Abstract
Genetic modification of genes such as recombination activating gene 2 (RAG2) or interleukin-2 receptor-γ (IL2RG) results in pigs exhibiting severe combined immunodeficiency (SCID). Pigs presenting a SCID phenotype are important animal models that can be used to establish xenografts and to study immune system development and various immune-related pathologies. However, due to their immunocompromised nature, SCID pigs have shortened lifespans and are notoriously difficult to maintain. The failure-to-thrive phenotype makes the establishment of a breeding population of RAG2/IL2RG double-knockout pigs virtually impossible. Here, to overcome this limitation, we investigated whether reconstituting the immune system of SCID piglets with a fetal bone allograft would extend their lifespan. Following intramuscular transplantation, allografts gave rise to lymphocytes expressing T cell (CD3, CD4 and CD8), B cell (CD79α) and natural killer cell (CD335) lineage markers, which were detected in circulation as well in the spleen, liver, bone marrow and thymic tissues. The presence of lymphocytes indicates broad engraftment of donor cells in the recipient SCID pigs. Unlike unreconstituted SCID pigs, the engrafted animals thrived and reached puberty under standard housing conditions. This study demonstrates a novel method to extend the survival of SCID pigs, which may improve the availability and use of SCID pigs as a biomedical animal model.
Similar content being viewed by others
Main
Pigs displaying severe combined immunodeficiency (SCID) are a valuable preclinical model in biomedical research. The lack of functional lymphocytes in these animals allows the successful engraftment of cells and tissues without host rejection1,2,3,4. Pigs lacking functional recombination activating gene 2 (RAG2) and interleukin-2 receptor subunit-γ (IL-2Rγ) do not have B, T and natural killer (NK) cell populations5, which resembles the phenotype of the NOD SCID gamma (The Jackson Laboratory) mouse models that have been extensively used for xenograft and disease studies6. SCID pigs have also been used for human xenograft and disease studies1,2,3,4,7; however, a limited number of studies has been performed using SCID pigs owing to difficulties producing and maintaining these animals8. Nevertheless, SCID pig models offer advantages over murine models due to their size and physiology being more comparable to humans, thus serving as a better context for the development of treatments and procedures that permit them to fill the niche for a more translational model9,10. One example is in cancer research, where SCID pigs can form tumors from human cells, which are of similar size to tumors found in humans and which are often impossible to grow in mice2. In addition, genetic variation in pigs better represents the genetic variation found in humans than highly inbred mouse strains, which is useful for the development of treatments and pharmaceuticals11.
Despite these advantages, current SCID pig models are expensive and resource intensive to produce; and even under intensive management practices, SCID pigs do not live long enough to reach sexual maturity5,12. This failure-to-thrive phenotype has prevented the establishment of breeding pigs that are homozygous for SCID mutations. Consequently, only founder animals are directly utilized for study, which could confound results owing to the prevalence of mosaicism13,14,15 or cloning defects16,17,18 depending on the route used to produce these animals. To avoid these issues, SCID pigs can be generated by breeding heterozygous animals; however, the process would take years due to the prolonged gestational length (114 days) of pigs and months to reach sexual maturity across multiple generations. Moreover, breeding heterozygous carriers produces a low fraction of homozygous knockout pigs (25%). Furthermore, the breeding scheme is not effective if more than one gene needs to be disrupted. For instance, pigs lacking functional RAG2 or IL-2Rγ do not thrive; therefore, obtaining RAG2/IL2RG double-knockout pigs through breeding is nearly impossible with an expectation of 1 in 16 piglets with double-null genotype after the breeding of double-heterozygous parents. Hematopoietic stem cell transplantations (HSCTs) have been performed to extend the lifespan of homozygous SCID pigs; however, few animals with successful engraftments have been reported4,19. Conventional HSCT requires conditioning of the recipient, T cell depletion of bone marrow and donor matching20,21. Performing total body irradiation or chemotherapy on large animals, such as pigs, is prohibitive due to the high expense and specialized facilities these procedures require.
In this study, we investigated the feasibility of a simplified process of reconstituting the immune system of RAG2/IL2RG double-knockout SCID pigs by engrafting bone fragments from immunocompetent fetal pigs. The reestablishment of a functioning immune system would allow SCID pigs to thrive long enough to serve as breeders, therefore enabling the production of SCID pigs from known genetic backgrounds and avoiding the use of founder SCID pigs that may carry unpredictable phenotypes for research. This strategy will substantially lower the cost and resources required to produce SCID pigs for biomedical research.
Results
Production of RAG2/IL2RG double-knockout SCID pigs
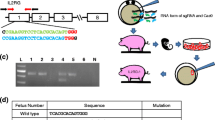
Microinjection of zygotes with the clustered regularly interspaced short palindromic repeats (CRISPR)–Cas9 system targeting RAG2 and IL2RG was performed to produce RAG2/IL2RG double-knockout piglets. Three litters were produced out of six embryo transfers (Supplementary Table 1). From the first litter, 12 piglets were delivered via cesarean section with all animals modified for RAG2 and IL2RG; the editing efficiency was consistent with our previous reports2,5. SCID pigs carrying modified RAG2 and IL2RG were used for transplantation. As expected, all SCID pigs lacked CD3e+ T cells when analyzed by flow cytometry (Supplementary Fig. 1a). Four piglets (n = 4, males) were selected on the basis of their genotypes and body condition to receive bone transplants at 9 days of age (Supplementary Figs. 1b and 2).
The second and third litters resulted in seven and ten piglets, respectively. PCR amplification of RAG2 and IL2RG genes followed by Sanger sequencing and flow cytometry analysis confirmed their SCID phenotype. Two (n = 2, males) and three (n = 3, males) piglets were selected from litter 2 and litter 3, respectively, to assess replicability and the proportions of immune reconstitution by fetal bone allografts.
Transplantation of fetal bone fragments into SCID pigs
Immunocompetent fetal piglets ubiquitously expressing the green fluorescent protein (GFP) gene (RRID NSRRC:0016)16,22 were selected as the source of fetal bone fragments to facilitate the detection of successful engraftment (Fig. 1a). Humerus and scapula bones were collected from day 79 fetal donors (Fig. 1b) and broken into fragments. A volume of 1 × 1 × 2 cm3 of bone fragments was inserted into the dorsal neck region of the recipient SCID pigs from the first litter (Fig. 1c–f). Surgical implantation of fetal bone fragments was successful, and no surgery-related complications were detected. Graft sites displayed no sign of infection following the surgery, and little to no redness or swelling was present during the healing process. After weaning, SCID pigs carrying the transplanted fetal bones appeared healthier and grew faster than their unreconstituted littermates (Fig. 2a). All recipient pigs were monitored for signs of graft-versus-host disease (GvHD) including weight loss, activity changes, coat condition, skin integrity, vomiting and diarrhea. No symptoms of GvHD were detected in the four piglets during the study. One pig, pig 4, died at 23 days post-transplantation of a suspected intestinal infection as discoloration, distension and edema were found in the abdomen upon necropsy, while the remaining pigs showed no signs of infection. One piglet, pig 7, was euthanized at 27 days post-transplantation to assess early engraftment in a healthy SCID pig. The remaining two pigs were maintained to assess transplantation outcomes over time (Fig. 2b).
a, Day 79 GFP+ transgenic fetal donors under a 440–460 nm wavelength excitation light source. b, Humerus and scapula bones collected from fetal donors were broken into fragments (arrow) before insertion. c, The insertion of GFP+ bone fragments (arrows) into the first implantation site on the left side of the incision site on the dorsal neck adjacent to the sixth cervical vertebra. d, GFP+ bone fragments (arrow) prior to insertion into the second implantation site on the opposite side of the incision. e, GFP+ bone fragments (arrow) after insertion into the second implantation site on the right side of the incision. f, A SCID piglet 1 week post-transplant with a palpable lump where the bone fragments were inserted. Arrow denotes the location of the first implantation site on the left side of the incision.
a, Weight gain in kilograms over time of recipient RAG2/IL2RG SCID pigs (n = 4, males; after day 36 n = 2) versus nonrecipient littermates (n = 4, 3 males, 1 female). After weaning at 21 days of age, growth rates varied. b, Recipient SCID pig from the first transplant using day 79 fetal bone at 3 months of age in a standard housing raised deck pen. c, CBC lymphocyte cell numbers over time in two recipient SCID pigs. Two wild-type control samples (n = 2, males) and clinical reference interval are included. d, Blood smears of isolated PBLs taken at 3 weeks post-transplantation from pig 5 compared with age-matched wild-type and GFP+ control pigs. Additional blood smear from pig 5 on day 42 following transplant (4× magnification; scale bar, 500 µm). DAPI stain was used to visualize the nuclei of all cells to compare to the GFP-expressing cell population. e, FACS of isolated PBLs to determine ratios of the population positive for GFP, CD3e, CD8α, CD21 and CD335 in the 5-month-old SCID recipient, pig 3 (5M) and age-matched wild-type (WT) and GFP control pigs. f, A representative flow cytometric analysis image of PBLs from pig 3 (5M). The PBLs were analyzed for their expression of GFP, CD3e, CD8α, CD21 and CD335 markers (n = 2; time points, 139 and 141 days). The PBLs were gated for live single cells, and forward scatter area (FSC) was used to measure the total number of collected events for each marker.
Contribution of fetal cells that originated from bone fragments
To assess the reconstitution of the immune system from the engrafted bone fragments, complete blood count (CBC) was performed on the surviving two pigs (Fig. 2c). Initial lymphocyte counts at 28 days post-transplantation were 0.36 × 103/µL and 0.43 × 103/µL, which were below the normal lymphocyte range (4.60–10.00 × 103/µL) of pigs. However, the lymphocyte level at 42 days post-transplantation increased by 3- to 4-fold (2.01 × 103/µL and 1.52 × 103/µL) in both pigs compared with the previous measurement. Pig 5 died unexpectedly at 63 days post-transplantation; post-mortem veterinary assessment suspected the sudden death was due to a febrile seizure. A CBC performed at the time of death showed that the level of lymphocytes in this pig had decreased to 0.92 × 103/µL compared with 1.52 × 103/µL at 42 days post-transplantation. The level of lymphocytes in the surviving pig, pig 3, continued to increase with counts of 2.71 × 103/µL and 3.47 × 103/µL at 70 and 84 days post-transplantation, respectively. A final CBC reading of 2.35 × 103 lymphocytes per microliter was collected when the pig was euthanized at 141 days post-transplantation after diagnosis with irreversible discospondylitis.
Blood collected 21 days after transplantation showed the presence of GFP+ cells in the circulation when analyzed by fluorescence microscopy (Fig. 2d) and flow cytometry (Fig. 2e). In addition, GFP+ cells remained present in circulation throughout the lifespan, and at 5 months of age, cells representing T and NK cells were observed in the peripheral blood of pig 3, but CD21+ cells representing mature B cells were not detected (Fig. 2e,f and Supplementary Table 2).
The distribution and the level of the transplanted fetal cells were analyzed by flow cytometry. GFP+ lymphocytes were detected in multiple organs including the spleen, liver, thymus and bone marrow from the ribs (Fig. 3a). Among GFP+ cells, CD3+ cells that were double negative, single positive and double positive for the CD4 and CD8 lineage markers were identified in the thymus, confirming that the transplanted fetal cells could give rise to both CD4+ and CD8α+ T cells. The presence of fetal cells in the host spleen was confirmed using immunohistochemistry (IHC) and hematoxylin and eosin (H&E) staining. While CD21+ B cells were not detected in tissues through flow cytometry, IHC staining confirmed that CD79α+ B cells were detected within white pulp structures along with CD3 T cells (Fig. 3b and Supplementary Fig. 3); CD79α+ and CD3+ cells and white pulp regions were absent or underdeveloped in control SCID pigs. Regions where CD79α+ and CD3+ cells were detected in transplanted SCID pigs corresponded with GFP+ signal in the spleen.
a, Single-cell flow cytometry to track the migration of cells at 27 days post-transplantation in pig 7. Lymphocyte populations were isolated and gated for GFP+, CD3+ and CD4+ or CD8α+ T cells in the recipient’s spleen, bone marrow, liver and thymus. Side scatter (SSC) and forward scatter (FSC) were used to isolate single lymphocytes from live cell populations, and the forward scatter was used to collect the total number of events for GFP+ and subsequent GFP+CD3+ cell populations. The isolated GFP+CD3+ T cells were then sorted into Q1 (CD8α+) Q2 (CD4+CD8+ double positive), Q3 (CD4+) or Q4 (double negative) T cell populations. b, IHC staining for CD3 T cells, GFP and CD79α B cells in the spleens of SCID pig 5 at 63 days post-transplantation and a wild-type pig (20× magnification; scale bar, 100 µm), and CD79α and CD3 IHC in a nonrecipient SCID pig littermate at 45 days of age (4× magnification; scale bar, 500 µm).
Structure of recovered bone allografts
Bone grafts were recovered from all recipient animals at post-mortem collections and appeared to undergo repair and bone remodeling processes that resulted in the formation of solid masses from the fragments. Bone grafts recovered from pig 4 at 23 days post-transplantation and pig 7 at 27 days post-transplantation were respectively 4.5 cm and 6 cm in length (Supplementary Fig. 4a,b). Of note, two grafts were recovered from pig 4 from the implantation sites on either side of the incision, while pig 7 possessed a single long graft across the vertebral column indicative that the engrafted bone fragments merged into one mass. Upon dissection, the pig 7 graft was found to consist of bone-encased GFP+ bone marrow of approximately 2 × 2 × 6 cm3. At 63 days post-transplantation, two similar grafts were recovered from pig 5 from both implantation sites (Supplementary Fig. 4c–e). Similar to pig 7, the oldest surviving graft recipient, pig 3, had a single graft that had embedded in the center of the dorsal neck across the vertebral column.
Histological analysis of H&E-stained bone grafts showed that the bone was actively undergoing growth and remodeling; sections displayed distinct zones of reserved cartilage, proliferation, maturation, hypertrophy, calcification and ossification (Fig. 4), which are consistent with naturally formed bones. The grafts displayed an architecture consistent with bone matrix, bone marrow cavities and vascular interfaces with the surrounding tissue. The density and structure of the transplanted bone were correlated with the duration of transplantation. The texture of bone grafts recovered from older SCID pigs appeared to be more rigid, compared with those recovered from younger SCID pigs. Indeed, histological analysis (Fig. 4) revealed that bone grafts recovered from the older two pigs appeared to be composed of denser and more mature bone. Interestingly, no signs of active bone growth were identified in the graft recovered from pig 3, the longest-surviving recipient, indicating that terminal differentiation had been reached.
a, Bone graft 27 days post-transplant displaying zones resembling epiphyseal growth plates including a reserve zone of cartilage (RZ), proliferative zone (PZ), hypertrophic zone (HT) and zone of calcification (CA). Bone matrixes filled with bone marrow (BM) could be observed. Regions around the bone were surrounded by muscle and other soft connective tissue. b, Bone graft at 63 days post-transplant showing denser bone regions with continued presence of growth zones. c, Bone graft recovered 141 days post-transplant displaying denser bone structures with large bone marrow cavities interfacing with connective tissue. d, Control humerus bone collected from a day 69 fetal pig displaying an epiphyseal growth plate. e, Cross section of a 5-month-old wild-type pig humerus bone for comparison to grafts. All images were taken at 4× magnification, with the scale bar denoting 500 µm.
Evidence of spermatogenesis
SCID pig 3 survived to 5 months of age. To verify if this boar was approaching sexual maturity, sections of the testis and epididymis were collected. Histological analysis identified sperm cells in both the testis and throughout the epididymis at various stages of production and maturation (Fig. 5), indicating that spermatogenesis was occurring and that the boar was reaching sexual maturity.
a, H&E staining of testes in a 5-month-old allograft recipient. The arrows show the presence of spermatogenesis. b, H&E staining of the epididymis of the recipient pig. The arrows show the presence of stored sperm. c, Fixed sperm from the recipient animal without staining. All images were taken at 40× magnification, with the scale bar denoting 50 μm.
Efficiency of the immune reconstitution by fetal bone transplantation
The consistency and effectiveness of the fetal bone transplantation were examined by using piglets from the second and third RAG2/IL2RG double-knockout litters. At 7 days of age, two pigs (n = 2, males) from the second litter were selected to receive allografts based on genotype and body condition using fetal bone allografts from day 110 GFP+ pigs (Supplementary Fig. 5). Both pigs survived to 2 months of age before being euthanized after developing fevers and joint inflammation. Increased neutrophil populations were detected from CBC and were confirmed by histology in the pigs, following trends seen in pig 3 in the first transplantation, which had an ongoing infection before being euthanized (Supplementary Fig. 6). A third set of transplantation surgeries were performed using three RAG2/IL2RG SCID pigs (n = 3, males) at 7 days of age from litter number 3 by using day 69 fetal bones. Two piglets were euthanized 5 days after the surgery due to skin infections. The remaining pig, 91-7, survived to 3 months of age before dying of a suspected stomach ulcer. Bone grafts recovered from all three pigs were comparable in structure to the initial cohort of transplanted pigs. Overall, 55% of pigs that received the transplant survived beyond 8 weeks of age compared with 0% of unreconstituted SCID pigs (Table 1).
Time-dependent increase in cells originated from the fetal bone grafts
To track cells originated from the fetal bone fragments, the distribution of GFP+ circulating peripheral blood leukocytes (PBLs) was monitored by using fluorescence-activated cell sorting (FACS) analysis. Before the transplantation, analysis of PBLs from RAG2/IL2RG SCID pigs revealed very few lymphocytes compared with wild-type pigs. Following the transplantation, we observed a steady increase in the level of GFP+ lymphocyte populations starting at 2 weeks post-transplantation. A significant increase in the GFP+ population was detected at 28 days post-transplantation compared with 2 weeks after transplantation (15.56% versus 6.09%, respectively), with the trend continuing at 42 and 70 days after the bone transplantation. While GFP+ PBLs in 10-week-old transplanted pigs was lower compared with the level in GFP pigs, the level of GFP+ cells remained steady after 6 weeks (Fig. 6 and Supplementary Table 3).
a, Representative FACS plots showing the immune-specific marker-presenting blood lymphocyte populations in wild-type and GFP control pigs compared with a SCID recipient (pig 3 from the first cohort shown) at transplantation and 10 weeks after transplantation. b, The ratio of GFP-, CD3e-, CD8α-, CD21- and CD335-presenting cells composing the isolated PBL populations of recipient pigs when sorted by FACS. Ratios of cell population in wild-type (WT) and GFP control pigs were compared to SCID recipients at birth (D0) and 2 weeks (2 W), 4 weeks (4 W), 6 weeks (6 W) and 10 weeks (10 W) post-transplantation. Except for the wild-type group (n = 6) and GFP+ group (n = 5), all groups were tested with three samples each (n = 3). Data were analyzed using a one-way ANOVA and Tukey’s multiple comparisons test and are presented as box-and-whisker plots. The different letters indicate statistically significant differences between each group and across timepoints (P < 0.05).
The level of immune cells also increased following the GFP fetal bone transplantation in RAG2/IL2RG SCID pigs. Flow cytometry analysis revealed a gradual increase in CD3e+ and CD8α+ T cells over time, reaching levels comparable to wild-type and GFP transgenic pigs between 6 and 10 weeks post-transplantation. CD21+ cells, a marker for mature B cells, remained undetectable throughout the post-transplantation period. The frequency of CD335+ cells, representing NK cells, varied; however, the level was statistically compatible with GFP control pigs from 2–6 weeks post-transplantation (Fig. 6 and Supplementary Fig. 7).
Distribution of immune cells in SCID pig PBLs that are originated from GFP fetal bone cells
The distribution of GFP+ immune cells in SCID pig PBLs was analyzed using double-gated FACS to investigate whether the immune cells are derived from cells that resided in the fetal bone fragments. Within the GFP+ population, the frequency of both CD3e+ and CD8α+ cells gradually increased, with the highest level at 10 weeks post-transplantation. The double-positive gating could identify GFP/CD21+ cells; however, the ratio did not increase and was significantly lower compared with the GFP control pigs, consistent with the previous detection of CD21 cells. The frequency of CD335+ cells within GFP+ cells varied but significantly increased when the pig was at 4–6 weeks post-transplantation (Fig. 7 and Supplementary Table 4).
a, Representative FACS plots showing dual channel gated by GFP and the immune-specific marker-presenting blood lymphocyte populations in wild-type and GFP control pigs compared with SCID recipients (pig 91-7 from the third cohort shown) at transplantation and 10 weeks after transplantation. b, The ratio of GFP+ CD3e-, CD8α-, CD21- and CD335-presenting cells composing the isolated PBL populations of recipient pigs when sorted by FACS. Ratios of cell population in wild-type (WT) and GFP control pigs were compared to SCID recipients at birth (D0) and 2 weeks (2 W), 4 weeks (4 W), 6 weeks (6 W) and 10 weeks (10 W) post-transplantation. Except for the wild-type group (n = 6) and GFP+ group (n = 5), all groups were tested with three samples each (n = 3). Data were analyzed using a one-way ANOVA and Tukey’s multiple comparisons test and are presented as box-and-whisker plots. The different letters indicate statistically significant differences between each group and across timepoints (P < 0.05).
Discussion
The limited lifespan of SCID pigs limits the use of these animals as models for long-term studies and prevents the propagation of homozygous lines. As SCID founders do not live long enough to reproduce naturally, direct microinjection of the CRISPR–Cas9 system2,5 or somatic cell nuclear transfer1,23, that is, cloning, is applied repeatedly to produce these models. Previously, HSCTs have been utilized to extend the lifespan of SCID pigs4,19; however, this is a challenging procedure in large animal models. As an alternative, we explored reconstituting the immune system of SCID pigs with hematopoietic stem cell (HSC)-containing fetal bones from immunocompetent pigs. The fetal allograft acts as a nonorthotopic source of HSCs. Following transplantation, recipient animals appeared to gain more weight and had better overall body condition than nonrecipient SCID littermates; thus, fetal allograft transplantation represents a viable strategy for improving the survival of SCID pigs.
The transplantation of exogenous tissues may induce acute GvHD conditions, a prevalent complication of allogenic HSCT24, which was a potential risk during this study. Recipient SCID pigs were monitored for signs of GvHD on the basis of grading scales found in the clinic25 and in rodent26 and porcine27 GvHD models. No clear evidence of GvHD was detected across all three cohorts. While GvHD could not be completely ruled out, we concluded the fetal allografts were not causing GvHD in the host SCID pigs.
Blood collected following transplantation showed the presence of circulating GFP+ PBLs, which increased over time. Not all lymphocytes in RAG2/IL2RG knockout pigs were GFP+; however, the majority of the lymphocytes were unlikely to be from an endogenous source as not all PBLs in circulation in GFP+ transgenic pigs were GFP+ and all blood samples from control SCID pigs were GFP–. Populations of GFP+ lymphocytes were likewise discovered in multiple tissues. Notably, while all SCID recipient pigs lacked a defined thymus, the thymic remnants in the pigs were found to contain CD4+ and CD8+ T cells, which is consistent with a previous report of allogenic lymphoid reconstitution in RAG2/IL2RG SCID pigs by delivering HSCs to SCID fetuses in utero28. This finding confirms that T cells were still able to be educated in the limited thymus of SCID pigs.
Results from our CBC analyses showed that lymphocyte numbers steadily increased over time following fetal bone allo-engraftment. However, no animals in the trial reached clinically normal lymphocyte levels, indicating that the engraftment may have not fully restored the lymphocyte production. Decreased lymphocyte counts in recipient animals around the time of death was suspected to be related to active infections as heightened neutrophil populations were also detected in the SCID pigs, which may have been the result of the small bone fragments not having a sufficient supply of primordial HSCs to support a rapidly growing pig. The FACS analysis of peripheral blood from engrafted pigs showed a consistent trend of increasing CD3+ and CD8a+ cells among lymphocytes. This reveals a preferential expansion of circulating T cells compared with other lymphocyte subsets. By contrast, NK cell levels decreased in older animals after an initial increase, suggesting that this population of lymphocytes had less impetus for expansion. Alternatively, this decrease may reflect innate immune system battling prolonged infection, resulting in NK cell exhaustion29.
The expanding T cell populations identified by flow cytometry were consistent with our IHC observations. In the spleen, CD3+ T cells stained positive for GFP and were widely spread out like in wild-type pigs. By contrast, GFP+CD79α+ cell distribution was sporadic and densely clustered. In addition, CD79α+ cells were generally not found outside these clusters or distributed as smoothly as in wild-type pigs, suggesting they may become trapped in the spleen and are unable to migrate through the tissue or into circulation. This is consistent with our inability to detect B cells in circulation, via anti-CD21 antibody staining, and with previous studies that used HSCT to reconstitute SCID pigs4,19. CD79α is a general B cell marker that is present before the pre-B cell stage30, while CD21 is predominantly expressed by mature B cells31. Naive B cells expressing CD79α leave the bone marrow and enter circulation to take up residence in secondary lymphoid organs where they functionally mature31. The presence of CD79α cells in the spleen without CD21 cells in circulation leads us to speculate that aberrant immune signaling in SCID pigs or the lack of complementary lymphoid organs such as lymph nodes may impair the ability of B cells to mature and survive reentry into circulation in RAG2/IL2RG pigs. This is consistent with clinical reports of HSCT in patients with SCID, which show a prevalence of delayed (median time to reach normal B cell function of 14.9 months) or absent donor B cell engraftment32. Further research into the biological pathways associated with B cell survival and maturation during immune reconstitution may overcome this obstacle.
The typical lifespan of SCID pigs housed under conventional housing system is 8 weeks or less1,2,5,8,23. The transplantation of fetal cells substantially extended lifespan of the pigs under standard housing, with our oldest SCID pig surviving until 5 months of age (on average, boars reach sexual maturity at 6 months of age). Although semen collection was not attempted before killing of the oldest pig, microscopic analysis of the testis and epididymis tissues revealed that this boar was producing sperm and, thus, beginning puberty. This boar was raised in conventional pig housing and therefore presumably exposed to a large number of microorganisms. In the future, the lifespan of transplanted pigs could be further extended by raising them in a controlled environment such as gnotobiotic or specific-pathogen-free housing, which is standard practice for housing SCID pigs2,5.
The fetal bone fragments used in this study were collected from fetuses at 69, 79 and 110 days of gestation. Compared with fragments from day 110 fetal donors, fetal bone grafts derived from younger donors (days 69 and 79) underwent a greater degree of remodeling after transplantation, with more complex bone matrixes that increased in density over time and higher lymphocyte counts on average at each time point. This is potentially due to the greater plasticity and capacity for bone growth and remodeling of bone at an earlier stage in development compared with bone at a later stage in the differentiation process. Although no direct comparison was conducted in this study, younger fetal donors may have different cellular programming capability that could stimulate expression of genes related to osteogenesis or HSC self-renewal and differentiation that results in better transplantation outcomes in SCID pigs than older donors. Further investigation into factors within fetal bone that influence growth and progenitor-cell proliferation would lead to more optimal approaches to reconstitute immune system in SCID pigs. In addition, previously reported bone marrow transplants in SCID pigs also used swine leukocyte antigen (SLA)-matched donors4,19. In this trial, we did not match the donors to recipients to assess the ability of these animals to engraft bone and immune cells regardless of SLA status. Potentially matching SLA may result in better engraftment outcomes.
In summary, the current work demonstrates the potential of using transplanted fetal bone fragments to extend the lifespan of immunodeficient SCID pigs to serve as a breeder to produce subsequent generations of SCID piglets with known genetic backgrounds. With further development, such as gnotobiotic housing and improved transplantation protocols, this approach may enable SCID pigs to reach sexual maturity, which would greatly reduce the time and resources needed to produce this valuable animal model for biomedical research.
Methods
Ethics statement
The use of animals during this study was in accordance with the approved protocol and standard operating procedures established by the Animal Care and Use Committee of the University of Missouri (protocol 23421).
Production of SCID pig models and animal husbandry
RAG2/IL2RG double-knockout pigs were generated via direct microinjection of the CRISPR–Cas9 system into one-cell stage embryos as described previously5. After 5 or 6 days in culture, blastocyst- and morula-stage embryos were surgically transferred into the ampullary–isthmic junction of a cycling gilt on day 4 or 5 of her estrous cycle. Pregnancy was monitored by heat checking and by ultrasound scans after day 25. On day 113 of gestation, piglets were delivered by cesarean section and processed individually in a sterile manner. Umbilical cord blood was collected for confirming the SCID phenotype by genotyping and flow cytometry. SCID pigs carrying modified RAG2 and IL2RG, and lacking CD3e+ T cells, were used for transplantation.
All animals were placed in individual pens within a peroxide fumigated (Bioquell), sterile room with positive air pressure. Animal handling was conducted with full personal protective equipment, including disposable coveralls, shoe covers, rubber boots, face masks, hair nets and gloves, to minimize opportunistic pathogen exposure. Piglets were given synthetic colostrum (NurseMate ASAP, Sterling Technology) after birth and then offered ultrahigh-temperature-pasteurized milk ad libitum. After weaning, the piglets were transferred to a diet of irradiated pellets. Some pigs with reconstituted lymphocytes after the transplantation were housed under conventional indoor housing, but personal protective equipment requirements remained consistent. Body weights were monitored daily from birth through weaning by placing piglets into a sterile tub on a scale. Post-weaning, pigs were weighed less frequently to minimize handling.
Genotyping of RAG2 and IL2RG
Genomic DNA was isolated from ear notches by using embryo lysis buffer as described previously15. All reagents were purchased from Sigma-Aldrich unless otherwise noted. Target regions were amplified by using primers designed to target RAG2 and IL2RG (Supplementary Table 5). A separate primer was designed for Sanger sequencing of the RAG2 and IL2RG amplicons. PCR conditions were as follows: initial denaturation at 95 °C for 2 min; 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min 15 s; final extension at 72 °C for 5 min. Amplicons were submitted to the University of Missouri Genomics Technology Core for Sanger sequencing.
Transplantation of allogenic fetal bone fragments
Day 79, 110 or 69 GFP+ fetuses were collected via cesarean section and placed into a sterile container (RRID NSRRC:0016). Each fetus was dissected in a biosafety cabinet, and the humerus and scapula bones were collected and crushed into fragments. The fragments were then transported in a sterile container to the surgery unit. Following preparation of the bone fragments, SCID piglets were anesthetized and prepped for intramuscular transplantation surgery. To insert the fetal bone fragments, a 3–5 cm incision was made on the dorsal neck region adjacent to the sixth cervical vertebra, and approximately three to four 1–2 × 1 × 1 cm3 bone fragments were transplanted into the incision site. A sterile bandage was placed over the incision site, and intramuscular injections of Ceftiofur crystalline-free acid (Excede, 100 mg/mL by Zoetis Animal Health) and Enrofloxacin (Baytril 100, 100 mg/mL by Bayer) were given at 5 mg/kg and 2.2 mg/kg of body weight, respectively, to prevent infection. After the transplantation surgery, piglets were recovered and housed in a newly hydrogen peroxide treated (Bioquell), sterile room. Incision sites were monitored closely and disinfected multiple times a day by using chlorhexidine until healed. All animals were checked multiple times a day to monitor any complications following the surgery. Blood samples were taken periodically to detect the presence and level of circulating lymphocytes.
Histological analysis
Tissue and graft samples were fixed in 10% neutral buffered formalin either overnight at room temperature (RT) or longer depending on the sample size. After formalin fixation, the samples were stored in 70% ethanol. Fixed tissues were submitted to the University of Missouri Veterinary Medical Diagnostic Laboratory to be embedded and stained with H&E for structural analysis of tissues. Bone grafts were decalcified for 2 min by using Decal Stat Decalcifier (StatLab, 1212-32) before staining. The presence of GFP, CD3 and CD79 cells in recipient tissues was analyzed through IHC by using anti-GFP antibody (Abcam, ab290), anti-CD3 antibody (Dako, A0452) and anti-CD79α (Bio-Rad, MCA2538H). Serial sectioning was performed to identify CD3, CD79α and GFP cells in the same regions. Heat-induced epitope retrieval was performed before the samples were loaded into a decloaking/pressure chamber with Diva Decloaker (Biocare) for anti-GFP and CD3, or Borg for CD79α in Biocare decloaker. Blocking was performed by using 3% hydrogen peroxidase followed by Sniper (Biocare). Anti-GFP antibody incubation was performed for 30 min at 37 °C and incubated with secondary rabbit HRP Envision (Dako) for 30 min. Romulin Red (Biocare) was used as the chromogen for 10 min with CAT hematoxylin (Biocare) used for 3 min for counterstaining. Staining for CD3 and CD79α were both performed for 45 min with secondary staining with rabbit HRP Envision (Dako) for CD3 and MACH 2 (Biocare) for CD79 each for 30 min.
Tissue preparation
Bone marrow, liver, spleen and thymic tissue from euthanized pigs was collected in Hanks’ Balanced Salt Solution (HBSS) (Gibco, 14170-112). Tissues were then dispersed into single-cell suspension using 20 mL glass homogenizers and filtered through a 80 µm mesh. Erythrocytes in the liver, spleen and thymus were lysed using an ammonium chloride-based lysis buffer. Bone marrow samples were not submitted to lysis buffer. Homogenized samples were centrifuged at 400g for 4 min at 4 °C, after which leukocytes were collected and washed once with HBSS. Finally, leukocytes were resuspended in HBSS at the desired concentration before flow cytometry analysis.
Flow cytometry
For isolation of PBLs, blood was collected in EDTA vacutainers. A volume of 1.5–2.0 mL of blood was transferred into a 50 mL conical tube containing 20 mL of an ammonium–chloride–potassium lysing buffer (Gibco, A10492-01) and allowed to incubate for 15 min at RT to remove red blood cells. White blood cells were collected by centrifugation at 300g for 5 min at RT. After removing the supernatant, cell pellets were resuspended with 5 mL of cold 1× Dulbecco’s phosphate-buffered solution (Gibco, 14190-136) and centrifuged at 300g for 5 min at 2–8 °C. The supernatant was removed, and the cell pellet was resuspended with FACS buffer (Invitrogen, 00-4222-26). Then, the isolated leukocytes were Fc blocked to prevent unwanted antibody binding to the fragment crystallizable (Fc) region of cell surface receptors using 1 mg/mL solution of rat IgG (Sigma-Aldrich, I4131) for 10 min at 4 °C followed by 30 min incubation at 4 °C with one of four antibody panels for flow cytometry analysis. Panel 1 contained antibodies against CD3e (SouthernBiotech, 4510-11) and CD8α (SouthernBiotech, 4520-09). Panel 2 contained antibodies against CD3e and CD21 (SouthernBiotech, 4530-09). Panel 3 contained antibodies against CD335 (Bio-Rad, MCA5972APC) and CD3e (SouthernBiotech, 4510-09). Panel 4 contained antibodies conjugated with phycoerythrin (PE) or allophycocyanin (APC) against CD3e, CD4 and CD8α using CD3e-PE-Cy7 (BD Biosciences, 561477), CD4-APC (SouthernBiotech, 4515-11) and CD8α-PE (SouthernBiotech, 4520-09). After cell surface staining, cells were fixed and permeabilized by using a BD Cytofix/Cytoperm kit (BD Biosciences, BD 554714) according to the manufacturer’s protocol. Stained cells were analyzed on an Attune Nxt Acoustic Focusing Flow Cytometer (Thermo Fisher Scientific). Dead cells were identified using LIVE/DEAD Fixable Near-IR Dead Cell Stain (Invitrogen, L34975), then cells were gated to isolate single cells and consistent event collection was verified before lymphocyte and antibody gating. Flow cytometry data were analyzed by using FlowJo version 10.8.1 software (FlowJo). Flow cytometry analyses of all SCID pigs across all time points were always accompanied by samples from immunocompetent pigs for positive controls. The gating strategies for lymphocyte populations are shown in Supplementary Fig. 8.
Statistical analysis
Each flow cytometry experiment was conducted with a minimum of three replicates. The statistical analyses were performed with the software GraphPad Prism 6.0. Percentage data were analyzed using one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test. Table data were expressed as the mean ± standard error of the mean. Statistical significance was determined as P < 0.05, unless stated otherwise.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available upon request from the corresponding authors.
References
Lee, K. et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc. Natl Acad. Sci. USA 111, 7260–7265 (2014).
Hendricks-Wenger, A. et al. Establishing an immunocompromised porcine model of human cancer for novel therapy development with pancreatic adenocarcinoma and irreversible electroporation. Sci. Rep. 11, 7584 (2021).
Basel, M. T. et al. Human xenografts are not rejected in a naturally occurring immunodeficient porcine line: a human tumor model in pigs. Biores Open Access 1, 63–68 (2012).
Boettcher, A. N. et al. Novel engraftment and T cell differentiation of human hematopoietic cells in ART−/− IL2RG−/Y SCID Pigs. Front. Immunol. 11, 100 (2020).
Lei, S. et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci. Rep. 6, 25222 (2016).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Boettcher, A. N. et al. Human ovarian cancer tumor formation in severe combined immunodeficient (SCID) pigs. Front. Oncol. 9, 9 (2019).
Boettcher, A. N., Loving, C. L., Cunnick, J. E. & Tuggle, C. K. Development of severe combined immunodeficient (SCID) pig models for translational cancer modeling: future insights on how humanized SCID pigs can improve preclinical cancer research. Front. Oncol. 8, 559 (2018).
Lossi, L., D’Angelo, L., De Girolamo, P. & Merighi, A. Anatomical features for an adequate choice of experimental animal model in biomedicine: II. Small laboratory rodents, rabbit, and pig. Ann. Anat. 204, 11–28 (2016).
Swindle, M. M., Makin, A., Herron, A. J., Clubb, F. J. & Frazier, K. S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49, 344–356 (2012).
Lunney, J. K. et al. Importance of the pig as a human biomedical model. Sci. Transl. Med. 13, eabd5758 (2021).
Powell, E. J. et al. Creating effective biocontainment facilities and maintenance protocols for raising specific pathogen-free, severe combined immunodeficient (SCID) pigs. Lab Anim. 52, 402–412 (2018).
Hai, T., Teng, F., Guo, R., Li, W. & Zhou, Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 24, 372–375 (2014).
Sato, M. et al. Direct injection of CRISPR/Cas9-related mRNA into cytoplasm of parthenogenetically activated porcine oocytes causes frequent mosaicism for indel mutations. Int. J. Mol. Sci. 16, 17838–17856 (2015).
Whitworth, K. M. et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol. Reprod. 91, 78 (2014).
Park, K. W. et al. Production of nuclear transfer-derived swine that express the enhanced green fluorescent protein. Anim. Biotechnol. 12, 173–181 (2001).
Lee, S. Y. et al. Comparative proteomic analysis associated with term placental insufficiency in cloned pig. Proteomics 7, 1303–1315 (2007).
Ao, Z. et al. Birth weight, umbilical and placental traits in relation to neonatal loss in cloned pigs. Placenta 57, 94–101 (2017).
Powell, E. J. et al. T cell lymphoma and leukemia in severe combined immunodeficiency pigs following bone marrow transplantation: a case report. Front. Immunol. 8, 813 (2017).
Grunebaum, E. et al. Bone marrow transplantation for severe combined immune deficiency. JAMA 295, 508–518 (2006).
Haddad, E. & Hoenig, M. Hematopoietic stem cell transplantation for severe combined immunodeficiency (SCID). Front. Pediatr. 7, 481 (2019).
Whitworth, K. M. et al. Method of oocyte activation affects cloning efficiency in pigs. Mol. Reprod. Dev. 76, 490–500 (2009).
Suzuki, S. et al. Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency. Vet. Immunol. Immunopathol. 178, 37–49 (2016).
Horowitz, M. M. et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75, 555–562 (1990).
Harris, A. C. et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 22, 4–10 (2016).
Naserian, S. et al. Simple, reproducible, and efficient clinical grading system for murine models of acute graft-versus-host disease. Front. Immunol. 9, 10 (2018).
Duran-Struuck, R. et al. Miniature swine as a clinically relevant model of graft-versus-host disease. Comp. Med. 65, 429–443 (2015).
Sper, R. B. et al. Allogeneic and xenogeneic lymphoid reconstitution in a RAG2−/−IL2RGy/− severe combined immunodeficient pig: a preclinical model for intrauterine hematopoietic transplantation. Front. Vet. Sci. 9, 965316 (2022).
Bi, J. & Tian, Z. NK cell exhaustion. Front. Immunol. 8, 760 (2017).
Mason, D. Y. et al. CD79a: a novel marker for B-cell neoplasms in routinely processed tissue samples. Blood 86, 1453–1459 (1995).
LeBien, T. W. & Tedder, T. F. B lymphocytes: how they develop and function. Blood 112, 1570–1580 (2008).
Haddad, E. et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 91, 3646–3653 (1998).
Acknowledgements
This work was supported by the National Institutes for Health, Office of the Director (R21OD027062) and funding for the National Swine Resource and Research Center is from the National Institute of Allergy and Infectious Disease, the National Heart, Lung and Blood Institute, and the Office of Research Infrastructure Programs, Office of the Director (U42OD011140).
Author information
Authors and Affiliations
Contributions
K.D.W., R.S.P., P.R.C. and K.L. conceived and designed the experiments. K.U., E.R. and L.D.S. generated the immunodeficient pigs. K.M., J.Y., E.R., D.C.R., M.S.S., S.S., K.M.W. and P.R.C. provided husbandry for immunodeficient pigs. K.M., E.R., J.-H.L., S.S. and P.R.C. performed transplantation of allogenic bones. K.M., J.Y., D.M.d.C.M., L.T., J.P.D. and P.R.C. analyzed immune system post-transplantation. K.M., J.Y. and P.R.C. wrote the initial draft. R.S.P. and K.L supervised the work. All authors have reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Tables 1–5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Monarch, K., Yoon, J., Uh, K. et al. Fetal bone engraftment reconstitutes the immune system in pigs with severe combined immunodeficiency. Lab Anim (2024). https://doi.org/10.1038/s41684-024-01439-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41684-024-01439-7
- Springer Nature America, Inc.