ATRA resistance22.
Ferroptosis is a distinct form of cell death characterized by iron-dependent ROS accumulation and phospholipid peroxidation. It is a nonapoptotic process that can be initiated by inhibiting the biosynthesis of glutathione or the activity of the glutathione-dependent antioxidant enzyme GPX442. In recent years, ferroptosis induction has emerged as a promising therapeutic strategy for treating various tumors, including lung cancer43. In our research, we demonstrated that RARA inhibited ferroptosis in LUAD by promoting TXN and PPM1F transcription (Fig. 7i, created with BioRender.com).
We first discovered that the induction of ferroptosis is more difficult in A549 and PC9 cell lines following treatment with RARA activators (Ch55, AM580, and ATRA), while cells with RARA knockdown or treated with RARA inhibitor (AGN193109) are more sensitive to ferroptosis. Among the RARA activators tested, ATRA had the most pronounced effect, potentially due to its known antioxidant properties that inhibit lipid peroxidation in addition to its ability to activate RARA. These findings suggested that resistance to ATRA in treating lung cancer may be related to the regulation of ferroptosis by RARA and that inhibition of RARA could be a potential target for combination therapy.
TXN is a potent antioxidant that mediates many biological processes, including redox signaling and is closely related to tumor pathogenesis and therapy44. In tumor tissues, the upregulation of redox homeostasis in intrinsic tumor cells leads to increased levels of TXN and GSH, which reduce the production of ROS. This limits the effectiveness of anti-ferroptosis drugs45. Previous studies have demonstrated that overexpression of TXN can reverse the decrease in GPX4 and GSH levels and inhibit ferroptosis29. PAK is an effector kinase of small GTPases implicated in the development of various tumors due to its dysregulated activity or expression. PPM1F, expressed in various tumor cell lines, has been shown to dephosphorylate and downregulate PAK activity, with reports indicating its role in modulating breast cancer cell invasion34. Our study has confirmed that TXN and PPM1F are downstream targets of RARA and that there was no interaction between the two. PPM1F further inhibits ferroptosis by negatively regulating PAK. The inhibitory effect of RARA could be reversed by overexpression of TXN and PPM1F in both in vivo and in vitro experiments, further highlighting the vital role of inhibiting RARA in the treatment of inducing ferroptosis in cancer. Interestingly, the reversal effect of PPM1F overexpression on the increased sensitivity of RARA-KD cells to ferroptosis was more pronounced than that of TXN overexpression. This result may be attributed to TXN being an antioxidant and inhibiting ferroptosis. The relationship between PPM1F and ferroptosis has not been clearly studied, but its negative regulation of PAK, which has been shown to promote ferroptosis, suggests that the effect of RARA regulating TXN to inhibit ferroptosis is more pronounced.
However, several issues warrant further discussion and investigation. Firstly, although RARA has been proposed as a promising therapeutic target, no specific drug targeting RARA has been developed. Thus, developing effective and safe RARA inhibitors represents an important area for future research. TXN and PPM1F have been identified as critical regulators of ferroptosis in our study, but the mechanisms by which TXN modulates iron metabolism in LUAD remain unclear. Further investigation into the roles of TXN in LUAD is therefore warranted.
Tumors resistant to conventional therapies or prone to metastasis have been found to exhibit a high degree of vulnerability to ferroptosis46,47, highlighting the role of ferroptosis in treating patients with malignancies. Therefore, applying FIN in combination with chemotherapy or other antitumor drugs may provide an alternative solution for cancer patients. In our research, applying RARA inhibitor or knockdown of RARA increased the sensitivity of LUAD cells to ferroptosis and evaluated the effect of chemotherapy. Thus, targeting the RARA pathway may offer a novel approach to the comprehensive management of LUAD patients and provide more evidence for overcoming ATRA resistance.
Methods
Cell culture and compounds
We obtained four LUAD cell lines (A549, PC9, H23, H2122) and human embryonic kidney 293 (HEK293T) cells from the cell bank of the Chinese Academy of Sciences, which were cultured in DMEM high glucose medium (Hyclone, UT, USA) added with 10% fetal bovine serum (Every Green, Hangzhou, Zhejiang, China), 0.1 mg/ml streptomycin and 100 U/ml penicillin (Sangon Biotech, Shanghai, China). The cells were kept in a humidified environment with 95% air and 5% CO2. The RSL3, Imidazole Ketone Erastin (IKE), Erastin, Ferric ammonium citrate (FAC), RARA inhibitor AGN193109, RARA activator Ch55 and AM580, PAK inhibitor FRAX486, cisplatin (CDDP), and pemetrexed were acquired from Topscience (Shanghai, China) and were dissolved in dimethyl sulfoxide (DMSO). ATRA was purchased from Beyotime (Zhejiang, China). Other reagents, including ferrostatin-1(fer-1), Z-VAD-FMK(Z-VAD), deferoxamine (DFO), and necrosulfonamide (necro), were obtained from TargetMol (MA, USA).
Transfection of siRNA, lentivirus, and gene overexpression
Two siRNAs (siRARA-1 and siRARA-2) targeting RARA and two negative control siRNAs (siNC-1and siNC-2), siRNA targeting PPM1F (siPPM1F) were designed by Ribobio (Ribobio, Guangzhou, China). They were transfected into cells using lipo8000 as transfection reagents (Beyotime, Zhejiang, China) and Opti-MEM (Thermo Fisher Scientific, MA, USA), following the manufacturer’s guidelines. The sequences of siRNAs are shown in Supplementary Table 2. Owing to their capacity to silence the RARA gene, two shRNAs were designed according to the siRARA sequences and subcloned into puromycin-resistant lentiviral vectors (Genechem, Shanghai, China). Subsequently, cells were transfected with the lentiviral vectors and subjected to puromycin selection for 48 h. Similarly, we constructed lentiviral vectors (Genechem) to mediate the overexpression of TXN and PPM1F and subsequently transfected them into cells.
RNA extraction and quantitative real-time PCR (qRT-PCR)
We used TRIzol reagent (Tiangen, Beijing, China) for total RNA extraction according to the manufacturer’s instructions. The Hifair® II First-strand cDNA Synthesis Kit (YEASEN, Shanghai, China) was used to transcribe RNA into complementary DNA. We performed qRT-PCR using the Hieff® qPCR SYBR Green Master Mix (YEASEN) in the ABI QuantStudio 5 real-time PCR system (Thermo Fisher, Waltham, MA, USA). Samples were added in three replicates, and mRNA expression was assessed using the 2-ΔΔCT parameter and β-actin as endogenous controls. All primers were purchased at Sangon Biotech (Shanghai, China) and can be seen in Supplementary Table 2.
Western blot (WB) experiments
Proteins were extracted from cells lysed on ice for 10 min using RIPA buffer (Beyotime, Shanghai, China) in conjunction with a protease and two phosphatase inhibitor cocktails (Topscience, Shanghai, China). Protein quantities were determined by BCA assay (YEASEN, Shanghai, China) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, YAMAY Biotech, Shanghai, China) before being transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were then blocked with 5% non-fat milk for 1 h at room temperature and incubated overnight with a specific primary antibody at 4 °C. After three treatments with Tris-buffered saline-Tween (TBST) solution, the membranes were incubated with horseradish peroxidase (HRP)-labeled secondary antibody (1:2500, Beyotime) in the chamber for 1 h. Protein bands were observed using the Moon Chemiluminescence Kit (Beyotime), and the primary antibodies utilized in this study are listed in Supplementary Table 3.
Cell viability assays
For cytotoxicity experiments, cells were seeded in 96-well plates at a density of 3000 cells per well and incubated for 24 h to allow for attachment and growth. After this initial incubation period, cells were pretreated with RARA activators and the inhibitor for 48 h before being exposed to various concentrations of RSL3 or IKE. Cell viability was assessed using the Cell Counting Kit-8 (Topscience), which measures the activity of cellular dehydrogenases as an indicator of cell metabolic activity. The optical density (OD) values were measured on a microplate reader to ascertain the relative number of viable cells in each well.
Malondialdehyde (MDA) level measurement
The degree of ferroptosis was determined by analyzing the levels of MDA in each experimental group. Briefly, cells were cultured in 6-well plates, and cell lysates by RIPA buffer (Beyotime) were used to measure MDA levels using the MDA Assay Kit (Beyotime), following the manufacturer’s guidelines. The protein quantification was conducted using a BCA assay (YEASEN). The MDA content was evaluated colorimetrically by the reaction with thiobarbituric acid.
Detection of Lipid Peroxidation (LPO) by BODIPY-C11
Cells cultured in 12-well plates were pretreated with various reagents and rinsed with phosphate-buffered saline (PBS, Beyotime). Cells are then resuspended in DMEM medium containing 200 μM BODIPY 581/591 C11 dye (Thermo Fisher, USA) for lipid peroxidation detection and incubated for 30 min at 37 °C. After rinsing three times with PBS, lipid peroxidation levels were assessed using the FITC channel of an Accuri 6 cytometer (BD, USA). Data were analyzed and displayed using FlowJo software (TreeStar, Woodburn, OR, USA).
Detection of Mitochondrial Membrane Potential (MMP)
The assessment of mitochondrial membrane potential (MMP, Δψ) was conducted using the tetramethylrhodamine methyl ester (TMRE) dye (Thermo Fisher, USA). Cells were cultured in 12-well plates and underwent a 24 h exposure to each specific treatment. This was followed by a 30 min incubation period at 37 °C with 200 nM of the fluorescent dye TMRE. After centrifugation at 13,00 rpm for 5 min, the cells were resuspended in 400 μl PBS and were analyzed with a PE channel of an Accuri 6 cytometer (BD, USA). Data were analyzed using FlowJo software (TreeStar, Woodburn, OR, USA).
Labile Iron Pool (LIP) assay
Labile iron pool (LIP) was measured using the calcein-acetoxymethyl ester (CA-AM) method. Cells were cultured in 12-well plates and collected with PBS. Then cells were incubated with 0.125 μM CA-AM (Thermo Fisher, USA) for 15 min at 37 °C. After washing with PBS, cells were treated with DFO (100 mM) or left untreated. Cells were analyzed with FACSAria III flow cytometry (BD). The mean fluorescence intensity (MFI) was calculated using FlowJo software (TreeStar, Woodburn, OR, USA). The difference in the MFI with and without DFO treatment reflects the LIP amount.
Microscopy of “Ballooning” phenotype
Cells were cultured in 12-well plates. After different pre-treatments, light microscopy was used to observe the “ballooning” phenotype of morphological changes in cells12, as a specific feature of ferroptosis. Images were captured by Olympus IX71 microscope (Olympus).
Chromatin immunoprecipitation (ChIP) analysis
Chromatin immunoprecipitation (ChIP) assays were performed using the SimpleChIP® Plus Enzymatic Chromatin IP Kit (Cell Signaling Technology, USA) according to the manufacturer’s protocol. Briefly, cells were treated with formaldehyde to crosslink DNA and associated proteins. Chromatin was then fragmented into 150–900 bp DNA/protein complexes using micrococcal nuclease digestion. Immunoprecipitation was performed using either control IgG or anti-RARA antibody (1:50 dilution, Cell Signaling Technology). Protein-DNA complexes were captured using protein G magnetic beads, and eluted chromatin was subjected to crosslink reversal. DNA fragments were purified using spin columns and quantified by qRT-PCR using the following primer pairs: TXN: Primer 1: F: CGTGGGCGTGTTCGATTCAG, R: AGGGCTTCGGCTCCTGTAAC; PPM1F: Primer 2: F: ACGGGATTAGAGGGCTGACG, R: ACCCATGTGAGCCTCGTCTC; Primer 3: F: AGAGAAGCAAGAGGCTGGTGACT, R: ACGGTGACTGCGGCCCTTTA.
Dual-luciferase reporter analysis
Luciferase reporter gene assays were performed to investigate the regulation of TXN and PPM1F expression. The promoter region of TXN spanning from -2000 to +200 base pairs and PPM1F, spanning from -2000 – +300 base pairs relative to the transcription start site, as well as the corresponding mutant sequence, was cloned into the phy-811 luciferase reporter vector (Hanyin Technology, Shanghai, China). HEK-293T cells with either NC and RARA knockdown were co-transfected with TXN-WT, PPM1F-WT, or Mut plasmids and a Renilla luciferase reporter plasmid using Lipo8000 (Beyotime) at 60–80% confluency. Dual-luciferase reporter assays were performed using a Luciferase Reporter Gene Assay kit (Beyotime) according to the manufacturer’s instructions after 48 h post-transfection. Luciferase activity was measured using a FlexStation 3 Microplate Reader (Molecular Devices, San Jose, California, USA).
Transmission electron microscopy
Treated cells cultured in 6 cm dishes were fixed with a solution containing 2.5% glutaraldehyde to preserve cellular structures by cross-linking proteins. After washing three times with 0.1 M phosphate buffer solution (PBS, pH 7.4) to remove excess glutaraldehyde, cells were further fixed with osmium acid in phosphate buffer. Following dehydration through a graded series of ethanol solutions and embedding in resin, samples were cured in a 60 °C oven for 48 h to allow the resin to harden. Ultrathin sections were then cut using an ultramicrotome and stained with lead citrate and uranyl acetate to enhance contrast. After drying overnight, sections were observed in a Hitachi transmission electron microscope (Hitachi, Japan) to visualize cellular ultrastructure.
Animal experiments
The Animal Ethics Committee of Zhongshan Hospital, affiliated with Fudan University approved all animal experiments. We have complied with all relevant ethical regulations for animal use. Six-week-old male nude mice were purchased from Shanghai Jie Sijie Laboratory Animal Factory and housed in a pathogen-free environment within a laminar flow cabinet. A549 cells (2 × 106) expressing NC, RARA-SH1, NC + TXN-PPM1F-OE, or RARA-SH1 + TXN-PPM1F-OE were resuspended in 100 μL PBS and subcutaneously injected into the right flank of each mouse. Once tumors reached approximately 50 mm3 in size, mice were randomly assigned to eight treatment groups. IKE (30 mg/kg) and CDDP(6 mg/kg) were administered intraperitoneally every three days for six times. Tumor size was measured weekly using vernier calipers, and tumor volume was calculated as (length × width2)/2. Mice were euthanized 4 weeks after tumor cell inoculation.
Patients and tumor specimens
With informed consent, paraffin-embedded specimens of the tumor and adjacent healthy tissues were collected from 100 LUAD patients who underwent surgery from 2016 – 2019 at the Department of Thoracic Surgery, Zhongshan Hospital, Fudan University. All the patient specimens had comprehensive clinicopathology and follow-up information (Supplementary Table 1). The pathologic classification was based on World Health Organization (WHO) guidelines, and the tumor stage was assessed using the eighth edition of the International Union Against Cancer (UICC) Cancer Staging Manual according to the tumor/lymph node/metastasis (TNM) classification. The Zhongshan Hospital Research Ethics Committee at Fudan University approved this project (B2022-180R). All ethical regulations relevant to human research participants were followed.
Immunohistochemistry (IHC)
According to the manufacturer’s protocol, paraffin-embedded tissues were dewaxed, hydrated, and stained using the GTVisionTM III Detection System (GeneTech, Shanghai, China). The antibodies can be seen in Supplementary Table 3. The detailed process can be found in the previous study48. PBS was employed as the negative control for the evaluation, which was determined based on staining intensity and the extent of positive staining. The staining intensity of colored cells was assessed using the immunoreactivity score (IRS) [IRS = Staining intensity × Proportion of positive-stained cells], with a scale of 0 (colorless/negative), 1 (light yellow/weak), 2 (dark yellow/moderate), and 3 (yellowish-brown/strong).
Statistics and reproducibility
All data analysis was performed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA) and R (version 4.1.2). Total RNA was extracted from cells using TRIzol reagent (TIANGEN) and subjected to sequencing. Reads were mapped to genes using TopHat (v.2.0.13) and HISAT2 (v.2.1.0), and raw data were normalized to fragments per kilobase of exon per million mapped reads (FPKM) for downstream analysis. Analysis and visualization of the differentially expressed genes were done using the limma and ggplot2 packages in R. Continuous variables were compared using Student’s t-test or two-way ANOVA, as appropriate. All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. The experiments were conducted in triplicate for every condition, and it was repeated at least 3 times.
Data availability
Source data supporting the findings of this study are available from the corresponding author upon reasonable request and Supplementary data. Numerical source data have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.25992559.v1). RNA-Seq data for A549 cells treated with FINs was deposited in Figshare (https://doi.org/10.6084/m9.figshare.25835980). RNA-Seq data for A549 cells with RARA-KD was deposited in Figshare (https://doi.org/10.6084/m9.figshare.25903963.v1).
The plasmids information are available in Addgene, the ID number is 220375. The gating strategy for all flow cytometry (FACS) plots can be seen in Supplementary Fig. 2. The uncropped WB blots can be seen in Supplementary Fig. 3.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 anccers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Zhang, X. et al. Behind the indolent facade: uncovering the molecular features and malignancy potential in lung minimally invasive adenocarcinoma by single-cell transcriptomics. Adv. Sci. (Weinh) 10, e2303753 (2023).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72, 409–436 (2022).
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398, 535–554 (2021).
Lin, X. et al. KIAA1429 promotes tumorigenesis and gefitinib resistance in lung adenocarcinoma by activating the JNK/ MAPK pathway in an m(6)A-dependent manner. Drug Resist Updat. 66, 100908 (2023).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Sacco, A. et al. Iron metabolism in the tumor microenvironment-implications for anti-cancer immune response. Cells 10, 303 (2021).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Wang, Y. et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 29, 2190–2202 (2022).
Battaglia, A. M. et al. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells 9, 1505 (2020).
Battaglia, A. M. et al. Iron administration overcomes resistance to erastin-mediated ferroptosis in ovarian cancer cells. Front Oncol. 12, 868351 (2022).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Liang, C., Zhang, X., Yang, M. & Dong, X. Recent progress in ferroptosis inducers for cancer therapy. Adv. Mater 31, e1904197 (2019).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Guo, J. et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res. Treat. 50, 445–460 (2018).
Sun, X. et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184 (2016).
Schubert, M. & Germain, P. Retinoic acid and retinoid X receptors. Cells 12, 0864 (2023).
di Masi, A. et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Aspects Med. 41, 1–115 (2015).
Quintero Barceinas, R. S., García-Regalado, A., Aréchaga-Ocampo, E., Villegas-Sepúlveda, N. & González-De la Rosa, C. H. All-trans retinoic acid induces proliferation, survival, and migration in A549 lung cancer cells by activating the ERK signaling pathway through a transcription-independent mechanism. Biomed. Res. Int. 2015, 404368 (2015).
Tomita, A., Kiyoi, H. & Naoe, T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int. J. Hematol. 97, 717–725 (2013).
García-Regalado, A., Vargas, M., García-Carrancá, A., Aréchaga-Ocampo, E. & González-De la Rosa, C. H. Activation of Akt pathway by transcription-independent mechanisms of retinoic acid promotes survival and invasion in lung cancer cells. Mol. Cancer 12, 44 (2013).
Tsuchiya, H. et al. Suppressive effects of retinoids on iron-induced oxidative stress in the liver. Gastroenterology 136, 341–350.e348 (2009).
Bi, G. et al. Retinol saturase mediates retinoid metabolism to impair a ferroptosis defense system in cancer cells. Cancer Res. 83, 2387–2404 (2023).
Han, L., Liu, Y., Lu, M., Wang, H. & Tang, F. Retinoic acid modulates iron metabolism imbalance in anemia of inflammation induced by LPS via reversely regulating hepcidin and ferroportin expression. Biochem. Biophys. Res. Commun. 507, 280–285 (2018).
Yoshikawa, O. et al. A retinoic acid receptor agonist tamibarotene suppresses iron accumulation in the liver. Obesity (Silver Spring) 21, E22–E25 (2013).
Bai, L. et al. Thioredoxin-1 rescues MPP(+)/MPTP-induced ferroptosis by increasing glutathione peroxidase 4. Mol. Neurobiol. 58, 3187–3197 (2021).
Koh, C. G., Tan, E. J., Manser, E. & Lim, L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr. Biol. 12, 317–321 (2002).
Wu, J. et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402–406 (2019).
Bai, L., Zhang, S., Zhou, X., Li, Y. & Bai, J. Brain-derived neurotrophic factor induces thioredoxin-1 expression through TrkB/Akt/CREB pathway in SH-SY5Y cells. Biochimie 160, 55–60 (2019).
Grimm, T. M. et al. Lockdown, a selective small-molecule inhibitor of the integrin phosphatase PPM1F, blocks cancer cell invasion. Cell Chem. Biol. 29, 930–946 (2022).
Susila, A. et al. The POPX2 phosphatase regulates cancer cell motility and invasiveness. Cell Cycle 9, 179–187 (2010).
Zhou, N. et al. FerrDb V2: update of the manually curated database of ferroptosis regulators and ferroptosis-disease associations. Nucleic Acids Res. 51, D571–d582 (2023).
Tsotakos, N. et al. All trans-retinoic acid modulates hyperoxia-induced suppression of NF-kB-dependent Wnt signaling in alveolar A549 epithelial cells. PLoS One 17, e0272769 (2022).
Bi, G. et al. miR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Mol. Ther. Nucleic Acids 28, 366–386 (2022).
Leelawat, K., Ohuchida, K., Mizumoto, K., Mahidol, C. & Tanaka, M. All-trans retinoic acid inhibits the cell proliferation but enhances the cell invasion through up-regulation of c-met in pancreatic cancer cells. Cancer Lett. 224, 303–310 (2005).
Hormi-Carver, K., Feagins, L. A., Spechler, S. J. & Souza, R. F. All trans-retinoic acid induces apoptosis via p38 and caspase pathways in metaplastic barrett’s cells. Am J Physiol. Gastrointest Liver Physiol. 292, G18–27, (2007).
Duong, V. & Rochette-Egly, C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta 1812, 1023–1031 (2011).
Cicconi, L. et al. Characteristics and outcome of acute myeloid leukemia with uncommon retinoic acid receptor-alpha (RARA) fusion variants. Blood Cancer J. 11, 167 (2021).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Chen, P. et al. Erianin, a novel dibenzyl compound in dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct Target Ther. 5, 51 (2020).
Zhang, J., Li, X., Zhao, Z., Cai, W. & Fang, J. Thioredoxin signaling pathways in cancer. Antioxid Redox Signal 38, 403–424 (2023).
Lin, Y. et al. Radiotherapy-mediated redox homeostasis-controllable nanomedicine for enhanced ferroptosis sensitivity in tumor therapy. Acta Biomater. 159, 300–311 (2023).
Tsoi, J. et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell 33, 890–904 e895 (2018).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Niu, H. et al. Knockdown of SMAD3 inhibits the growth and enhances the radiosensitivity of lung adenocarcinoma via p21 in vitro and in vivo. Int. J. Biol. Sci. 16, 1010–1022 (2020).
Acknowledgements
This research was funded by the Special Foundation for Supporting Biomedical Technology of Shanghai, China (No. 22S11900300) and Innovation Fund for Science and Technology of Zhongshan Hospital, Fudan University (2023-2ZSCX24).
Author information
Authors and Affiliations
Contributions
(I) Conceptualization and design were contributed by Cheng Zhan and Qun Wang; (II) Administrative support was contributed by Qun Wang; (III) Experiments were conducted by Yunyi Bian, Guangyao Shan, and Jiaqi Liang; (IV) Collection and assembly of data were contributed by Yunyi Bian, Qihai Sui and Guoshu Bi; (V) Data analysis and interpretation were contributed by Zhengyang Hu, Haochun Shi, Guoshu Bi, and Yunyi Bian; (VI) Manuscript writing was contributed by Yunyi Bian; (VII) Final approval of the manuscript was contributed by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Flavia Biamonte, Spencer Gibson, Steven de Jong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bian, Y., Shan, G., Liang, J. et al. Retinoic acid receptor alpha inhibits ferroptosis by promoting thioredoxin and protein phosphatase 1F in lung adenocarcinoma. Commun Biol 7, 751 (2024). https://doi.org/10.1038/s42003-024-06452-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06452-7
- Springer Nature Limited