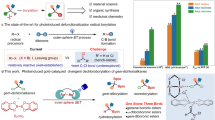
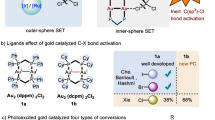
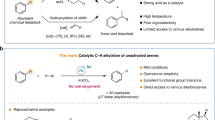
Abstract
The use of alkyl chlorides as C(sp3)-hybridized electrophiles for the construction of C(sp3)–C(sp3) bonds represents one of the most challenging issues in organic synthesis, associated with their low reduction potential and strong bond dissociation energy. Here we report a divergent radical transformation of a rich library of structurally diverse gem-dichloroalkanes by the controllable dechloroalkylation of alkenes by excited-state dinuclear gold catalysis. The gem-dichloroalkanes can be used to assemble C(sp3)–C(sp3) bonds as a chloroalkyl radical, an alkyl radical cation and a carbene equivalent precursor for carbon-chain propagation, or formal [4+1] and [2+1] cyclization of alkenes. The use of commercially available deuterium-labelled polychloromethanes provides a practical method to incorporate CD2Cl, CDCl2, CDCl and CD2 moieties into organic scaffolds. Studies of the mechanism have revealed an inner-sphere single electron transfer pathway for the homolytic cleavage of a strong C–Cl bond.








Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2157587 (1a) and CCDC 2157457 (1b). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Data related to materials and methods, optimization of conditions, experimental procedures, mechanistic experiments, density functional theory calculations and spectra are provided in the Supplementary Information. All data are available from the corresponding authors upon reasonable request.
References
Meijere, A. D. & Diederich, F. Metal-catalyzed Cross-coupling Reactions 2nd edn, 217–315 (Wiley-VCH, 2008).
Geist, E., Kirschning, A. & Schmidt, T. sp3–sp3 Coupling reactions in the synthesis of natural products and biologically active molecules. Nat. Prod. Rep. 31, 441–448 (2014).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Pitre, S. P., Weires, N. A. & Overman, L. E. Forging C(sp3)–C(sp3) bonds with carbon-centered radicals in the synthesis of complex molecules. J. Am. Chem. Soc. 141, 2800–2813 (2019).
Juliá, F., Constantin, T. & Leonori, D. Applications of halogen-atom transfer (XAT) for the generation of carbon radicals in synthetic photochemistry and photocatalysis. Chem. Rev. 122, 2292–2352 (2022).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Cybularczyk-Cecotka, M., Szczepanik, J. & Giedyk, M. Photocatalytic strategies for the activation of organic chlorides. Nat. Catal. 3, 872–886 (2020).
Vasudevan, D. Direct and indirect electrochemical reduction of organic halides in aprotic media. Russ. J. Electrochem. 41, 310–314 (2005).
Chintawar, C. C., Yadav, A. K., Kumar, A., Sancheti, S. P. & Patil, N. T. Divergent gold catalysis: unlocking molecular diversity through catalyst control. Chem. Rev. 121, 8478–8558 (2021).
Xia, S. & Xie, J. Energy transfer in gold photocatalysis. Gold Bull. 55, 123–127 (2022).
Ye, L.-W. et al. Nitrene transfer and carbene transfer in gold catalysis. Chem. Rev. 121, 9039–9112 (2021).
Zheng, Z. et al. Homogeneous gold-catalyzed oxidation reactions. Chem. Rev. 121, 8979–9038 (2021).
Huang, B., Hu, M. & Toste, F. D. Homogeneous gold redox chemistry: organometallics, catalysis, and beyond. Trends Chem. 2, 707–720 (2020).
Witzel, S., Hashmi, A. S. K. & Xie, J. Light in gold catalysis. Chem. Rev. 121, 8868–8925 (2021).
Hopkinson, M. N., Tlahuext-Aca, A. & Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 49, 2261–2272 (2016).
Fu, W.-F., Chan, K.-C., Miskowski, V. M. & Che, C.-M. The intrinsic 3[dσ*pσ] emission of binuclear gold(I) complexes with two bridging diphosphane ligands lies in the near UV; emissions in the visible region are due to exciplexes. Angew. Chem. Int. Ed. Engl. 38, 2783–2785 (1999).
Wan, S. G. & Lu, W. Reversible photoactivated phosphorescence of gold(I) arylethynyl complexes in aerated DMSO solutions and gels. Angew. Chem. Int. Ed. Engl. 56, 1784–1788 (2017).
Schmidbaur, H. & Raubenheimer, H. G. Excimer and exciplex formation in gold(I) complexes preconditioned by aurophilic interactions. Angew. Chem. Int. Ed. Engl. 59, 14748–14771 (2020).
Che, C.-M., Kwong, H.-L., Yam, V. W.-W. & Cho, K.-C. Spectroscopic properties and redox chemistry of the phosphorescent excited state of [Au2(dppm)2]2+ [dppm bis(diphenylphosphino)-methane]. J. Chem. Soc. Chem. Commun. 885−886 (1989).
Zidan, M., Rohe, S., McCallum, T. & Barriault, L. Recent advances in mono and binuclear gold photoredox catalysis. Catal. Sci. Technol. 8, 6019–6028 (2018).
Revol, G., McCallum, T., Morin, M., Gagosz, F. & Barriault, L. Photoredox transformations with dimeric gold complexes. Angew. Chem. Int. Ed. Engl. 52, 13342–13345 (2013).
Zidan, M., McCallum, T., Swann, R. & Barriault, L. Formal bromine atom transfer radical addition of nonactivated bromoalkanes using photoredox gold catalysis. Org. Lett. 22, 8401–8406 (2020).
Xie, J. et al. A highly efficient gold-catalyzed photoredox C(sp3)-H alkynylation of tertiary aliphatic amines with sunlight. Angew. Chem. Int. Ed. Engl. 54, 6046–6050 (2015).
Zhang, L., Si, X., Rominger, F. & Hashmi, A. S. K. Visible-light-induced radical carbo-cyclization/gem-diborylation through triplet energy transfer between a gold catalyst and aryl iodides. J. Am. Chem. Soc. 142, 10485–10493 (2020).
Si, X. et al. Visible light-induced α-C(sp3)-H acetalization of saturated heterocycles catalyzed by a dimeric gold complex. Org. Lett. 22, 5844–5849 (2020).
Nzulu, F. et al. A dinuclear gold(I) complex as a novel photoredox catalyst for light-induced atom transfer radical polymerization. Polym. Chem. 6, 4605–4611 (2015).
Yu, H., Zhang, X. & Zhang, S. Mechanism of photocatalytic cyclization of bromoalkenes with a dimeric gold complex. Organometallics 37, 1725–1733 (2018).
Li, D., Che, C.-M., Kwong, H.-L. & Yam, V. W.-W. Photo-induced C-C bond formation from alkyl halides catalysed by luminescent dinuclear gold(I) and copper(I) complexes. J. Chem. Soc. Dalton Trans. 3325−3329 (1992).
Wang, W. et al. Dinuclear gold catalysis. Chem. Soc. Rev. 50, 1874–1912 (2021).
Liu, K., Li, N., Ning, Y., Zhu, C. & Xie, J. Gold-catalyzed oxidative biaryl cross-coupling of organometallics. Chem 5, 2718–2730 (2019).
Zhang, S., Wang, C., Ye, X. & Shi, X. Intermolecular alkene difunctionalization via gold catalyzed oxyarylation. Angew. Chem. Int. Ed. Engl. 59, 20470–20474 (2020).
Chintawar, C. C., Yadav, A. K. & Patil, N. T. Gold-catalyzed 1,2-diarylation of alkenes. Angew. Chem. Int. Ed. Engl. 59, 11808–11813 (2020).
Zeineddine, A. et al. Rational development of catalytic Au(I)/Au(III) arylation involving mild oxidative addition of aryl halides. Nat. Commun. 8, 565 (2017).
Ma, C., Chan, C. T. L., To, W. P., Kwok, W. M. & Che, C.-M. Deciphering photoluminescence dynamics and reactivity of the luminescent metal–metal-bonded excited state of a binuclear gold(I) phosphine complex containing open coordination sites. Chem. Eur. J. 21, 13888–13893 (2015).
Jandik, P., Schubert, U. & Schmidbaur, H. Methylene bridging of two gold atoms through double oxidative addition of methylene dihalides to a cyclic ylide complex. Angew. Chem. Int. Ed. Engl. 21, 73–73 (1982).
Xia, Z. et al. Photosensitized oxidative addition to gold(I) enables alkynylative cyclization of o-alkylnylphenols with iodoalkynes. Nat. Chem. 11, 797–805 (2019).
Csok, Z., Vechorkin, O., Harkins, S. B., Scopelliti, R. & Hu, X. Nickel complexes of a pincer NN2 ligand: multiple carbon–chloride activation of CH2Cl2 and CHCl3 leads to selective carbon–carbon bond formation. J. Am. Chem. Soc. 130, 8156–8157 (2008).
Xu, W., Zheng, P. & XU, T. Dual nickel- and photoredox-catalyzed reductive cross-coupling of aryl halides with dichloromethane via a radical process. Org. Lett. 22, 8643–8647 (2020).
Werth, J. & Uyeda, C. Cobalt-catalyzed reductive dimethylcyclopropanation of 1,3-dienes. Angew. Chem. Int. Ed. Engl. 57, 13902–13906 (2018).
Uyeda, C. & Kalb, A. E. Catalytic reductive carbene transfer reactions. Chem Catal. 2, 667–678 (2022).
Li, Y. et al. Organophotocatalytic selective deuterodehalogenation of aryl or alkyl chlorides. Nat. Commun. 12, 2894 (2021).
Huang, G., Yu, J.-T. & Pan, C.-D. Recent advances in polychloromethylation reactions. Adv. Synth. Catal. 363, 305–327 (2021).
Liang, Y.-Y., Lv, G.-F., Ouyang, X.-H., Song, R.-J. & Li, J.-H. Recent developments in the polychloroalkylation by use of simple alkyl chlorides. Adv. Synth. Catal. 363, 290–304 (2021).
Zhou, F., Liu, Y.-L. & Zhou, J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381–1407 (2010).
Simmons, H. E. & Smith, R. D. A new synthesis of cyclopropanes from olefins. J. Am. Chem. Soc. 80, 5323–5324 (1958).
del Hoyo, A. M., Herraiz, A. G. & Suero, M. G. A. Stereoconvergent cyclopropanation reaction of styrenes. Angew. Chem. Int. Ed. Engl. 56, 1610–1613 (2017).
Kumar, N., Reddy, R. R., Eghbarieh, N. & Masarwa, A. α-Borylalkyl radicals: their distinctive reactivity in modern organic synthesis. Chem. Commun. 56, 13–25 (2020).
Ameen, D. & Snape, T. J. Chiral 1,1-diaryl compounds as important pharmacophores. MedChemComm. 4, 893–907 (2013).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. Engl. 57, 1758–1784 (2018).
Marcus, R. A. On the theory of oxidation-reduction reactions involving electron transfer. J. Chem. Phys. 24, 966–978 (1956).
Ren, H. et al. How does iridium(III) photocatalyst regulate nickel(II) catalyst in metallaphotoredox-catalyzed C–S cross-coupling? Theoretical and experimental insights. ACS Catal. 9, 3858–3865 (2019).
Li, R.-H. et al. Photocatalytic C(sp3)–O/N cross-couplings by NaI–PPh3/CuBr cooperative catalysis: computational design and experimental verification. ACS Catal. 11, 6633–6642 (2021).
Xu, J., Yang, Y., Zhao, X., Liu, C. & Zhang, D. DFT mechanistic study of Ir(III)/Ni(II)-metallaphotoredox-catalyzed difluoromethylation of aryl bromides. Inorg. Chem. 60, 8682–8691 (2021).
Saveant, J. M. A simple model for the kinetics of dissociative electron transfer in polar solvents. Application to the homogeneous and heterogeneous reduction of alkyl halides. J. Am. Chem. Soc. 109, 6788–6795 (1987).
Saveant, J. M. Electron transfer, bond breaking, and bond formation. Acc. Chem. Res. 26, 455–461 (1993).
Saveant, J.-M. Dynamics of cleavage and formation of anion radicals into and from radical and nucleophiles. Structure–reactivity relationships in SRN1 reactions. J. Phys. Chem. 98, 3716–3724 (1994).
Truhlar, D. G., Garrett, B. C. & Klippenstein, S. J. Current status of transition-state theory. J. Phys. Chem. 100, 12771–12800 (1996).
Acknowledgements
We thank the National Natural Science Foundation of China (22122103 and 21971108 to J.X., 22101130 to J.H., 21971111 and 21732003 to C.Z.), the National Key Research and Development Program of China (2022YFA1503202 and 2021YFC2101901 to J.X.), Natural Science Foundation of Jiangsu Province (grant no. BK20190006 to J.X.), Fundamental Research Funds for the Central Universities (020514380252 and 020514380272 to J.X.) for financial support. S. Wang, Y. He, Y. Li and N. Li are acknowledged for their reproduction of the experimental procedures for products 4aa, 6a, 7i and 8g. All theoretical calculations were performed at the High-Performance Computing Center (HPCC) of Nanjing University.
Author information
Authors and Affiliations
Contributions
C.-L.J., J.H. and J.X. conceived the work and designed the experiments. C.-L.J., T.L. and C.-G.Z. performed the experiments and analyzed the experimental data. T.L. and C.-L.J. performed the EPR measurements. J.H. performed the density functional theory calculations and discussed the results with C.-L.J. and C.Z., and J.X. co-wrote the manuscript with input from all the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Rongxiu Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–7, Figs. 1–285 and Discussion.
Supplementary Data 1
Crystallographic data for compound 1a.
Supplementary Data 2
Crystallographic data for compound 1b.
Supplementary Data
Atomic coordinates of the optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, CL., Han, J., Li, T. et al. Photoinduced gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes. Nat Catal 5, 1098–1109 (2022). https://doi.org/10.1038/s41929-022-00881-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00881-5
- Springer Nature Limited
This article is cited by
-
Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes
Nature Communications (2024)
-
Gold-catalyzed four-component multifunctionalization of alkynes
Nature Communications (2023)
-
Photoinduced copper-catalyzed C–N coupling with trifluoromethylated arenes
Nature Communications (2023)
-
Excited-state gold catalyzed activation of inert C–Cl bonds
Gold Bulletin (2023)