Abstract
Lobopodians represent a key step in the early history of ecdysozoans since they were the first animals to evolve legs within this clade. Their Cambrian representatives share a similar body plan with a typically cylindrical annulated trunk and a series of non-jointed legs. However, they do not form a monophyletic group and likely include ancestors of the three extant panarthropod lineages (Tardigrada, Onychophora, Euarthropoda). Some species display astonishing protective devices such as cuticular plates and spines. We describe here the armor and molting process of Microdictyon from the early Cambrian of China. Microdictyon secreted ovoid paired cuticular sclerites that were duplicated in a non-synchronous way along the animal’s body. The reticulated pattern and cuticular architecture of these sclerites have similarities to extant armored tardigrades that recently served in hypothesizing that tardigrades are possibly miniaturized lobopodians. Ecdysis and hard cuticular protection are now well documented in the whole spectrum of early Cambrian ecdysozoans such as soft-bodied scalidophorans, lobopodians and fully articulated euarthropods. We hypothesize that the secretion of sclerotized cuticular elements periodically renewed via ecdysis was a key innovation that opened large-scale evolutionary opportunities to invertebrate animal life, specifically ecdysozoans, both in terms of anatomical functionalities and ecological success.
Similar content being viewed by others
Introduction
Ecdysozoans form a huge animal clade1 that includes euarthropods (hexapods, crustaceans, myriapods, chelicerates), onychophorans, tardigrades, and a variety of scalidophoran (e.g. priapulids) and nematoid vermiform organisms, and have a very rich fossil record from the early Cambrian onwards. In contrast to all other animals that grow in a gradual manner, all ecdysozoans undergo stepwise molting stages during which their cuticle is shed and renewed. The intermolt stage is characterized by the expansion of internal tissues, whereas the external size of the exoskeleton remains unchanged, thus giving the ecdysozoan growth a unique incremental pattern, well-exemplified by modern euarthropods. Ecdysis in crustaceans and insects is controlled by a complex gene regulatory network, the endocrine system and specific neurosecretors, resulting in the biosynthesis of ecdysteroids such as ecdysone (E) and 20-hydroxyecdysone (20E) (e.g. refs. 2,3,4). The molting pathway of other ecdysozoan groups, noticeably the scalidophorans, is, however, far less understood. Comparative biochemical, genomic and transcriptomic analyses3 revealed that the required genes responsible for the biosynthesis of ecdysteroids are present in non-arthropod and even in non-ecdysozoan groups, suggesting that key genetic elements of the molting pathway probably evolved prior to the rise of arthropods (early Cambrian) and perhaps among some of the deepest branches of the animal tree (e.g. ecdysis-related neuropeptids5). Although fossil ecdysozoans can rarely be observed during the act of molting (e.g. Cambrian euarthropods such as Marrella6 and Alacaris7, loriciferans8 and worms such as Cricocosmia9), exuviae do occur in the fossil record. Several criteria can be used to confidently distinguish exoskeleton molts (exuviae) from carcasses (whole bodies), especially in trilobites10,11 that grew through incremental growth stages as modern arthropods. Large accumulations of assumed exuviae suggest that the mid-Cambrian Burgess Shale euarthropods Alalcomenaeus and Canadaspis performed synchronized molting12 as do extant crustaceans such as krill in which molting and mating occur simultaneously13. Recently, ecdysis has been described in 535-million-year-old scalidophorans from China14. These vermiform organisms molted in a manner similar to that of extant priapulids, i.e., by turning their old cuticle inside out or by extricating themselves smoothly from their exuvia. These fossil exuviae provide the oldest known direct evidence of ecdysis in animals and show that molting was already operational among one the most basal ecdysozoan groups (e.g. ref. 15), well before the divergence of panarthropods.
We focus here on Cambrian lobopodians that, in most recent phylogenies (e.g. ref. 16), stand in a relatively basal position and represent the earliest leg-bearing animals, and describe their molting process in detail. Lobopodians are non-segmented panarthropods17 resembling modern onychophorans, characterized by soft legs and, in many taxa, by a paired dorsal armature made of rigid sclerites (e.g. Microdictyon) or spines with a cone-in-cone structure (e.g. Hallucigenia18,19). Their molting behavior has received little attention from scientists, although duplicated sclerites have been reported in Cambrian small shelly fossils (SSF) assemblages20,21 and Burgess-Shale-type (BST) Lagerstätten22, and interpreted as possibly resulting from the overlap of old and newly secreted sclerites. We describe here almost complete specimens of the lobopodian Microdictyon sinicum (see refs. 22,23,24) that were buried alive in sediment during the pre-molt stage. Although lobopodians have no direct counterpart among modern animals, their sclerite structure and segmental distribution resemble that of extant armored limno-terrestrial tardigrades, the echiniscids. We analyze these similarities for the first time in light of recent evolutionary scenarios25. Finally, the secretion of sclerotized elements coupled with ecdysis are seen as key innovations that might have shaped the early evolution of animal life and ecosystems.
Results and discussion
Description of fossil specimens
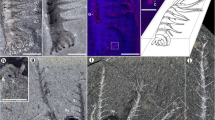
The fossil specimens described here have the diagnostic features of Microdictyon sinicum (e.g. refs. 26,27) such as a tubular body tapering anteriorly into a limbless curved projection, nine pairs of ovoid trunk sclerites bearing a hexagonal mesh, cylindrical perforations and spiky nodes (Figs. 1a–h, 2; Supplementary Fig. 1). Each pair of sclerites is inserted in line with a pair of slender legs bearing terminal claws (Fig. 1i, j). The limbless extension of Microdictyon most probably represents the anterior part of the animal, based on comparisons with other lobopodians (see ref. 28 for homologies), such as Hallucigenia in which eyes and pharynx are accommodated within a comparable anterior feature18,19. The number of appendages is likely to be nine pairs26 rather than ten27. The terminal pair of limbs is the shortest one and its right and left counterparts often lie within the same plane, thus creating the false impression of two distinct pairs of appendages (see ref. 27, Fig. 18). The sclerites of Microdictyon were most probably organic, possibly chitinous, as are the rigid spikes of Hallucigenia18.
a–c General anatomy of the animal Microdictyon; complete specimen (YRCP0041, Jiucun section, Chengjiang City); lateral view, details of mouth and pharynx area, and trunk annulation, respectively. d General view of a laterally compressed specimen (YRCP0040, Xiaolantian section) showing duplicated sclerites (anterior part points to left). e, f Duplicated sclerites (5th pair; see location in d) from different angles of illumination. g, h Duplicated sclerites (4th pair; see location in d) from different angles of illumination. i, j Idealized diagram to show overlapping old and new sclerite on both sides of the body, in life attitude and after burial (body rotated ca. 90° anticlockwise); grey arrows indicate compaction in sediment. k Laterally compressed specimen showing right and left sets of sclerites. l Sclerite overlapping represented by colored areas (new sclerites larger than old ones). Sclerites are numbered 1–9 from anterior to posterior. an annulation, ANT anterior end, ap appendage, bo body, cu cuticle, dt digestive tract, L left body side, no node, ns new sclerite, ns(l) new sclerite (left), ns(r) new sclerite (right), nr negative relief; os old sclerite, os(l) old sclerite (left), os(r) old sclerite (right), POST posterior end, pr positive relief, R right body side, ri ring, se sediment, tc terminal claw, w wall of reticulum. Scales bars: 1 cm in (a); 5 mm in (b, d); 2 mm in (c); 1 mm in (e–h).
a, b General view showing circular perforations and nodes with a polygonal distribution; quadra-, penta-, hexa- and heptagonal pattern highlighted in yellow, blue, red and green color; hexagonal pattern dominant. c, d Details of cuticular wall around perforations showing mushroom-like features at nodes; yellow lines indicate central axis of elevated features at nodes. e, f Details on the inner surface showing rimmed circular perforations; yellow circles indicate the basis of nodes. All SEM images, courtesy of Bing Pan. mls mushroom-like structure, no node, pe perforation, 4–7 quadragonal to heptagonal pattern. Scale bars: 200 µm in (a, b, e, f); 100 µm in (c, d).
Specimen YRCP0040 (Xiaolantian section; Figs. 1d, 3b) displays a complete set of paired sclerites preserved in situ, some of them being duplicated. Pairs 5th–9th show a slight overlap of the right sclerites over the left ones. This configuration results from the collapse of soft tissues after death, followed by lateral compression after burial in sediment, causing the four rigid, duplicated sclerites to come into contact with a slight offset. The lack of duplication in the 1st and 2nd pairs suggests that the molting process was not completely achieved when the animal died and was buried in the sediment. Specimen CJ-MZ19007(1) (Jiucun section; Figs. 3a, 4a) is well-preserved with nine series of sclerites distributed in pairs along the body. The 1st–3rd, 8th and 9th pairs show a moderate overlap of the left sclerite over the right one. Pairs 4th–7th are duplicated, each with two sets of stalked sclerites. Each duplicated set consists of a larger elliptical element topped with a smaller one, i.e. that the new sclerite is larger than the old one (exuvia). Lateral compression resulted in bringing the two sets of sclerites into contact, the right and left one being made up of concave and convex stacked elements, respectively. Specimen CJ-MZ19007(1) is interpreted as being fossilized in the process of molting. CJ-MZ19007(2) (Jiucun section; Fig. 4a) is an incomplete specimen (anterior part missing) that similarly exhibits four pairs (4th–7th) of duplicated sclerites in the midpart of the body, each with a set of concave and convex elements either stacked or displaced (Fig. 4b–f). YMYD082 (Maotianshan section; Supplementary Fig. 1) is an additional incomplete specimen showing duplicated sclerites most distinctly on the third-to-fifth pairs.
a Complete specimen at intermolt stage (CJHMD-MZ19007; see Fig. 2a, specimen 1); note sight overlap of right and left sclerites (1st–4th pairs). b Complete specimen at molting stage (YRCP0040; see Fig. 1d) showing sclerite duplication in the middle part of the body. c Incomplete specimen at molting stage (CJHMD-MZ19007; see Fig. 4a, specimen 2 and Fig. 4b) showing sclerite duplication in the middle part of the body. Sclerites are numbered 1–9 from anterior (black arrow) to posterior. Body in light gray. Sclerites along the right body side are represented in red (old sclerite) and orange (new sclerite), and those along the left side in blue (old sclerite) and green (new sclerite); see explanation Fig. 1i–k. ant anterior end, bo body, post posterior end. Scale 5 mm for all specimens.
a, b General view of two laterally compressed specimens on the same slab (Jiucun section; CJHMD-MZ19007), part and counterpart (note that only one part of the rock slab is shown), respectively; specimen 1 with 9 pairs of sclerites, intermolt stage; specimen 2 with duplicated sclerites, molting stage. c, d Details of 5th sclerites (see location in a, part) from different angles of illumination. e, f Detail of 3rd and 2nd sclerites, respectively (see location in b, counterpart). Sclerites are numbered 1–9 from anterior to posterior in specimen 1, tentatively 3–8 in specimen 2 (counterpart, 3*–8* in b), the anterior polarity being uncertain. ant anterior end, ns new sclerite, op old sclerite, post posterior end. Scale bars: 5 mm in (a, b); 1 mm in (c–f).
Molting process in lobopodians
The four specimens of Microdictyon described here confirm that lobopodians renewed their cuticle and grew through successive molting stages and reveal previously unknown details of their molting process. The duplicated right and left sets of sclerites suggest that Microdictyon did not shed off its old sclerites immediately after the new ones were formed and that new and old sclerites both remained associated for a while. Isolated sets of stacked sclerites found in other lobopodian species such as Microdictyon jinshaense, M. chinense and Quadratopora zhenbaensis among early Cambrian SSF assemblages29 similarly indicate the temporary retention of the old sclerite over the new one. The presence of both duplicated and non-duplicated sclerites along the same specimen (e.g. Fig. 3) suggests that ecdysis did not take place synchronously along the body but, instead, possibly started in the central region and then spread anteriorly and posteriorly. Interestingly, this recalls the biphasic molting seen in both terrestrial and marine isopods (e.g. refs. 30,31). In contrast with the vast majority of crustaceans that perform monophasic molting, these isopods renew their exoskeleton following two steps- i.e. its posterior part is shed before the anterior one due to the differential responses of the anterior and posterior part of the animal to ecdysteroids. The hypothetical asynchronous molting of Microdictyon might find its origin in comparable variations of the biochemical signal.
The molting mechanism of lobopodians, in its general principles, seems to have been comparable with that of other ecdysozoans (e.g. modern crustaceans). However, it applied to a heterogenous cuticular system made of rigid sclerites and a thinner flexible cuticle that lined the whole annulated body and appendages. We suggest that molting possibly took place through the following sequence (Supplementary Fig. 2): (1) At the intermolt stage the animal was fully protected by a thin and flexible cuticular layer secreted by underlying epidermal cells; this layer was locally thickened and sclerotized to form paired rigid elliptical sclerites; (2) Based on the molting process of extant ecdysozoans, we assume that the secretion of fluid between the epidermis and the cuticle (apolysis) resulted in lysing the basal part of the cuticle, thus initiating ecdysis; (3) Cell division occurred within the epidermal layer and new cuticular material was secreted, including new sclerites; however, old and new sclerites were not completely dissociated from each other at that stage; (4) The final step was characterized by the breakup of the old cuticle and the release of the exuvia whereas the newly secreted sclerites kept growing thicker and possibly harder (sclerotization). The hypothesis that molting may have initiated in the central part of the body would suggest that the animal pulled itself out through a possible mid-dorsal split (Supplementary Fig. 3) and via repeated muscular contractions, as observed in extant crustaceans (e.g. ref. 32) and insects, keeping in mind that a great variety of molting mechanisms occurs among both groups. This mechanical process would have facilitated the shedding of the thin and relatively long ventral appendages. As in the vast majority of present-day euarthropods, Alacaris mirabilis, an early Cambrian fuxianhuiid7 molted its exoskeleton dorsally through a large gap that appeared at the base of its head shield. Microdictyon is probably no exception to the rule, with a newly molted animal emerging dorsally and not ventrally, as assumed for the lobopodian Xenusion33.
The duration of the whole molting process (premolt, ecdysis, postmolt) of extant euarthropods varies according to species and environmental conditions, from several days to months34. However, the active phase of shedding, i.e. when the animal extricates itself from its old cuticle, is relatively short (15 minutes in crayfish32). The duration of the whole molting process is impossible to estimate in Microdictyon. What we observe in the case of Microdictyon corresponds to the pre-molt stage preceding ecdysis.
Composition and function of lobopodian sclerites
Lobopodian sclerites often occur in small shelly fossils (SSF) assemblages as secondarily phosphatized isolated elements, but no evidence points to an originally biomineralized composition. Secondary phosphatization affects both fully organic and biomineralized (e.g. calcified) tissues. For example, the grasping spines of Cambrian chaetognaths, although mineralized in calcium phosphate in SSF assemblages, show an organic composition in Burgess-Shale-type Lagerstätten (see ref. 35) and are chitinous in modern species36,37. Similarly, Steiner et al.38 showed that the sclerites of Microdictyon sinicum, Hallucigenia hongmeia, H. fortis and Onychodictyon ferox are mainly enriched in Fe and C and devoid of detectable traces of Ca and P. These latter chemical elements may have been removed during diagenesis or, more likely, were originally absent. The dorsal spines of Hallucigenia from both BST localities and small carbonaceous fossils (SCF) have remarkably well-preserved microstructures such as internal nested construction and external ornamentation made of irregularly distributed microscales18,19. These spines show an overwhelmingly organic composition with only very faint traces of P in C-rich areas. Similarly, small amounts of P occur in the cuticle of modern arthropods (e.g. insects39 and isopods40) as nano-sized granules of amorphous calcium phosphate that do not constitute a mineralized layer sensu stricto but may simply enhance cuticular hardness. The diffused presence of P in lobopodian sclerites from BST-localities may result from a comparable original cuticular composition. We agree with other authors38,41 that the sclerites of all known Cambrian lobopodians were originally mainly organic, possibly chitinous, irrespective of their morphology (e.g. spines, cones, perforated sclerites). This, together with a consistent dorsal location in pairs aligned to appendages, supports the hypothesis38 that these various cuticular elements are all homologous.
The function of the Microdictyon sclerites has been subject to various interpretations (e.g. ref. 29). Chen et al.42 and Budd43 suggested that they served as anchoring areas for muscles. However, no potential attachment areas such as scars are visible along their internal surface (e.g. ref. 44; Fig. 2e, f) and rare lobopodians with well-preserved muscles45 show no oblique fibers attached dorsally to the inner wall of the cuticle. The hexagonal pattern of the Microdictyon sclerites has been compared with that of arthropod compound eyes and interpreted as structures possibly supporting eye lenses46. Multiple eyes are known in Cambrian arthropods (e.g. Opabinia47,48) but are concentrated in the head. As pointed out by Zhang et al.29, if Microdictyon had multiple eyes, then it would make it a unique case among lobopodians that in their great majority do not possess perforated sclerites (e.g. Hallucigenia). Above all, the strongest argument against this hypothesis is that true eyes occur in the head region of several lobopodians as tiny ocelli (e.g. Miraluolishania, Hallucigenia and Cardiodictyon; see refs. 49,50,51,52). A protective function remains by far the most plausible option28. All types of dorsal sclerites, a fortiori the pointed ones (e.g. Hallucigenia) might have had a defensive or deterrent function at least against potential predators of comparable size.
Comparisons with Cambrian worms
Yu et al.9 described the molting process of Cricocosmia jinningensis, a palaeoscolecidomorph worm that is particularly abundant in the early Cambrian Chengjiang localities. Before complete ecdysis, this worm displayed duplicated longitudinal rows of dorsal sclerites that clearly indicate an on-going splitting process between old and new cuticle identical in every way to that seen in Microdictyon. Whether the sclerites of Cricocosmia are homologous to those of Microdictyon remains uncertain. However, one could not exclude that the genes involved in the dorsal, serial and symmetrical location and secretion of these trunk sclerites were similar in both ecdysozoan groups. How Cricoscomia got rid of its old cuticle via an assumed ecdysial break between the introvert and the trunk as in modern priapulids14, or in a different way, remains unclear9. However, its lack of paired legs and the extremely elongated muscular trunk would suggest that the ecdysial process of palaeoscolecidomorph worms was different from that of lobopodians.
Similarities with the cuticular plates of modern tardigrades
Tardigrades are microscopic panarthropods with four pairs of legs ending in well-developed claws, or more rarely, suction disks and pads in marine forms53,54, many species living in mosses and lichens. The cuticle of most limno-terrestrial heterotardigrades is locally thickened and forms an armor made of dorsal and dorsolateral sclerotized elements such as the cephalic, segmental and median plates that all display bilateral symmetry (Fig. 5a–c; see examples in refs. 55,56,57,58). Although non-ovoid, these paired segmental plates aligned with appendages (Fig. 5a) recall those of Microdictyon (Fig. 1a; Supplementary Fig. 1a, b), Other similarities may concern the structure of the cuticle itself. The cuticle of heterotardigrades is complex (Fig. 5d–k)59,60,61 and exhibits important variations both in external appearance and ultrastructure (e.g. pores, pseudopores, “spongy” layer59). One of the most original features of the heterotardigrade cuticle and particularly evident in dorsal plates, is probably the vertical pillars that link the lower part of the procuticle to the most external layer, the outer epicuticle (Fig. 5d, e; see refs. 56,62). These pillars may interconnect via thin bridges called striae or a more extensive layer (Fig. 5e, f), and outer elements of epicuticle are often distributed to form more or less regular polygonal patterns (e.g. hexagonal; Fig. 5j, k). This pillar structure creates a complex network of internal hollows within the cuticular plates that is either closed (“spongy” structure) or open to the exterior (e.g. via pores63). Interestingly, the sclerites of Microdictyon, although ca. 25 times larger (width), share important features with those of tardigrades, such as (1) a polygonal pattern (4- to 7-, hexagonal dominant; compare Figs. 2b and 5j, k; see ref. 63) with spiky or mushroom-shaped nodes44 (Fig. 2c, d) reminiscent of the pillar structure (Fig. 5d–h) seen in tardigrades; (2) a cuticular architecture made of circular perforations (Fig. 2) and thin walls that correspond to the hollow structure of the tardigrade cuticle (Fig. 5d). The similarities between lobopodians and tardigrades, although rarely explored25, go far beyond their plates and concern other key aspects of their body plan such as their soft (ancestrally telescopic) legs terminated with claws, often terminal mouth, pharyngeal structure and the lack of strong head differentiation25. Thus, close phylogenetic relationships between tardigrades and luolishaniid lobopodians have recently been hypothesized25, suggesting that tardigrades and luolishaniid lobopodians share a common ancestor, echoing earlier studies (e.g. Antennacanthopodia64, Onychodictyon ferox19, and Aysheaia65). Kihm et al.25 suggested that luolishaniids, a group of Cambrian lobopodians without sclerites, had sister-relationships with Tardigrada, thus challenging evolutionary models that instead favor relations with onychophorans19,52. In their phylogenetic tree, luolishaniid lobopodians appear as a possible stem-group Tardigrada, based on characters shared with modern tardigrades (differentiation of legs into two types, dorso-lateral paired structures on mid-head). Luolishania has a rounded head separated from the trunk by a slight constriction. The trunk bears 16 annulated, relatively long legs, each being seemingly associated with a pair of rounded bumps interpreted as sclerites26,27. Surprisingly, other lobopodians bearing sclerites, such as Microdictyon, Hallucigenia, Cardiodictyon and Onychodictyon were resolved in a more basal position before the divergence of Euarthropoda and Onychophora. Although a new phylogenetic study is out of the scope of our paper, Kihm et al.’s phylogeny25 raises questions. The presence of paired sclerites in Microdictyon and allied forms, comparable to those of modern tardigrades, appears as a new character (not considered in Kihm et al.25), and is either a convergence given that the echiniscoideans are a highly specialized heterotardigrade clade adapted to limno-terrestrial environments or, alternatively, might be interpreted as plesiomorphic. Sclerotized dorsal and ventral plates also occur in marine heterotardigrades that are considered as retaining ancestral tardigrade morphology. If so, this character may be of potential significance to resolve the ancestry of tardigrades and, more generally, the placement of lobopodians along the three branches of the panarthropod tree. The detailed description of ca. 250–350 µm long tardigrade-like microfossils from a Middle Cambrian Orsten-type locality in Siberia66,67 may provide new information on the anatomy of early ecdysozoans and how they differ or not from modern representatives of Tardigrada (Gąsiorek and collaborators, in-progress).
a–c General morphology of Echiniscus pellucidus in lateral and dorsal views (cephalic and segmental plates highlighted in colors). d, g, h Transverse sections through a plate of Barbaria ganczareki showing pillars and internal hollows within the cuticle. e, f Pillars in the cuticle of Cornechiniscus madagascariensis. i Intermediate dorsal view of Echiniscus pellucidus showing plates and internal hollows within the cuticle. j, k Details of plate cuticular structure in Claxtonia mauccii showing pillar-like epicuticular bumps distributed in polygons (blue). a–h are SEM images, i–k photographs in phase contrast microscopy. cp cephalic plate, cu cuticle, l1–l4 first to fourth pair of legs, mp median plate, pi pillar, s1–s4 segmental plates (s1 = scapular plate; s4 = caudal plate, s2 and s3 are paired plates with a and b being the left and right elements, respectively) Scale bars: 50 µm in a–c; 20 µm in i–k; 5 µm in e, f; 1 µm in d, g, h. d, g, h, courtesy of Łukasz Michalczyk (see also refs. 56,57,58,59,60,61,62,63,76).
The rise and evolutionary importance of cuticular protection
Direct evidence for molting is now available for many ecdysozoan groups that co-existed in the early Cambrian such as scalidophorans9,14, lobopodians23 and euarthropods10,11. Body and trace fossils suggest that worm-like animals (ecdysozoan or not) lived in the Ediacaran (e.g. Ikaria68) and were the potential makers of numerous sub-horizontal trails and shallow burrows. Treptichnus69,70 is a widespread complex burrow system still used as a marker to define the Precambrian–Cambrian transition. Its detailed morphology and experimental ichnology69 using extant priapulids (Priapulus) both suggest that its maker was a tubular, annulated and contractible worm with an introvert, scalid rows and terminal mouth, thus closely resembling scalidophoran worms. Although the nature of the last common ancestor of ecdysozoans remains hypothetical15, the secretion of a cuticle may be seen as a key innovation that preceded or was contemporaneous with the radiation of ecdysozoans. Schmidt-Rhaesa71 suggested that a preexisted matrix (e.g. glycocalyx; glycoproteins linked to polysaccharide chains) secreted by epidermal cells served as a matrix for the deposition of other molecules such as collagen or chitin, thus giving rise to a cuticular layer. Non-ecdysozoan animals have no chitinous cuticle and use other various methods such as mucous (e.g. flatworms) or more resistant secretions (e.g. annelids) to create a protective boundary layer around their body.
We suggest that three major successive steps may have taken place during the Precambrian–Cambrian transition: (1) Cuticular secretion evolved from precursors with a ciliated epidermis and a glycocalyx; (2) Molting lifted the mechanical constraints imposed by the non-extensible cuticle and incompatible with continuous body growth, i.e. natural selection selected ecdysis as a successful option that reconciled protection and optimal body growth; (3) Sclerotization in the form of rigid cuticular sclerites evolved among vermiform organisms into a variety of complex cuticular 3D-structures such as conical or plate-like sclerites (e.g. palaeoscolecids9,72,73). Whereas the cuticle is an efficient physical barrier that prevents damage due to friction with sediment, sclerites provide additional protection and increase anchoring to sediment. Indeed, sclerotization is likely to have promoted burrowing lifestyles (see evidence from trace fossils; e.g. refs. 69,70,74).
Lobopodians not only inherited the capacity of molting from their probable vermiform legless ancestors but also that of secreting paired sclerites (e.g. spines or plates as in Microdictyon). Whereas sclerites were crucial to the early bioturbators of sediment, they probably played an equally important role for the pioneer epibenthic walkers, that of protecting their soft body dorsally against predators and physical damage. The crucial role of cuticular secretion/sclerotization associated with molting is even more evident in euarthropods that evolved more sophisticated articulated systems involving joints and muscles such as those already seen in early Cambrian megacheirans (arthropods with a great appendage; see, e.g. ref. 16) and more advanced forms. The success of euarthropods shown by the remarkable increase of their diversity and ecological expansion at the beginning of the Cambrian era, would not have been possible without the major innovations of molting and sclerite secretion that preceded their radiation.
Methods
All studied fossil specimens were collected from the Xiaolantian, Jiucun, and Maotianshan sections, ca. 8 km from Chengjiang, Yunnan Province, China, that all belong to the Yu’anshan Member of the Qiongzhusi Formation, and correspond to the Eoredlichia-Wutingaspis biozone, Cambrian Stage 3. Microscopic observations and light photography of fossil specimens were made with a Leica M205C stereomicroscope and a Canon EOS 5D Mark IV digital camera. Details on the methods (scanning electron microscopy, SEM) used for imaging Microdictyon (Fig. 2) are in Pan et al.44. All fossil specimens are housed in the collections of the following Chinese institutions: Yuxi Normal University Research Center of Paleobiology (YRCP numbers), Yuxi, Yunnan Province; Chengjiang Science Museum of the Management Committee of the Chengjiang Fossil Site World Heritage (CJHMD-MZ numbers), Yunnan Province; Yuxi Museum (YM-YD numbers), Yuxi, Yunnan Province. Tardigrades prepared for SEM were first fixed in ethanol and then dehydrated in an ethanol/acetone series prior to critical point drying in liquid CO2. Dried specimens were mounted on stubs, coated with gold, and later observed in SEM (for more details see ref. 75). Information concerning the fossil material and extant tardigrades can be obtained from Ailin Chen (ailinchen@yxnu.edu.cn) and Piotr Gąsiorek (piotr.lukas.gasiorek@gmail.com), respectively.
References
Giribet, G. & Edgecombe, G. D. Current understanding of Ecdysozoa and its internal phylogenetic relationships. Integr. Comp. Biol. 57, 455–466 (2017).
Schumann, I., Kenny, N., Hui, J., Hering, L. & Mayer, G. Halloween genes in panarthropods and the evolution of the early moulting pathway in Ecdysozoa. R. Soc. Open Sci. 5, 180888 (2018).
de Sena Oliveira, I. et al. Functional morphology of a lobopod: case study of an onychophoran leg. R. Soc. Open Sci. 6, 191200 (2019).
Campli, G. et al. The moulting arthropod: a complete genetic toolkit review. EcoEvoRxiv. Preprint at https://doi.org/10.32942/X2DS4S.
Zieger, E., Robert, N. S. M., Calcino, A. & Wanninger, A. Ancestral role of ecdysis-related neuropeptides in animal life cycle transitions. Curr. Biol. 31, 207–213 (2021).
García-Bellido, D. & Collins, D. Moulting arthropod caught in the act. Nature 429, 40 (2004).
Yang, J., Ortega-Hernández, J., Drage, H. B., Du, K. & Zhang, X. Ecdysis in a stem-group euarthropod from the early Cambrian of China. Sci. Rep. 9, 5709 (2019).
Peel, J., Stein, M. & Kristensen, R. M. Life cycle and morphology of a Cambrian stem-lineage loriciferan. PLoS ONE 8, e73583 (2013).
Yu, C., Wang, D. & Han, J. Cambrian palaeoscolecidomorph Cricocosmia caught in the act of moulting. Hist. Biol. 1–7 https://doi.org/10.1080/08912963.2024.2324427 (2024).
Daley, A. C. & Drage, H. B. The fossil record of ecdysis and trends in the moulting behaviour of trilobites. Arthropod Struct. Dev. 45, 71–96 (2016).
Drage, H. B. & Daley, A. C. Recognising moulting behaviour in trilobites by examining morphology, development and preservation: comment on Błażejowski et al. 2015. Bioessays 38, 981–990 (2016).
Haug, J. T., Caron, J.-B. & Haug, C. Demecology in the Cambrian: synchronized molting in arthropods from the Burgess Shale. BMC Biol. 11, 64 (2013).
Tarling, G. A. & Cuzin-Roudy, J. Synchronization in the molting and spawning activity of northern krill (Meganyctiphanes norvegica) and its effect on recruitment. Limnol. Oceanogr. 48, 2020–2033 (2003).
Wang, D. et al. Origin of ecdysis: fossil evidence from 535-million-year-old scalidophoran worms. Proc. R. Soc. B 286, 20190791 (2019).
Howard, R. J. et al. The Ediacaran origin of Ecdysozoa: integrating fossil and phylogenomic data. J. Geol. Soc. 179, jgs2021–jgs2107 (2022).
Aria, C. The origin and early evolution of arthropods. Biol. Rev. 97, 1786–1809 (2022).
Ortega-Hernández, J. Lobopodians. Curr. Biol. 25, R845–R875 (2015).
Caron, J.-B., Smith, M. R. & Harvey, T. H. P. Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians. Proc. R. Soc. B 280, 20131613 (2013).
Smith, M. R. & Ortega-Hernández, J. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366 (2014).
Topper, T. P., Brock, G., Skovsted, C. B. & Paterson, J. R. Microdictyon plates from the lower Cambrian Ajax Limestone of South Australia: implications for species taxonomy and diversity. Alcheringa 35, 427–443 (2011).
Topper, T. P., Skovsted, C. B., Peel, J. S. & Harper, D. A. T. Moulting in the lobopodian Onychodictyon from the lower Cambrian of Greenland. Lethaia 46, 490–495 (2013).
Chen, J.-Y., Zhou, G. & Ramsköld, L. The Cambrian lobopodian Microdictyon sinicum and its broader significance. Bull. Natl Mus. Nat. Sci. 5, 1–93 (1995).
Hou, X.-G. & Bergström, J. Cambrian lobopodians, ancestors of extant onychophorans? Zool. J. Linn. Soc. 114, 3–19 (1995).
Bergström, J. & Hou, X.-G. Cambrian Onychophora or Xenusians. Zool. Anz. 240, 237–245 (2001).
Kihm, J.-H. et al. Cambrian lobopodians shed light on the origin of the tardigrade body plan. PNAS 120, e2211251120 (2023).
Hou, X.-G. et al. The Cambrian fossils of Chengjiang, China. In The Flowering of Early Animal Life (Blackwell, 2004).
Hou, X.-G. et al. The Cambrian fossils of Chengjiang, China. In The Flowering of Early Animal Life (Wiley, 2017).
Ramsköld, L. & Hou, X.-G. New early Cambrian animal and onychophoran affinities of enigmatic metazoans. Nature 351, 225–228 (1991).
Zhang, X.-G. & Aldridge, R. J. Development and diversification of trunk plates of the lower Cambrian lobopodians. Palaeontology 50, 401–415 (2007).
Vani Sahadevan, A., Priya, J. & Kappalli, S. Biphasic moulting in isopods confers advantages for their adaptation to various habitats and lifestyle. Biologia 77, 1067–1081 (2022).
Montesanto, G. & Cividini, S. The moult cycle of the terrestrial isopod Armadillo officinalis Duméril, 1816 (Crustacea: Isopoda: Oniscidea). Acta Zool. 99, 263–273 (2018).
Phlippen, M. K., Webster, S. G., Chung, J. S. & Dircksen, H. Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J. Exp. Biol. 203, 521–536 (2000).
Dzik, J. & Krumbiegel, G. The oldest ‘onychophoran’ Xenusion: a link connecting phyla? Lethaia 22, 169–181 (1989).
Brusca, R. C., Giribet, G. & Moore, W. Invertebrates, 4th edn (Oxford University Press, 2023).
Briggs, D. E. G. & Caron, J.-B. A large Cambrian chaetognath with supernumerary grasping spines. Curr. Biol. 27, 2536–2543 (2017).
Bone, Q., Ryan, K. P. & Pulsford, A. L. The structure and composition of the teeth and grasping spines of chaetognaths. J. Mar. Biol. Assoc. UK 63, 929–939 (1983).
Vannier, J., Steiner, M., Renvoisé, E., Hu, S.-X. & Casanova, J.-P. Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proc. R. Soc. B 274, 627–633 (2007).
Steiner, M., Hu, S.-X., Liu, J.-N. & Keupp, H. A new species of Hallucigenia from the Cambrian Stage 4 Wulongqing Formation of Yunnan (South China) and the structure of sclerites in lobopodians. Bull. Geosci. 87, 107 (2012).
Cribb, B. W., Rasch, R., Barry, J. & Palmer, C. M. Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae). Arthropod Struct. Dev. 34, 41–48 (2005).
Hild, S. et al. Ultrastructure and mineral distribution in the tergal cuticle of the terrestrial isopod Titanethes albus. Adaptations to a karst cave biotope. J. Struct. Biol. 168, 426–436 (2009).
Steiner, M. Taphonomy of Early Cambrian phosphatic fossil remains from South China. Erlanger Geol. Abh. 6, 64–65 (2008).
Chen, J.-Y., Hou, X.-G. & Lu, H.-L. Early Cambrian netted scale-bearing worm-like sea animal. Acta Palaeontol. Sin. 28, 1–16 (1989).
Budd, G. E. Why are arthropods segmented. Evol. Dev. 3, 332–342 (2001).
Pan, B., Topper, T. P., Skovsted, C. B., Miao, L. & Li, G. Occurrence of Microdictyon from the lower Cambrian Xinji Formation along the southern margin of the North China Platform. J. Paleontol. 92, 59–70 (2018).
Zhang, X.-G., Smith, M. R., Yang, J. & Hou, J. Onychophoran-like musculature in a phosphatized Cambrian lobopodian. Biol. Lett. 12, 20160492 (2016).
Dzik, J. Early Cambrian lobopodian sclerites and associated fossils from Kazakhstan. Palaeontology 46, 93–112 (2003).
Whittington, H. B. The enigmatic animal Opabinia regalis, Middle Cambrian, Burgess Shale, British Columbia. Philos. Trans. R. Soc. B 271, 1–43 (1975).
Briggs, D. E. G. Extraordinary fossils reveal the nature of Cambrian life: a commentary on Whittington (1975) ‘The enigmatic animal Opabinia regalis, Middle Cambrian, Burgess Shale, British Columbia. Philos. Trans. R. Soc. B 370, 20140313 (2015).
Liu, J.-N., Shu, D.-G., Han, J. & Zhang, Z. A rare lobopod with well-preserved eyes from Chengjiang Lagerstätte and its implications for origin of arthropods. Chin. Sci. Bull. 49, 1063–1071 (2004).
Liu, J.-N., Shu, D.-G., Han, J. & Zhang, Z. Comparative study of Cambrian lobopods Miraluolishania and Luolishania. Chin. Sci. Bull. 53, 87–93 (2008).
Liu, J.-N. et al. An armoured Cambrian lobopodian from China with arthropod-like appendages. Nature 470, 526–530 (2011).
Smith, M. R. & Caron, J. B. Hallucigenia’s head and the pharyngeal armature of early ecdysozoans. Nature 523, 75–78 (2015).
Fontoura, P., Bartels, P. J., Jørgensen, A., Kristensen, R. M. & Hansen, J. G. A dichotomous key to the genera of the marine heterotardigrades (Tardigrada). Zootaxa 4294, 1–45 (2017).
Møbjerg, N., Jørgensen, A., Kristensen, R. M. & Neves, R. C. Morphology and functional anatomy. In Water Bears: The Biology of Tardigrades. Zoological Monographs, (ed. Schill, R. O.) Vol. 2, 57–94 (Springer, 2018).
Kristensen, R. M. Generic revision of the Echiniscidae (Heterotardigrada), with a discussion of the origin of the family. In Biology of Tardigrades, Selected Symposia and Monographs, (ed Bertolani, R.) 261–335 (Mucchi, Modena, 1987).
Gąsiorek, P. & Michalczyk, Ł. Revised Cornechiniscus (Heterotardigrada) and new phylogenetic analyses negate echiniscid subfamilies and tribes. R. Soc. Open Sci. 7, 200581 (2020).
Gąsiorek, P., Bochnak, M., Vončina, K. & Michalczyk, Ł. Phenotypically exceptional Echiniscus species (Heterotardigrada: Echiniscidae) from Argentina (Neotropics). Zool. Anz. 294, 210–228 (Springer, 2021).
Gąsiorek, P. Water bear with barbels of a catfish: a new Asian Cornechiniscus (Heterotardigrada: Echiniscidae) illuminates evolution of the genus. Zool. Anz. 300, 47–64 (2022).
Michalczyk, Ł. & Kaczmarek, Ł. Echiniscus ganczareki, a new species of Tardigrada (Heterotardigrada: Echiniscidae, bigranulatus group) from Costa Rica. Zootaxa 1471, 15–25 (2007).
Czerneková, M. & Vinopal, S. The tardigrade cuticle. Limnol. Rev. 21, 127–146 (2021).
Schuster, R. O., Grigarick, A. A. & Toftner, E. C. Ultrastructure of tardigrade cuticle. In International Symposium on Tardigrades, 1974 (ed. Higgins, R. P.). Mem. Ist. Ital. Idrobiol. 32 (Suppl.), 337–375 (1975).
Grollman, M. M., Jørgensen, A. & Møbjerg, N. Actinarctus doryphorus (Tanarctidae) DNA barcodes and phylogenetic reinvestigation of Arthrotardigrada with new A. doryphorus and Echiniscoididae sequences. Zootaxa 5284, 351–363 (2023).
Gąsiorek, P. et al. Echiniscidae (Heterotardigrada) of South Africa. Zootaxa 5156, 1–238 (2022).
Ma, X.-Y., Edgecombe, G. D., Legg, D. A. & Hou, X.-G. The morphology and phylogenetic position of the Cambrian lobopodian Diania cactiformis. J. Syst. Palaeontol. 12, 567–575 (2014).
Caron, J.-B. & Aria, C. Cambrian suspension-feeding lobopodians and the early radiation of panarthropods. BMC Evol. Biol. 17, 29 (2017).
Maas, A. & Waloszek, D. Cambrian derivatives of the early arthropod stem lineage, pentastomids, tardigrades and lobopodians, an ‘Orsten’ perspective. Zool. Anz. 240, 451–459 (2001).
Müller, K. J., Waloszek, D. & Zakharov, A. “Orsten” type phosphatized soft-integument preservation and a new record from the Middle Cambrian Kuonamka Formation in Siberia. N. Jahrb. Geol. Paläontol. Abh. 197, 101–108 (1995).
Evans, S. D., Hughes, I. V. & Gehling, J. G. Discovery of the oldest bilaterian from the Ediacaran of South Australia. PNAS 117, 7845–7850 (2020).
Vannier, J., Calandra, I., Gaillard, C. & Żylińska, A. Priapulid worms: Pioneer horizontal burrowers at the Precambrian–Cambrian boundary. Geology 38, 711–714 (2010).
Kesidis, G., Slater, B. J., Jensen, S. & Budd, G. E. Caught in the act: priapulid burrowers in early Cambrian substrates. Proc. R. Soc. B 286, 20182505 (2019).
Schmidt-Rhaesa, A. The Evolution of Organ Systems (Oxford University Press, 2007).
Topper, T. P., Brock, G., Skovsted, C. B. & Paterson, J. R. Palaeoscolecid scleritome fragments with Hadimopanella plates from the early Cambrian of South Australia. Geol. Mag. 147, 86–97 (2010).
Han, J., Liu, J.-N., Zhang, Z., Zhang, X. & Shu, D.-G. Trunk ornament on the palaeoscolecid worms Cricocosmia and Tabelliscolex from the Early Cambrian Chengjiang deposits of China. Acta Palaeontol. Pol. 52, 423–431 (2007).
Mangano, M. G. & Buatois, L. A. The Cambrian revolutions: trace-fossil record, timing, links and geobiological impact. Earth Sci. Rev. 173, 96–108 (2017).
Degma, P. Field and laboratory methods. In Water Bears: The Biology of Tardigrades. Zoological Monographs Vol. 2, 349–369 (ed. Schill, R. O.) (Springer, 2018).
Gąsiorek, P., Degma, P. & Michalczyk, Ł. Hiding in the Arctic and in mountains: a (dis)entangled classification of Claxtonia (Heterotardigrada: Echiniscidae). Zool. J. Linn. Soc. 200, 60–86 (2024).
Acknowledgements
This project was supported by the National Natural Science Foundation of China (42262004, 41662003; 42202009 to D.W) and the State Key Laboratory of Palaeobiology and Stratigraphy, NIGPAS (no. 193104). J.V. thanks the Région Auvergne Rhône Alpes and the Université Claude Bernard Lyon 1 (PAI grant) for financial support. We thank Bing Pan (Nanjing) and Łukasz Michalczyk (Kraków, Poland) for kindly providing SEM images of Microdictyon and extant tardigrades, respectively, and Harriet Drage and Russell Garwood for their constructive reviews.
Author information
Authors and Affiliations
Contributions
A.C., D.W., J.H. and J.V. designed the research. A.C., J.G. and W.M. collected the fossil specimens. D.W., A.C., J.G. and W.M. imaged them. P.G. provided key information and images on extant tardigrades. D,W. made elemental mapping of the specimens. J.V. produced the figures and wrote the manuscript with inputs from all co-authors. All authors discussed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Harriet Drage and Russell Garwood for their contribution to the peer review of this work. Primary Handling Editors: Katie Davis and Luke Grinham.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, A., Vannier, J., Guo, J. et al. Molting in early Cambrian armored lobopodians. Commun Biol 7, 820 (2024). https://doi.org/10.1038/s42003-024-06440-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06440-x
- Springer Nature Limited