Abstract
Ammonia and its amine-containing derivatives are widely found in natural decomposition byproducts. Here, we conducted biased chemoreceptor screening to investigate the mechanisms by which different concentrations of ammonium salt, urea, and putrescine in rotten fruits affect feeding and oviposition behavior. We identified three ionotropic receptors, including the two broadly required IR25a and IR76b receptors, as well as the narrowly tuned IR51b receptor. These three IRs were fundamental in eliciting avoidance against nitrogenous waste products, which is mediated by bitter-sensing gustatory receptor neurons (GRNs). The aversion of nitrogenous wastes was evaluated by the cellular requirement by expressing Kir2.1 and behavioral recoveries of the mutants in bitter-sensing GRNs. Furthermore, by conducting electrophysiology assays, we confirmed that ammonia compounds are aversive in taste as they directly activated bitter-sensing GRNs. Therefore, our findings provide insights into the ecological roles of IRs as a means to detect and avoid toxic nitrogenous waste products in nature.
Similar content being viewed by others
Introduction
Nitrogen is an essential building block for the synthesis of DNA and protein and is the most abundant element in the Earth’s atmosphere. Therefore, the nitrogen cycle is instrumental in maintaining healthy ecosystem dynamics. Animals and many plants produce nitrogenous wastes throughout their life histories. Ammonia and urea are the decomposition byproducts of protein1, whereas rotten fruits are rich in polyamines such as putrescine2,3,4. These organic compounds can be recycled as a source of nitrogen groups such as amines, amides, and anilines, which are central for developmental and physiological processes in both plants and animals5. In nature, ammonia concentration is in the range of 10–20 mM in the cassava plant leaf and the cattle manure6,7.
Animals, including insects, rely on chemoreception for feeding, mating, and escaping from predators8,9,10,11. Moreover, although the anatomy and molecular basis of taste perception in vertebrates and invertebrates are evolutionarily distinct, they share a few similar fundamentals12,13,14. Fruit flies (Drosophila melanogaster) possess specialized taste neurons on their labella, legs, pharynx, wing margins, and ovipositor15,16,17. Flies can sense sweetness, bitterness, sourness, saltiness, and water18,19,20,21,22. Major gustatory organs, such as the labellum and legs, have evolved to recognize chemicals via several channels and receptors, such as gustatory receptors (GRs), odorant receptors (ORs), ionotropic receptors (IRs), transient receptor potential (TRP) channels, and pickpocket ion channels (PPKs)23,24,25,26,27,28. Most taste sensilla harbor four distinct GRNs, of which two are attractive GRNs (sweet-sensing and water-sensing GRNs) and two are aversive GRNs (bitter-sensing or calcium-sensing GRNs)18,29,30,31. Moreover, these neuronal circuits have been found to be distinct, as attractive or aversive GRNs can be independently activated and behaviorally controlled by artificially expressing temperature-activated TRPA1, capsaicin-activated rat TRPV1, or light-activated channelrhodopsin-229,32,33.
Among these chemoreceptors, IRs are broadly expressed in the peripheral sensory systems involved in chemosensation, thermosensation, and hygrosensation16,23,34,35. Recent studies on the mechanisms of taste perception indicate that saltiness, sourness, amino acids, and other chemical cues are directly sensed by taste IRs15,20,36,37. In nature, amine-containing compounds not only elicit aversive responses but have also been identified as important kairomones in host-seeking insects. For instance, insects and some disease vectors are attracted by the odor of ammonia and amines, which are excreted through sweat38,39,40,41. Ammonia and putrescine are olfactory cues for many anthropophilic insects, including Anopheles mosquitoes41. However, high concentrations of ammonia and urea have been found to dramatically decrease the fecundity and egg viability of Drosophila suzukii42. The production of urea in insects has been attributed to the catalysis of arginine hydrolysis43. In contrast, blood-feeding insects such as Aedes aegypti can efficiently detoxify ammonia-containing compounds. Specific mechanisms have evolved to tightly regulate the synthesis and excretion of nitrogenous waste and avoid the toxic effects that may result from abnormally high ammonia concentrations in tissues44.
The above-described studies highlight the critical role of nitrogen-containing compound perception in insects, in addition to the more widely characterized perception of sweetness, sourness, saltiness, bitterness, and water. Previous studies have investigated the mechanisms of ammonia and amino group olfactory perception in Drosophila antennae and other insects38; however, the cellular and molecular mechanisms that enable the taste perception of these compounds remain largely uncharacterized. Here, we elucidated the gustatory mechanisms by which Drosophila (fruit flies) sense ammonia and its derivatives in plant- and animal-derived decay products. Using a combined behavioral and electrophysiology approach, we discovered that flies perceive and avoid ammonia and its derivatives as bitter tastants via bitter-sensing GRNs. We also elucidated molecular sensors that are required for the gustatory perception of naturally occurring nitrogenous waste products.
Results and discussion
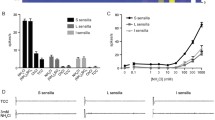
The perception of ammonia as an olfaction cue has been investigated in flies and mosquitoes23,45,46. However, only a few studies have assessed the gustatory mechanisms of ammonia perception21,47. The labellum is the major taste organ in D. melanogaster and possesses 31 taste sensilla in each hemisphere16. Each sensillum is named according to its length and position (“L4,” “I8,” “S6” indicate “long 4,” “intermediate 8,” and “short 6,” respectively). All sensilla have one sweet gustatory receptor neuron (GRN), but only S-type and I-type sensilla have bitter-sensing GRNs16. We found that nitrogenous wastes such as ammonium sulfate [(NH4)2SO4], ammonium chloride (NH4Cl), urea, and putrescine can activate S6 sensilla, but not L4, in a dose-dependent manner (Fig. 1a). Moreover, ammonium sulfate and ammonium chloride strongly activate S5, S6, and S7 sensilla but not L- or I-type sensilla38 (Supplementary Fig. 1a). The positive and negative control responses for L4 were measured with sucrose and caffeine (Supplementary Fig. 1b, c). The humidified materials of nitrogenous wastes are in the range of 10–20 mM, but we believe that these nitrogenous wastes can be easily dried to generate much higher concentration in the wild condition. GRNs are mainly classified into four different categories16. These GRNs include the sweet-sensing Gr5a-GAL4, bitter-sensing Gr66a-GAL4 or Gr33a-GAL4, calcium-sensing ppK23-GAL4, and water-sensing ppK28-GAL4 neurons18,29,30,31,48. Sugar and water are generally attractive, whereas bitterness and calcium are aversive. To perform an unbiased test, we expressed the inwardly rectifying potassium channel (Kir2.1) gene to inhibit each category. All four chemicals induced action potentials in the S6 sensilla of the controls (w1118 and UAS-Kir2.1/+) (Fig. 1a, b). These action potentials were only inhibited by the ablation of bitter-sensing GRNs (Gr33a-GAL4/UAS-Kir2.1 and Gr66a-GAL4/UAS-Kir2.1), whereas the other neurons exhibited similar responses to those of the controls6,7,49. The results of these electrophysiological tip recordings were further confirmed by our behavioral assays (Fig. 1d–f). Flies avoided ammonium sulfate, ammonium chloride, urea, and putrescine in a dose-dependent manner (Fig. 1d). Female flies also avoided laying their eggs on surfaces containing 50 mM of each chemical, and this response was mediated by bitter-sensing GRNs (Fig. 1e). However, Gr66a-GAL4/UAS-Kir2.1 female flies laid slightly more eggs on ammonia-containing food. This made us measure the pH of each chemical. Ammonium sulfate (pH 5.8), ammonium chloride (pH 5.9), and putrescine (pH 5.5) are slightly acidic in solution, whereas urea (pH 7.6) is slightly basic. To address whether the pH affected oviposition behavior and binary food choice assay, we tested ammonium sulfate which were adjusted to neutral pH by adding ammonium hydroxide (Supplementary Fig. 1d, e). The aversions of wild-type flies were not affected by the slight change of pH. Next, the slight attraction of bitter-sensing GRNs-ablated flies to the chemicals in oviposition may be affected by olfaction, because a population of OR neurons and projection neurons are activated by ammonia40. To test any possible roles of antenna in oviposition (Fig. 1e), we tested normal and antenna-removed female flies with the same genotypes in Fig. 1e (Supplementary Fig. 1f). We found that antenna-removed controls and Gr66a-GAL4/UAS-Kir2.1 flies showed slightly increased oviposition index, but not statistically significant, compared to normal flies (Supplementary Fig. 1f). These results confirmed that the bitter-sensing GRNs in taste had a major role in affecting oviposition on the ammonia-containing media, though ammonia odor may partially influence female flies to select egg laying sites if any. Furthermore, to confirm the putative aversiveness of nitrogenous wastes and their role in activating bitter-sensing GRNs, the response of flies to each chemical was characterized via an adaptation of the proboscis extension reflex (PER) assay. This PER response was evaluated after a pre-stimulus with sucrose (Fig. 1f). As expected, this paradigm provided cellular-level evidence of the involvement of bitter-sensing GRNs, but not calcium-sensing GRNs, in the aversive response to nitrogenous waste products.
a Tip recording performed on the taste sensilla (solid colored lines are S6 and dashed colored lines are L4) from the labella of control (w1118) flies with indicated concentrations of ammonium sulfate [(NH4)2SO4], ammonium chloride (NH4Cl), urea [CO(NH2)2], and putrescine [NH2(CH2)4NH2] (n = 10). b Frequencies of the action potentials elicited from S6 sensilla using 100 mM of the indicated chemicals in control (w1118), UAS-Kir2.1/+, and each neuron-ablated fly, including Gr5a-GAL4, Gr33a-GAL4, Gr66a-GAL4, ppk23-GAL4, and ppk28-GAL4 with UAS-Kir2.1 (n = 10–13). c Representative sample traces from panel (b). d Two-way choice feeding assay showing the preferences of control (w1118) flies upon presentation of 100 mM sucrose alone versus 100 mM sucrose laced with the indicated concentrations of (NH4)2SO4, NH4Cl, CO(NH2)2, and NH2(CH2)4NH2 (n = 6). e Ovipositional preference assay of control (w1118), UAS-Kir2.1/+, and bitter-sensing GRN-ablated flies (Gr66a-GAL4/UAS-Kir2.1) between 100 mM sucrose food and food containing 100 mM sucrose laced with 50 mM (NH4)2SO4, NH4Cl, CO(NH2)2, and NH2(CH2)4NH2, respectively (n = 6). The line colors indicate each chemical, as in panel (a). f Average percentage of flies exhibiting the proboscis extension response (PER) after applying 100 mM of the indicated chemical stimulus to the labellum of the control (w1118), UAS-Kir2.1/+, and two aversive GRN-ablated flies (ppk23-GAL4/UAS-Kir2.1 and Gr66a-GAL4/UAS-Kir2.1). A pretest was conducted using 100 mM sucrose alone. The taste stimuli consisted of 100 mM sucrose and 100 mM of (NH4)2SO4, NH4Cl, CO(NH2)2, and NH2(CH2)4NH2, respectively (n = 6). All error bars represent the SEMs. Multiple comparisons were conducted using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance compared with the control (*P < 0.05, **P < 0.01).
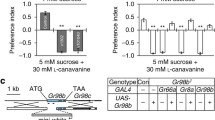
Further, we performed labellar tip recordings with 100 mM ammonium sulfate using candidate IRs to identify the molecular basis of ammonium sulfate, ammonium chloride, urea, and putrescine perception20,23,29 (Fig. 2a). We found deficits in three mutants of the broadly required IR25a and IR76b receptors, in addition to a newly identified IR51b mutant from 28 Ir mutants, which were expressed in the labellum and taste sensilla that surround the legs and wing margins (Fig. 2a and Supplementary Table 1). Tip recordings of the response of other candidate GR mutants to ammonium sulfate were conducted to further confirm the results of our screening experiments50 (Supplementary Fig. 2a). Once again, we found that only three IRs were required for ammonium sulfate perception. We also tested neutral ammonium sulfate for control and three IR mutants in electrophysiology (Supplementary Fig 2b). We confirmed that the pH of the ammonia had no role in the residual spikes in mutant flies. Next, tip recordings were conducted to assess the responses to the remaining chemicals. Using a 0–100 mM range, we found that the same IRs are required for sensing ammonium chloride, urea, and putrescine (Fig. 2b–d). To further support the indispensable role of these three IRs to respond to nitrogenous wastes, rescue experiments were conducted using a targeted gene expression approach via the GAL4/UAS system. These genetic experiments indicated that the deficits of Ir25a2 and Ir76b1 mutants to sense ammonium sulfate, urea, and putrescine were recovered through the expression of each wild-type cDNA under the control of the Gr33a-GAL4 bitter-sensing GRNs48 and its respective GAL4, as demonstrated by our electrophysiology experiments (Fig. 2e, f). Furthermore, we rescued the Ir51b1 deficit by expressing Ir51b cDNA under the control of Ir25a-GAL4 or Gr33a-GAL4 (Fig. 2g).
a Average number of spikes per second when the S6 sensilla on the labellum were stimulated with 100 mM (NH4)2SO4 [control (w1118) and 28 Ir mutant lines] (n = 10). b–d Average frequencies of action potentials obtained by performing tip recordings on the S6 sensilla of the control (w1118) and three candidate Ir mutant flies in responses to the indicated doses (mM) of (b) ammonium salts, (c) CO(NH2)2, and (d) NH2(CH2)4NH2, respectively (n = 10–11). e–g Rescue of tip recording defects in (e) Ir25a2, (f) Ir76b1, (g) Ir51b1 mutants by expressing the respective wild-type cDNA under the control of the indicated GAL4 promoters. Action potentials were elicited on S6 sensilla from the labellum with 100 mM of (NH4)2SO4, CO(NH2)2, and NH2(CH2)4NH2 (n = 10–12). All error bars represent the SEMs. Multiple comparisons were conducted using single-factor ANOVA coupled with Scheffe’s post hoc test. The colored asterisks in panels (e–g) indicate a significant difference between the same-colored bars compared to the control (**P < 0.01).
To assess the molecular basis of ammonium-induced aversive behaviors, the response of the candidate IRs Ir25a, Ir51b, and Ir76b to a 100 mM sucrose solution versus 100 mM sucrose + 50 mM ammonium sulfate were examined via two-way choice feeding assays (Fig. 3a). The results of these behavioral assays supported the physiological evidence that mutations in Ir25a, Ir51b, and Ir76b only resulted in diminished avoidance to 50 mM ammonium sulfate, whereas mutation of other Irs had no significant effect on ammonium sulfate avoidance in the feeding assay40. Three IRs may also affect olfaction, while taste is likely a main determinant of ammonia aversion in feeding behavior which is highly attributed to changes in taste physiology.
a Screening of Ir mutants using the binary food choice assay. The flies were given a choice between 100 mM sucrose versus 100 mM sucrose laced with 50 mM (NH4)2SO4 (n = 6). b, c Binary food choice assay results showing the rescue of feeding defects in Ir25a2 mutants in response to (b) 50 mM (NH4)2SO4, 50 mM CO(NH2)2, and (c) 50 mM NH2(CH2)4NH2 when wild-type Ir25a cDNA was expressed under the control of Ir25a-GAL4 as well as Gr33a-GAL4 (n = 6–11). d, e Behavioral rescue of feeding defects in Ir76b1 mutants exposed to (d) 50 mM (NH4)2SO4, 50 mM CO(NH2)2, and (e) 50 mM NH2(CH2)4NH2 expressing wild-type Ir76b cDNA under the control of Ir76a-GAL4 or Gr33a-GAL4 (n = 6–11). f, g Binary food choice assays to assess the rescue the feeding defects of Ir51b1 mutants in response to (f) 50 mM (NH4)2SO4, 50 mM CO(NH2)2, and (g) 50 mM NH2(CH2)4NH2 when wild-type Ir51b cDNA was driven under the control of Ir25a-GAL4 or Gr33a-GAL4 (n = 6–10). All error bars represent the SEMs. Multiple comparisons were conducted using single-factor ANOVA coupled with Scheffe’s post hoc test. The colored asterisks indicate statistical significance with the same-colored bar values compared to the control (**P < 0.01).
Similar to the electrophysiological responses, we confirmed that the same Ir mutants exhibited behavioral responses to ammonium sulfate, urea, and putrescine concentrations ranging from 0 to 75 mM (Supplementary Fig. 3a–c). Additionally, upon rescuing the deficits of these mutants via genetic experiments, the results of our behavior assays were consistent with those of our electrophysiology recordings (Fig. 3b–g).
We previously reported that IR25a was co-expressed extensively with the IR76b reporter29. Further, a previous study indicated that the Ir51b-GAL4 reporter was not detected in the labellum15. In addition, Ir51b is detected in the RNA-seq analysis of the Drosophila antenna46. Therefore, to investigate whether Ir51b RNA is expressed in the labellum, we performed reverse transcriptase-polymerase chain reaction (RT-PCR) using labellum, leg, and antenna samples (Fig. 4a and Supplementary Fig. 5a). We identified the predicted 1.7 kb band in the wild-type labella and legs, but not antennae. This band was confirmed by DNA sequencing. Furthermore, repeated experiments for three independent sample preparation were quantified by ImageJ and normalization (Fig. 4b). This suggests that IR51b acts as a contact-mediated chemosensor. However, we do not completely exclude any possible role of IR51b in antenna, because other group detects low-level expression of Ir51b by RNA-seq. Next, we expressed the cell death gene UAS-hid under the control of sweet-sensing (Gr5a-GAL4) or bitter-sensing (Gr33a-GAL4) receptors to detect whether Ir51b participates in the GRN-mediated perception of bitter substances using tubulin as an internal control (Fig. 4c, d and Supplementary Fig. 5b). We found that Ir51b RNA was almost completely eliminated in the Gr33a-GAL4/UAS-hid mutants (Fig. 4c, d and Supplementary Fig. 5b). Next, we generated a second Ir51b allele using ends-out homologous recombination (Supplementary Fig. 4). The in-frame knock-in of GAL4 was generated with a 1017 bp deletion of the exon; however, we failed to recapitulate the expression pattern of Ir51b. Therefore, this mutant was named Ir51b2. This second allele also showed similar deficits to those of Ir51b1 (Fig. 2b–d), thus suggesting the role of IR51b in the GRN-mediated perception of bitter-tasting (aversive) compounds.
a Gel picture of RT-PCR results showing Ir51b expression in the labellum and legs, but not in the antennae. Amplified tubulin products were used as control. “M” indicates the DNA ladder marker. b Quantification of Ir51b RNA levels which was normalized by tubulin in the same reaction in panel (a). The density of Ir51b RNA was divided by tubulin RNA level in each batch and control (whole body) was set to 100 by same fold change in the batch. Three repeated experiments were provided. c Gel picture of Ir51b expressed in bitter-sensing GRNs. The 1.7 kb Ir51b gene was amplified using RT-PCR in no-DNA template, the control (w1118), UAS-hid, Gr5a-GAL4 (sugar-sensing GRNs), Gr33a-GAL4 (bitter-sensing GRNs), Gr5a-GAL4/UAS-hid (sugar-sensing GRNs ablated), and Gr33a-GAL4/UAS-hid (bitter-sensing GRNs ablated) flies. Amplified tubulin products were used as internal control of PCR reaction. “M” indicates the DNA ladder marker. d Quantification of Ir51b RNA levels which was normalized by tubulin in the same reaction in panel (c). Three repeated experiments were provided. The density of Ir51b RNA was divided by tubulin RNA level in each batch and control (w1118) was set to 100 by same fold change in the batch. All error bars represent the SEMs. Multiple comparisons were conducted using single-factor ANOVA coupled with Scheffe’s post hoc test. The asterisks indicate statistical significance, compared to the control (whole-body sample in (b) and control in (d)) (**P < 0.01).
To investigate the genetic recapitulation of ammonia-taste receptors, we assessed whether these three genes were sufficient to elicit taste-induced avoidance of nitrogenous compounds in flies. I-type sensilla in the fly’s labellum possess only two GRNs, whereas L-type and S-type sensilla harbor four GRNs (Fig. 5a–c). We then induced the expression of wild-type Ir51b cDNA in the bitter-sensing I-type cells or sweet-sensing L-type cells where they are normally not expressed based on our mapping results (Supplementary Fig. 1a). The action potentials of the I-type sensilla of flies carrying either the UAS-Ir51b or Gr33a-GAL4 mutations were not significantly different from those of the w1118 strain. In contrast, ammonium sulfate elicited a significant increase in the I8 and I9 sensilla action potential frequency of UAS-Ir51b/Gr33a-GAL4 flies (Fig. 5d, e). Furthermore, similar to our experiments where only the expression of Ir51b cDNA was induced, expression of all three cDNAs with Gr33a-GAL4 elicited similar responses from I8 and I9 sensilla when presented with a 100 mM ammonium sulfate stimulus. These results were consistent with our previous report that IR25a and IR76b were involved in the GRN-mediated perception of bitter compounds29. Next, we expressed UAS-Ir51b only or with UAS-Ir25a and UAS-Ir76b under the control of the sweet-sensing Gr5a-GAL4 and recorded from L-type sensilla (Fig. 5f). However, no significant differences from w1118 were observed. Therefore, our findings indicated that three IRs (IR25a, IR51b, and IR76b) were involved in the gustatory detection of nitrogenous waste products via heteromultimeric channel formation; however, ammonia sensing could not be recapitulated in sweet-sensing GRNs.
a–c Cartoons of three different types of gustatory sensilla and their GRNs on the labellum of Drosophila. a Hetero multimeric association of Ir25a, Ir51b, and Ir76b in S-type sensilla for ammonia taste processing. b Misexpression of Ir51b cDNA in I-type sensilla. c Misexpression of Ir51b cDNA in L-type sensilla. d UAS-Ir51b alone or UAS-Ir25a, UAS-Ir51b, and UAS-Ir76b were expressed in bitter-sensing GRNs under the control of Gr33a-GAL4. Average action potentials were generated on I8 and I9 sensilla from the labellum of the indicated genotypes with 100 mM (NH4)2SO4 (n = 20). e Representative sample traces from panel (d). f Overexpression of Ir25a, Ir51b, and Ir76b cDNA in sugar-sensing GRNs in L-type sensilla under the control of Gr5a-GAL4. Recordings of nerve responses were performed on L4 and L6 sensilla in the labellum of the indicated flies with 100 mM (NH4)2SO4 (n = 20). All error bars represent SEMs. Multiple comparisons were conducted using single-factor ANOVA coupled with Scheffe’s post hoc test (**P < 0.01).
Overall, we identified three IRs to sense nitrogenous waste compounds in S-type. Furthermore, three IRs were enough to induce ammonium sulfate response in I-type sensilla. However, the combination of three IRs was insufficient to elicit physiological responses to ammonium sulfate in L-type sensilla when driven with Gr5a-GAL4. This indicates that additional channel subunits required for the responses to the nitrogenous waste compounds must be present in S-type and I-type GRNs. Therefore, this study has a limitation to claiming that the IRs are receptor ion channels for nitrogenous waste compounds, because we cannot rule out the function of IRs in transduction pathways. Future works should focus on finding additional channel subunits to prove that nitrogenous waste compounds can directly activate these IRs by heterologous expression.
Chemical sensation is an essential modulator of physiology and behavior. In invertebrates, the vast majority of chemical stimuli in the environment are recognized by members of two evolutionarily related chemosensory receptors: the ORs and the GRs51. However, recent studies have indicated that IRs are likely the most ancient chemoreceptors and thus predate ORs and GRs, as their existence can be traced back prior to the deuterostome-protostome split16,27.
Ammonia can act as a kairomone, and therefore some species, such as flour mites, are attracted to the microbial degradation products of certain amino acids52. Females of some uricotelic muscid flies are reportedly attracted to ammonia when searching for suitable oviposition sites53. In this case, ammonia acts as a chemical attractant that enables some parasitic organisms to detect their hosts. However, ammonia can also be used to kill or repel bed bugs, ants, rats, fleas, and snakes. Insect survival may also vary depending on ecological niche or host characteristics; however, the proliferation of insect populations is generally thought to be highly host-dependent. Here, we demonstrated that fruit flies avoided nitrogenous waste products both when laying eggs and when selecting their food, as these compounds are potentially toxic. Despite the differences in the mechanisms of chemical sensation between arthropods and humans, the identification of ammonia-associated taste sensors in insects provides important insights into how animals perceive and react to specific chemicals.
Methods
Fly strains
Unless otherwise indicated, all flies were maintained at 25 °C under a 12 h light/12 h dark cycle. Both male and female flies were used randomly in our experiments. The control strain used in this study was w1118.
We previously described the Ir7a1, Ir47a1, Ir52a1, Ir56a1, Ir60b3, Ir94a1, Ir94c1, and Ir94h1 strains20. The Ir7g1 (BL42420), Ir10a1 (BL23842), Ir21a1 (BL17171), Ir48a1 (BL26453), Ir48b1 (BL23473), Ir51b1 (BL10046), Ir52b1 (BL25212), Ir56b1 (BL27818), Ir62a1 (BL32713), Ir67a1 (BL56583), Ir75d1 (BL24205), Ir92a1 (BL58205), Ir94b1 (BL23424), Ir94d1 (BL33132), Ir94g1 (BL25551), and Ir100a1 (BL31853). Gr2a1 (BL18415), Gr10a1 (BL29947), Gr22f1 (BL43859), Gr23a1 (BL19287), Gr28bMi (BL24190), Gr36b1 (BL24608), Gr36c1 (BL26496), Gr58b1 (BL29065), Gr59a1 (BL26125), Gr77a1 (BL26374), Gr93d1 (BL27800), Gr94a1 (BL17550), Gr97a1 (BL18949), Ir8a1 (BL41744), Ir85a1 (BL24590), UAS-hid (BL65403), and UAS-Kir2.1 (BL6596) strains were obtained from the Bloomington Drosophila Stock Center. Additionally, we obtained the following mutants from the Korea Drosophila Resource Center: Gr28a1, Gr36a1, Gr39b1, Gr59c1, and Gr89a1. We previously described the Gr8a1 54, Gr33a1 48, Gr47a1 55, Gr66aex83 56, Gr33aGAL4 48, Gr98b1 57, Ir76b1 29, Ir76b-GAL4 29, and UAS-Ir76b 29. K. Scott provided ppk23-GAL458 and ppk28-GAL418. H. Amrein gave ∆Gr32a, Gr66a-GAL4, and Gr5a-GAL459,60. L. Vosshall provided Ir25a223. We obtained Gr22e1 (140936) from Kyoto Drosophila Stock Center.
Chemical reagents
Sucrose (CAS No 57-50-1, Cat No S9378), sulforhodamine B (CAS No 3520-42-1, Cat No 230162), ammonium sulfate (CAS No 7783-20-2), ammonium chloride (CAS No 12125-02-9), putrescine (CAS No 333-93-7), and urea (CAS No 57-13-6) were purchased from Sigma-Aldrich Co. Brilliant blue FCF (CAS No 3844-45-9, Cat No 027-12842) was purchased from Wako Pure Chemical Industry Ltd.
Generation of Ir51b 2 mutant lines
The Ir51b2 knock-in-GAL4 mutant line was created via ends-out homologous recombination as previously described61. Concretely, we amplified 2.94 kb upstream and 3.05 kb downstream of genomic fragments through PCR and subcloned the DNA into the pw35-GAL4 vector48. Right arm extension included 3050 bp and left arm extension included 2944 bp along with the ATG start codon. GAL4 was inserted by replacing 1017 bp of genomic regions to preserve the reading frame of the ATG start codon. The construct was injected into w1118 embryos by the Korea Drosophila Resource Center (KDRC).
RT-PCR
Labellum, leg, and antenna samples were dissected from approximately 30 control, Gr5a-GAL4/UAS-hid, and Gr33a-GAL4/UAS-hid adult flies. Total RNA was extracted using TRIzol (Invitrogen) and cDNA was synthesized using AMV reverse transcriptase (Promega). To perform the RT-PCR, we used the following Ir51b primers: 5′-GGC GCT AAC AAA CGC TGC TTAC -3′ and 5′-CAG AGC TGA CAG TAT CCA ACC AA-3′. The tubulin primers were 5′-TCC TTG TCG CGT GTG AAA CA-3′ and 5′-CCG AAC GAG TGG AAG ATG AG-3′. RT-PCR products were obtained after 35 cycles. Each samples were repeated at least three times. Intensity measurement was done by using ImageJ (Fiji) application and then Ir51b RNA level in each sample was normalized by the internal control, tubulin.
Two-way food choice assay
To perform binary food choice assays, we followed a previously described protocol29. First, 5–7-day-old mixed gender (males and females were randomly selected) flies were starved in a vial containing water-soaked Kimwipe paper for 16–18 h in a dark and humid chamber. Each experiment was conducted using 50–70 flies. We then prepared two food options, both containing 1% agarose: one contained only sucrose and the other contained sucrose mixed with nitrogen-containing chemicals. These food sources were colored with either blue (brilliant blue FCF, 0.125 mg/mL) or red food-grade dye (sulforhodamine B, 0.1 mg/mL). These two food preparations were dispensed into a 72-well microtiter dish (Thermo Fisher Scientific, Cat No 438733) in an alternative position. We briefly anesthetized the starved flies and introduced them into the food dish, after which we immediately transferred them to an incubator for 90 min. The flies were euthanized in a −20 °C freezer for at least 2 h. Then, the abdomen color was classified as “blue,” “red,” or “purple” using a stereomicroscope. The preference index (PI) was calculated using following equations:
depending on the dye/tastant combinations. At least six replicates were performed for each fly strain.
Electrophysiology
Tip recording assays were conducted as described in a previous study29. First, 4–6-day-old flies were anesthetized on ice. A reference glass electrode filled with Ringer’s solution (3 mM CaCl2, 182 mM KCl, 46 mM NaCl, and 10 mM Tris-base; pH 7.2) was inserted into the thorax of the flies. The glass electrode was gently pushed towards their proboscis without causing any severe damage to the GRNs on the proboscis. Approximately 4–6 flies were used for each experiment. Using an electrophysiology system, we activated the S-type, I-type, and L-type taste sensilla on the labella of flies for 5 s using a mixture of tastants with 30 mM tricholine citrate (TCC). The recording electrode (10–20 μm tip diameter) was connected to a preamplifier (Taste PROBE, Syntech, Hilversum, The Netherlands), and the signals were collected and amplified by 10x using a signal connection interface box (Syntech) in conjunction with a 100–3000 Hz band-pass filter. Recordings of action potentials were acquired using a 12 kHz sampling rate and analyzed using the Autospike 3.1 software (Syntech). We then counted the action potentials for 50–550 ms and presented doubled values of the period per second in the figures. Each consecutive recording was performed with an approximately 1 min gap between each stimulation. The sample numbers (n) in each experiment indicate the number of animals. The same procedure was repeated on different days and using different setups.
Proboscis extension reflex (PER) assay
The PER assay was performed as previously described62. The flies were first starved for 20–24 h in a vial with water-soaked Kimwipe paper. The flies were then briefly anesthetized on ice and fixed on a glass slide using glue. A fine Kimwipe paper wick was then used to deliver the initial 100 mM sucrose stimulus to the flies. Only flies that showed a positive PER to sucrose were considered for the next test. Taste stimuli were delivered to the labellum at least three times to avoid false-positive responses. At this point, only the flies that exhibited a positive PER to the experimental solutions (i.e., 100 mM ammonia in 100 mM sucrose) were deemed PER positive. A total of 10–15 flies were evaluated per experiment, after which PER percentages were calculated. At least six replicates were performed for each strain.
Oviposition preference assay
Oviposition preference assays were conducted as described in a previous study63. A total of 15 female and 15 male newly hatched flies were transferred into a new food vial supplemented with dry yeast and kept in a normal light/dark cycle for two days. Prior to the assays, the experimental animals were acclimatized in 1% agarose containing a test food choice for 5–6 h. Two food options were then provided, one containing only sucrose and another containing a mixture of sucrose and a nitrogen-containing chemical, both of which were dispensed on a Petri dish (35 mm diameter, Product No. 351007) divided into two equal halves. The agarose food was allowed to solidify and then transferred to an egg-laying chamber (Code No. FEC-50200, Hansol Tech, Republic of Korea); the acclimated flies were transferred into the chamber thereafter. The flies were then allowed to lay eggs overnight inside of the incubator. The next day, the number of eggs deposited on each side of the chamber (i.e., each containing a different food option) was counted, and the ovipositional preference index was calculated as follows:
At least six replicates were performed for each strain.
Statistics and reproducibility
We selected D. melanogaster as a model animal. Both sexes of experimental animals were considered randomly for the experiments we performed. All the experiments were conducted at laboratory conditions. Based on the previous studies, we determined at least six replicates per genotype were enough to verify behavioral data, where as at least 10 animals per genotype were enough in electrophysiological recordings. We performed three replicates to analyze Ir51b expression for RNAi analysis. We met enough sample size to make our data more reliable in each figure. Each experiment was conducted for at least two different days. No data was excluded from the analysis. Each data points represents a real value. Average of all the replicates for that specific genotypes were presented. All error bars represent the standard error of the mean (SEM). Multiple comparisons were then evaluated using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01). Statistical analyses were performed using Origin Pro 8 for Windows (ver. 8.0932; Origin Lab Corporation, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets used in this paper are preserved with corresponding author, which are available upon resonable request. The source data for the individual values and scripts used to generate figures are attached to this paper as Supplementary Data 1.
References
Mohiuddin, S. S. & Khattar, D. Biochemistry, Ammonia (StatPearls [Internet], 2019).
Mustafavi, S. H. et al. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant 40, 1–19 (2018).
Mattoo, A. K., Minocha, S. C., Minocha, R. & Handa, A. K. Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 38, 405–413 (2010).
Hussain, A. et al. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 14, e1002454 (2016).
Kutvonen, H., Rajala, P., Carpén, L. & Bomberg, M. Nitrate and ammonia as nitrogen sources for deep subsurface microorganisms. Front. Microbiol. 6, 1079 (2015).
Sudarman, A., Nurul Amalia, R. & Apri Astuti, D. Effect of molasses, rice bran and tapioca flour as additives on the quality and digestibility of cassava leaf silage. J. Int. Soc. Southeast Asian Agric. Sci. 22, 40–49 (2017).
Chaoui, H., Montes, F., Rotz, C. A. & Richard, T. L. Dissociation and mass transfer coefficients for ammonia volatilization models. Paper number 083802. In ASABE Conference 2008 (ASABE, 2008), Dr. Garey Fox (Editor in Chief).
Joseph, R. M. & Carlson, J. R. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 31, 683–695 (2015).
Bruce, T. J., Wadhams, L. J. & Woodcock, C. M. Insect host location: a volatile situation. Trends Plant Sci. 10, 269–274 (2005).
Wyatt, T. Pheromones and Animal Behaviour: Communication by Smell and Taste (Cambridge University Press, 2003).
Rimal, S., Sang, J., Dhakal, S. & Lee, Y. Cucurbitacin B activates bitter-sensing gustatory receptor neurons via gustatory receptor 33a in Drosophila melanogaster. Mol. Cells 43, 530 (2020).
Bargmann, C. I. Comparative chemosensation from receptors to ecology. Nature 444, 295–301 (2006).
Gevi, F., D’Alessandro, A., Rinalducci, S. & Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD–SAGM. J. Proteom. 76, 168–180 (2012).
Yarmolinsky, D. A., Zuker, C. S. & Ryba, N. J. Common sense about taste: from mammals to insects. Cell 139, 234–244 (2009).
Koh, T.-W. et al. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 83, 850–865 (2014).
Rimal, S. & Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 27, 1–7 (2018).
Chen, Y.-C. D. & Dahanukar, A. Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep. 21, 2978–2991 (2017).
Cameron, P., Hiroi, M., Ngai, J. & Scott, K. The molecular basis for water taste in Drosophila. Nature 465, 91–95 (2010).
Zhang, Y. V., Ni, J. & Montell, C. The molecular basis for attractive salt-taste coding in Drosophila. Science 340, 1334–1338 (2013).
Rimal, S. et al. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 26, 1432–1442. e1434 (2019).
Weiss, L. A., Dahanukar, A., Kwon, J. Y., Banerjee, D. & Carlson, J. R. The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272 (2011).
Isono, K., Ueno, K., Ohta, M. & Morita, H. Drosophila sweet taste receptor. Pure Appl. Chem. 74, 1159–1165 (2002).
Benton, R., Vannice, K. S., Gomez-Diaz, C. & Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009).
Suh, G. S. et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–859 (2004).
Su, C.-Y., Menuz, K. & Carlson, J. R. Olfactory perception: receptors, cells, and circuits. Cell 139, 45–59 (2009).
Croset, V. et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6, e1001064 (2010).
Rytz, R., Croset, V. & Benton, R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 43, 888–897 (2013).
Dhakal, S. & Lee, Y. Transient receptor potential channels and metabolism. Mol. Cells 42, 569 (2019).
Lee, Y., Poudel, S., Kim, Y., Thakur, D. & Montell, C. Calcium taste avoidance in Drosophila. Neuron 97, 67–74. e64 (2018).
Slone, J., Daniels, J. & Amrein, H. Sugar receptors in Drosophila. Curr. Biol. 17, 1809–1816 (2007).
Wang, Z., Singhvi, A., Kong, P. & Scott, K. Taste representations in the Drosophila brain. Cell 117, 981–991 (2004).
Kim, S. H. et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl Acad. Sci. USA. 107, 8440–8445 (2010).
Mann, K., Gordon, M. D. & Scott, K. A pair of interneurons influences the choice between feeding and locomotion in Drosophila. Neuron 79, 754–765 (2013).
Chen, Y.-C. D. & Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci. 77, 1087–1101 (2020).
Ni, L. The structure and function of ionotropic receptors in Drosophila. Front. Mol. Neurosci. 13, 266 (2020).
Croset, V., Schleyer, M., Arguello, J. R., Gerber, B. & Benton, R. A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci. Rep. 6, 1–13 (2016).
Chen, Y.-C. D., Park, S. J., Joseph, R. M., William, W. J. & Dahanukar, A. A. Combinatorial pharyngeal taste coding for feeding avoidance in adult Drosophila. Cell Rep. 29, 961–973. e964 (2019).
Delventhal, R. et al. The taste response to ammonia in Drosophila. Sci. Rep. 7, 1–12 (2017).
Geier, M., Bosch, O. J. & Boeckh, J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem. Senses 24, 647–653 (1999).
min, S., Ai, M., Shin, S. A. & Suh, G. S. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc. Natl Acad. Sci. USA 110, E1321–E1329 (2013).
Zwiebel, L. & Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 34, 645–652 (2004).
Belloni, V., Galeazzi, A., Bernini, G., Mandrioli, M., Versace, E. & Haase, A. Evolutionary compromises to metabolic toxins: ammonia and urea tolerance in Drosophila suzukii and Drosophila melanogaster. Physiol. Behav. 191, 146–154 (2018).
Scaraffia, P. Y. et al. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA 105, 518–523 (2008).
Scaraffia, P. Y., Zhang, Q., Thorson, K., Wysocki, V. H. & Miesfeld, R. L. Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect Physiol. 56, 1040–1049 (2010).
Meijerink, J., Braks, M. & Van Loon, J. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol. 47, 455–464 (2001).
Menuz, K., Larter, N. K., Park, J. & Carlson, J. R. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 10, e1004810 (2014).
Hiroi, M., Meunier, N., Marion‐Poll, F. & Tanimura, T. Two antagonistic gustatory receptor neurons responding to sweet‐salty and bitter taste in Drosophila. J. Neurobiol. 61, 333–342 (2004).
Moon, S. J., Lee, Y., Jiao, Y. & Montell, C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 19, 1623–1627 (2009).
Farkaš, A., Rožić, M. & Barbarić-Mikočević, Ž. Ammonium exchange in leakage waters of waste dumps using natural zeolite from the Krapina region, Croatia. J. Hazard. Mater. 117, 25–33 (2005).
Sang, J., Rimal, S. & Lee, Y. Gustatory receptor 28b is necessary for avoiding saponin in Drosophila melanogaster. EMBO Rep. 20, e47328 (2019).
Robertson, H. M., Warr, C. G. & Carlson, J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 14537–14542 (2003).
Levinson, H. Z., Levinson, A. R. & Müller, K. Functional adaptation of two nitrogenous waste products in evoking attraction and aggregation of flour mites (Acarus siro L.). Anz. f.ür. Sch.ädlingskunde, Pflanzenschutz, Umweltschutz 64, 55–60 (1991).
Browne, L. B. An analysis of the ovipositional responses of the blowfly Lucilia cuprina to ammonium carbonate and indole. J. Inse. Physi 11, 1131–1143 (1965).
Lee, Y. et al. Gustatory receptors required for avoiding the insecticide L-canavanine. J. Neurosci. 32, 1429–1435 (2012).
Lee, Y., Moon, S. J., Wang, Y. & Montell, C. A Drosophila gustatory receptor required for strychnine sensation. Chem. Senses 40, 525–533 (2015).
Moon, S. J., Köttgen, M., Jiao, Y., Xu, H. & Montell, C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 16, 1812–1817 (2006).
Shim, J. et al. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat. Commun. 6, 8867 (2015).
Thistle, R., Cameron, P., Ghorayshi, A., Dennison, L. & Scott, K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151 (2012).
Dunipace, L., Meister, S., McNealy, C. & Amrein, H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835 (2001).
Thorne, N., Chromey, C., Bray, S. & Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079 (2004).
Gong, W. J. & Golic, K. G. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl Acad. Sci. USA 100, 2556–2561 (2003).
Poudel, S. & Lee, Y. Gustatory receptors required for avoiding the toxic compound coumarin in Drosophila melanogaster. Mol. Cells 39, 310 (2016).
Poudel, S., Kim, Y., Kim, Y. T. & Lee, Y. Gustatory receptors required for sensing umbelliferone in Drosophila melanogaster. Insect Biochem. Mol. Biol. 66, 110–118 (2015).
Acknowledgements
This work was supported by grants to Y.L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A2B6004202 and NRF-2021R1A2C1007628) and the Korea Environmental Industry and Technology Institute (KEITI) grant funded by the Ministry of Environment of Korea. S.D. and B.A. were supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea.
Author information
Authors and Affiliations
Contributions
Y.L. designed the research. S.D. performed the majority of research and analyzed the data. J.S. and B.A. performed important behavioral and electrophysiological experiments and analyzed. Y.L. commented on the results. S.D. and Y.L. combined the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks Makoto Hiroi and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editor: Caitlin Karniski. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dhakal, S., Sang, J., Aryal, B. et al. Ionotropic receptors mediate nitrogenous waste avoidance in Drosophila melanogaster. Commun Biol 4, 1281 (2021). https://doi.org/10.1038/s42003-021-02799-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02799-3
- Springer Nature Limited
This article is cited by
-
The proton channel OTOP1 is a sensor for the taste of ammonium chloride
Nature Communications (2023)
-
Molecular sensors in the taste system of Drosophila
Genes & Genomics (2023)
-
The power of Drosophila genetics in studying insect toxicology and chemical ecology
Crop Health (2023)