Abstract
Although it is generally recognized that sleep quality, depressive symptoms, and cognitive functions are related respectively, the main ambiguity comes from difficulties in determining their cause-effect relationships. The present study aimed to explore the longitudinal causation relationships among sleep quality, depressive symptoms, and cognitive functions in older people with mild cognitive impairment (MCI). A total of 134 patients from 24 communities in Ningbo City, Zhejiang Province, China with MCI were interviewed at baseline, while 124 of them were re-interviewed 2 months later, and 122 were re-interviewed 4 months later. The Patient Health Questionnaire-9, the Pittsburgh Sleep Quality Index and the Montreal Cognitive Assessment Scale were assessed in the interview. Cross-lagged models were tested to disentangle the relationships among sleep quality, depressive symptoms, and cognitive functions using structural equation modeling with latent variables on the four-mouth longitudinal data. The correlation coefficients between sleep quality and depressive symptoms were significant showing the stability across time points of assessment, while the correlation coefficient of cognitive function was not significant (r = 0.159, p > 0.05). The results of index of model fit indicated that the cross-lagged model was acceptable (CFI = 0.934, TLI = 0.899, RMSEA = 0.075, χ2/df = 1.684). The results of cross-lagged model analysis supported the complete mediating role of depressive symptoms in the association between sleep quality and cognitive functions, where worse sleep quality may lead to more severe depressive symptoms, which in turn leads to more severe cognitive decline. In Conclusion, sleep quality is significantly correlated with cognitive functions in patients with mild cognitive impairment, which association is fully mediated by depressive symptoms. Approaches addressing sleep quality and depressive symptoms are recommended and hold promise for the management of mild cognitive impairment.
Similar content being viewed by others
Introduction
With the rapid process of population aging, age-related diseases are becoming more and more prevalent. Dementia, as the most important kind of senile disease, has become the fourth major chronic disease threatening the health of the elderly1. According to the World Health Organization (WHO), there are currently about 50 million people in the world with dementia, and nearly 60% of them live in low—and middle-income countries. Epidemiological data show that the proportion of dementia has risen to 6.4% in the aging population, and it is expected that by 2040, the number of patients with dementia in China (about 24 million) will be equal to the whole population with dementia in all developed countries2. However, the lack of resources and approaches for dementia care and treatment remain challenges in China, and all over the world.
Mild cognitive impairment is defined as the early stage of dementia, which is manifested as impairment in one or more cognitive domains but does not meet the clinical diagnosis criteria of dementia3,4. As mild cognitive impairment is a transitional state between normal aging and dementia, people in this stage reserve a variety of possible outcomes, including remain stable or get better, or further progress into dementia5. A follow-up study at the Mayo Clinic Alzheimer's Center found that the conversion rate for dementia was only 1% to 2% per year in healthy controls, while the conversion rate for mild cognitive impairment was about 10% to 15% per year6,7. It highlights the urgent need of developing effective interventions to slow or prevent the progression from mild cognitive impairment to dementia, and identifying risk factors that contribute to the conversion to dementia may provide ideas for dementia prevention and intervention.
Studies have found that sleep disorders may be important risk factors for cognitive impairment8,9,10. Although sleep changes often occur in normal aging process, they are more common in people with mild cognitive impairment, including the changes in sleep latency, sleep efficiency, and duration11. Research evidence shows that many mild cognitive impairment patients are affected by sleep disorders, and the prevalence ranges from 35 to 48%12. In addition, the prevalence of sleep disorders generally increases with the progression of cognitive disorders. Studies have pointed out that the prevalence of sleep disorders in patients with mild to moderate Alzheimer's disease is 25%13,14, while about 50% of patients with moderate to severe Alzheimer's disease are affected by sleep disorders13.
Moreover, the progression of cognitive decline has been observed to be significantly correlated with poorer sleep quality15. For example, a longitudinal study found that insomnia in middle age (OR = 1.24, 95% CI [1.02, 1.50]) and later life (OR = 1.94, 95% CI [1.08, 3.49]) were both associated with a higher risk of dementia16. Therefore, some scholars have proposed that sleep disorders may predict the risk of mild cognitive impairment turning into dementia17, with accelerating the process of cognitive impairment as a complication of mild cognitive impairment18. Though a growing body of research recognize the interplay between sleep quality and cognitive functions, the underlying mechanisms remain unknown and need further investigation to provide reliable theoretical framework for intervention strategies aiming at delaying cognitive decline in patients with mild cognitive impairment.
To the best of our knowledge, current investigations into the relationship between sleep quality and cognition encompass both physiological and psychosocial factors. Physiologically, brain structures such as the precentral cortex, lateral orbitofrontal cortex, and hippocampus19,20,21 are primarily implicated; however, further research is required to establish definitive connections.
Psychological factors often play important roles in the occurrence and development of aging-related diseases, such as such as loneliness22, anxiety23, and depression24,25. Depression is the most common mental health problem among the elderly and is closely related to sleep disorders26,27. Though many studies have reported the associations between sleep quality and depression28,29,30, the causal link between the two variables is not clear. Traditionally, sleep disorders have been considered a concomitant symptom of depression, but recent evidence argue that sleep disorders may precede depression31,32. For example, a recent longitudinal cohort study showed that short sleep duration was associated with an increased risk of depressive symptoms in elderly Chinese women33, and another study implied that sleep disorders might play as independent risk factors for depression recurrence in the elderly34. Theoretically, according to the learned helplessness model35, if individuals are constantly troubled and hit by insomnia, they believe that they cannot solve the problem themselves, and thus negative emotions were produced. Thus, we hypothesize that sleep disturbances may increase individuals’ learned helplessness, which may lead to more pronounced depression.
Relationships between depression and mild cognitive impairment are also well investigated. A survey showed that 32% of individuals with mild cognitive impairment were depressed or showed significant depressive mood36. In addition to the high prevalence, research evidence shows that depression can damage the instantaneous memory and delayed recall ability of mild cognitive impairment patients37, lead to the decline of working memory, episodic memory and non-velocity executive function38, and even affect their daily function and quality of life39. The Impaired Disengagement Hypothesis explains the impact of depression on cognitive functions40: Depressive experiences are often accompanied by rumination41, and individuals pay particular attention to negative stimuli, immersing themselves in attention resources and making it difficult to withdraw from them, thus decreasing executive control ability42. Similar hypotheses have been examined recently, in which depression is supposed to lead to attentional control deficits43,44,45, and thus impair cognitive functions.
Taken together, the associations between depression, sleep quality, and cognitive functions are complex and multifaceted. The main aim of the present study was to explore the longitudinal causation relationships of the three variables in older people with mild cognitive impairment. We assume that depressive symptoms serve as a partial mediator in the relationships between sleep quality and cognitive functions. We believe this work will help us to refine the theoretical basis for the treatment and management of mild cognitive impairment and provide scientifically feasible guidance for future practice.
Methods
Participants and procedure
The participants were elderly residents aged 60 and above from 24 communities in a township of Ningbo City, Zhejiang Province. 10 trained research assistants accessed older residents whether in the primary care clinics, senior citizens activity centers, or their homes, introducing the research, inviting them to participate, evaluating for eligibility and making the study interview. The interview content included the participants’ demographic characteristics, sleep quality, depressive symptoms and cognitive functions, and it took about 30 min to complete the interview. Those who were screened positive for mild cognitive impairment, defined as the Montreal Cognitive Assessment score ≤ 24 (+ 1 for < 6 years education)46,47,48 and not demented with Clinical Dementia Rating (CDR) < 149, in the preliminary cognitive screening procedure were invited to participate in the follow-up survey. Assessments were conducted at baseline (T1), two months (T2) and four months later (T3) evaluating their depressive symptoms, sleep quality and cognitive functions. Specially, cognitive functions were only assessed at T1 and T3 as the study was initially designed to explore the potential pathways related to the cognitive changes between two time points, T1 and T3.
The research was performed in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Ningbo Huamei Hospital (Reference No. YJ-KYSB-NBEY-2020-220-01). Written informed consent was obtained from all the participants.
The inclusion criteria were as follows: (i) community-dwelling residents; (ii) aged ≥ 60 years; (iii) no severe problems with vision, hearing, or speaking reported by the resident or informant (i.e., families) or observed by the research assistants; and (iv) willing to give written informed consent.
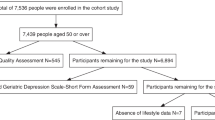
The number of individuals with mild cognitive impairment interviewed at the three time points were 134 (T1), 124 (T2) and 122 (T3) respectively, and only data of the final 122 participants who complete all the three surveys were included for analysis (See Fig. 1).
Analyses of variance were conducted to determine differences between dropouts and those completed the questionnaires. They did not differ significantly (ps < 0.05) at socio-demographic variables, depressive symptoms, sleep quality and cognitive functions.
Sample size calculation
Specific recommendations were made for the sample size of structural equation model as to the number of the samples needed to be estimated per parameter, a common number being 1050. Given the numbers of parameters estimated in the hypothesis model was 12, according to the rule, the recommended minimum sample size was 120, the sample size of 122 in this study conforms to the criteria.
Measures
Basic information
A self-designed questionnaire was utilized to gather demographic information including age, gender, educational background, marital status, and living conditions.
Depressive symptoms
Depressive symptoms were measured using the Patient Health Questionnaire-9 (PHQ-9). The PHQ-9 was compiled by Spitzer et al.51 according to the nine diagnostic criteria of the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, DSM-IV), which scores each of the criteria as “0” (not at all) to “3” (nearly every day). A higher score indicates more severe depression. PHQ-9 has sample content and strong operability and has been validated for use in the elderly population52. It is not only a screening tool for depression but it is used to monitor the severity of depression53. The item on sleep quality in the PHQ-9 scale was excluded and the total score of the remaining 8 items was calculated to assess depressive symptoms to eliminate possible overlapping confusion in the test. The Cronbach's α coefficients of the remaining eight items and the entire PHQ-9 scale in this study was 0.770 and 0.771, respectively.
Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality, which was compiled by Buysse et al.54. PSQI is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval for patients with sleep disorders and mental disorders and also for general people54. The scale has 19 items divided into seven components, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each dimension is scored from 0 to 3, and the sum of these seven components yields one global score, which ranges from 0 to 21 points. The Cronbach's α coefficient of PSQI scale was 0.816.
Cognitive functions
Cognitive functions were measured by the Montreal Cognitive Assessment Scale (MoCA). MoCA is a rapid and effective tool for screening cognitive dysfunction. It was revised by Nasreddine et al.48 based on clinical experience and by referring to cognitive items and scores of Mini-mental State Examination (MMSE). The scale includes 8 cognitive domains, including visuospatial and executive function, naming, memory, language, attention, abstraction, delayed recall, and orientation, with a total score of 30. In this study, the reliability Cronbach's α of this scale was 0.803.
Statistical analysis
We conducted with SPSS 22.0: (1) descriptive analyses to characterize the sample; and (2) bivariate analyses to assess sleep quality in relation to depressive symptoms and cognitive functions, separately. We employed structural equation modeling to test the specified models. Cross-lagged models is appropriate for studies that aim to determine the relative importance of prospective reciprocal influencing factors, i.e., assume that the effects spread over time. We used AMOS 21.0 to analyze a series of competing cross-lagged models for sleep quality, depressive symptoms, and cognitive functions to explore the longitudinal interaction between the three variables. Age, sleep quality, depressive symptoms, and cognitive functions were included as continuous variables in the equations, while the remaining variables were treated as categorical.
Model 1 (M1) is the baseline model to estimate the stability coefficient of the relationship among the three variables, which correlated the error terms among variables. Based on M1, Model 2 (M2) adds pathways from cognitive functions to depressive symptoms and from depressive symptoms to sleep quality. Model 3 (M3) adds pathways from sleep quality to depressive symptoms and from depressive symptoms to cognitive functions based on M1. Model 4 (M4) is based on M1, adding all paths of cross lagged between the three variables. Figure 2 presents the M3 as an example. In this model, a variable at T2 is predicted by the same variable at T1 (autoregressive path) and the other variables at T1 (cross-lagged path). Similarly, a variable at T3 is predicated by the same variable at T2 (autoregressive path) and the other variables at T2 and T1 (cross-lagged path). Considering the gender, age and years of education might affect the cognitive functions, these covariates were added in the models to control the hybrid effect. The number of bootstrap samples was chosen to be 1000, under the bias-corrected 95% confidence interval (CI).
Three waves, three variables, cross-lagged effects model of sleep quality, depressive symptoms, and cognitive functions. The standardized coefficients were displayed. Dotted straight lines represent insignificant paths and solid straight lines represent significant paths. PSQI Pittsburgh Sleep Quality Index, PHQ9 Patient Health Questionnaire-9, MoCA Montreal Cognitive Assessment Scale, e measurement error. ***p < 0.001, **p < 0.01, *p < 0.05.
Model fit was evaluated using the Comparative Fit Index (CFI), Tucker Lewis Index (TLI), and Root Mean Square Error of Approximation (RMSEA). The ratio of chi-square statistic (χ2) to degree of freedom (df) was also used as an indicator of model fit, since χ2 is sensitive to the number of parameters in the model and to sample size. The CFI and TLI values greater than 0.90 are typically taken to reflect acceptable and excellent fits to the data respectively55, and RMSEA values greater than 0.10 are unacceptable56. In addition, the current study used ΔCFI, ΔTLI, and ΔRMSEA to compare measurement model. The ΔCFI and ΔTLI values < 0.01, respectively and ΔRMSEA values < 0.015, indicating that fit between the models was equivalent57. The Akaike information criterion (AIC) and Bayesian Information Criterion (BIC) were also used to assist in selecting the best fit model.
Results
Demographic characteristics
As shown in Table 1, the mean age of subjects was 70.1 (SD = 3.8) years. Most of the participants were female (73.8%) and nearly 93% of them had education of primary school or below. Most are married (77.5%) while 22.5% are widowed. 75.4% of them live with their spouse; 18.9% live alone and others live with other people.
Descriptive statistics and correlations
Means, standard deviations, and correlations for the measures of sleep quality, depressive symptoms and cognitive functions are shown in Table 2. The correlation coefficients of sleep quality in three evaluating times were significant and show stability across time. The depressive symptoms behaved similarly. However, there was no significant correlation between cognitive function at the two measurement points (r = 0.159, p > 0.05).
The correlation coefficients between sleep quality and depressive symptoms were significant at different time points, indicating close relationships between them. The correlation between cognitive function and sleep quality was also significant at T1 and T3 respectively, revealing a close relationship between sleep quality and cognitive function. The correlation between cognitive function and depressive symptoms was significant only at T1, but cognitive function at T3 was significantly correlated with depressive mood at T2, suggesting that there may be a potential pathway for depressive symptoms to predict future cognitive decline.
The cross-lagged model modeling sleep quality, depressive symptoms and cognitive functions
Table 3 summarizes the results of the model fitting for the cross-lagged relationships among sleep quality, depressive symptoms, and cognitive functions. As demonstrated, M3 has the best model fit, and significantly makes the fit better relative to the other models. The final cross-lagged model results of the three variables are shown in Fig. 2.
The relative stability coefficients were 0.73 and 0.83 for sleep quality (all ps < 0.001), and 0.27 and 0.18 for depressive symptoms (all ps < 0.05). In terms of cross-lagged effects, the standardized regression coefficients of sleep quality at T1 on depressive symptoms at T2 (β = 0.33, p < 0.001, 95% CI [0.10, 0.32]) indicated that worse sleep quality predicted more severe depressive symptoms at the next time point. Similarly, worse sleep quality at T2 significantly predicted more severe depressive symptoms at T3 (β = 0.22, p = 0.023, 95% CI [0.02, 0.26]). As expected, depressive symptoms at T2 significantly and negatively predicted cognitive functions at T3 (β = -0.25, p = 0.008, 95% CI [− 0.56, − 0.02]), but sleep quality at T1 did not predict cognitive function at T3 (β = − 0.07, p = 0.43, 95% CI [− 0.19, 0.07]). It suggests that depressive symptoms at T2 is a complete intertemporal mediator between sleep quality at T1 and cognitive functions at T3.
Discussion
The purpose of the present study was to provide empirical evidence on the link between sleep quality, depressive symptoms, and cognitive functions in older people with mild cognitive impairment. The causal relationships among these three constructs remain elusive. Although it is generally agreed that these variables are related to each other, the main ambiguity arises from difficulties in determining their case-effect relationships: does sleep quality promote depressive symptoms and then accelerate cognitive decline, or does cognitive impairment promote depression and then cause sleep disturbance? Clarification of the directionality of causal effects among these constructs has important implications for the mental health research and the treatment of cognitive impairment.
Main findings of the current study were that, sleep quality was significantly correlated with cognitive functions in patients with mild cognitive impairment, which association was fully mediated by depressive symptoms. The longitudinal mediation model examined in the study added to the theoretical knowledge regarding the complex relationships between sleep, depression and cognitive functions, in which sleep quality had impact on cognitive functions total through the mediation effect of depressive symptoms.
Our results supported the prospective effects model, in which each construct exerted a causal influence on another one over time. It means that, in people with mild cognitive impairment, sleep quality predicted subsequent levels of depressive symptoms, and then influence subsequent levels of cognitive functions, controlling for prior depressive status and cognitive functions. For the elderly with mild cognitive impairment, when their sleep quality is decreased, it will obviously cause or aggravate depression, which will lead to the decline of cognitive functions. To be specific, poor sleep quality significantly impaired cognitive functions by exacerbating depression within 2 to 4 months.
From biological perspective, prefrontal cortex function may explain the casual influence between sleep quality and depressive symptoms. Loss of sleep may lead to decreased inhibitory control of negative emotions through disruption of prefrontal cortex function and thus increase of negative emotions58. In previous functional magnetic resonance imaging (fMRI) studies, sleep deprived individuals showed significantly increased amygdala activation in response to negative emotional stimuli (60% higher volume level) compared to controls (normal sleep); There was also reduced connectivity between the amygdala and the medial prefrontal cortex (a region that has been found to be involved in inhibitory control and highly predictive of amygdala inhibition), and stronger connectivity between the amygdala and the locus coeruleus59. In other words, reduced metabolic activity in the medial prefrontal cortex may impair normal regulation of emotion-related amygdala responses, leading to elevated processing of negative emotions. However, further studies are needed to examine the mediating and/ or moderating roles of prefrontal cortex function in the relationship between sleep quality and depression.
As for the relation of depressive symptoms and cognitive functions, dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis observed in depressed patients may lead to increased production of glucocorticoids60, increased amounts of amyloid plaques (i.e., beta-amyloid and tau proteins), and chronic inflammation61, all of which can lead to hippocampal atrophy62, or abnormal frontal striatum63. These changes are highly associated with cognitive declines64. However, it is important to note that these above biological changes have been indicated in several studies with heterogeneity in terms of samples recruited. Therefore, further investigation is needed to determine whether these changes are more likely to be promoted in patients with MCI or dementia compared to those with normal cognitive function.
According to the results of correlation analysis, sleep quality and depression showed a certain horizontal and vertical stability, while cognitive functions showed a decline tendency. It is worth noting that the correlation between the cognitive function evaluations at T1 and T3 was not significant, which implied the relatively rapid progress on cognitive decline in individuals with mild cognitive impairment and the urgent need of addressing this issue.
Overall, this study revealed the mediating role of depressive symptoms in sleep quality affecting cognitive functions in patients with mild cognitive impairment, where the worse sleep quality led to a more severe depressive symptoms resulting in more serious cognitive decline. Interventions for older adults with mild cognitive impairment are suggested to include both sleep disorders and depressive symptoms for consideration.
This study had some limitations. First, despite the longitudinal nature of the data sets, the models tested in this research do not necessarily represent causal relationships between the variables contained in these models. In addition, the sample of this study was from a township of Ningbo City, so our findings may not be generalizable to other areas. The future studies should consider recruiting a larger and more diverse sample, encompassing a broader range of regions. Moreover, the study was limited by using only self-report measures, more objective measurement tools are recommended in future studies. Furthermore, the collection and integration of modifiable factors such as anxiety, mental stress, and lifestyle into the analysis were omitted. Given the study's objective to investigate the mediating role of depressive symptoms between sleep quality and cognitive function, the inclusion of these extra variables could potentially obscure or attenuate the direct association between depressive symptoms and cognitive functions, as they may act as potential mediators or moderators in the relationship between the two variables. Finally, the inclusion of elderly individuals with mild cognitive impairment in this study without clinical diagnosis may have impacted the accuracy of the results, thus a more rigorous diagnostic process should be implemented to further validate the findings presented in this study.
Conclusions
In a word, this study contributed to the controversial topic of the complex relationships among three common mental health related issues in the aging population. Sleep quality was significantly correlated with cognitive functions in patients with mild cognitive impairment, which association was fully mediated by depressive symptoms in a longitudinal pattern. The research revealed the mechanism of depressive symptoms in the relationship between sleep quality and cognitive functions in older adults with mild cognitive impairment. Understanding this relationship may help to the primary prevention and treatment of these problems among older people. The intervention of elderly mild cognitive impairment patients with sleep disorders should not only consider the improvement of sleep quality, but also focus on the management of depression.
Data availability
The datasets generated for this study are available on request to the corresponding authors.
References
Patterson, C., World Alzheimer Report 2018. 2018.
Chan, K. Y. P. et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet (British Edn.) 381(9882), 2016–2023 (2013).
Petersen, R. C. et al. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 275(3), 214–228 (2014).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7(3), 270–279 (2011).
Petersen, R. C. et al. Current concepts in mild cognitive impairment. Arch. Neurol. 58(12), 1985 (2001).
Solfrizzi, V. et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology 63(10), 1882–1891 (2004).
Radler, K. H. et al. Rate of progression from mild cognitive impairment to dementia in an essential tremor cohort: A prospective, longitudinal study. Parkinson. Relat. Disord. 74, 38–42 (2020).
Bubu, O. M. et al. Sleep, cognitive impairment, and Alzheimer’s disease: A systematic review and meta-analysis. Sleep https://doi.org/10.1093/sleep/zsw032 (2017).
Smith, L. et al. Sleep problems and mild cognitive impairment among adults aged ≥50 years from low- and middle-income countries. Exp. Gerontol. 154, 111513–111513 (2021).
Diem, S. J. et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am. J. Geriatr. Psychiatry 24(3), 248–258 (2016).
Hu, M. et al. Sleep disturbance in mild cognitive impairment: A systematic review of objective measures. Neurol. Sci. 38(8), 1363–1371 (2017).
Palmer, K. et al. Sleep disturbance in mild cognitive impairment and association with cognitive functioning. A case-control study. Front. Aging Neurosci. 10, 360 (2018).
Peter-Derex, L. et al. Sleep and Alzheimer’s disease. Sleep Med. Rev. 19, 29–38 (2014).
Petit, D., et al., Alzheimer Disease and Other Dementias. Principles and practice of sleep medicine (6th ed.), ed. M.H. Kryger, T. Roth, and W.C. Dement. Philadelphia: Principles and Practice of Sleep Medicine. 935–943. (2017).
Economou, N. T. et al. From amnestic mild cognitive impairment towards Alzheimer’s disease: A progression based on sleep features. J. Neurol. 257, S167 (2010).
Sindi, S. et al. Sleep disturbances and dementia risk: A multicenter study. Alzheimer’s Dement. 14(10), 1235–1242 (2018).
Kim, S. J. M. D. et al. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am. J. Geriatr. Psychiatry 19(4), 374–381 (2011).
Cassidy-Eagle, E. L. & Siebern, A. Sleep and mild cognitive impairment. Sleep Sci. Pract. 1(1), 1–5 (2017).
You, J. C. et al. Association of β-amyloid burden with sleep dysfunction and cognitive impairment in elderly individuals with cognitive disorders. JAMA Netw. Open 2(10), e1913383–e1913383 (2019).
Luo, Y. et al. Functional brain connectivity in mild cognitive impairment with sleep disorders: A study based on resting-state functional magnetic resonance imaging. Front. Aging Neurosci. 14, 812664 (2022).
Li, Y. et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat. Aging 2(5), 425–437 (2022).
Smith, L. et al. Is loneliness associated with mild cognitive impairment in low-and middle-income countries?. Int. J. Geriat. Psychiatry 36(9), 1345–1353 (2021).
Liew, T. M. Subjective cognitive decline, anxiety symptoms, and the risk of mild cognitive impairment and dementia. Alzheimer’s Res. Ther. 12, 1–9 (2020).
Zhu, T. et al. Social support and depression related to older adults’ hypertension control in rural China. Am. J. Geriatr. Psychiatry 27(11), 1268–1276 (2019).
Wang, J. et al. Mediating effects of depressive symptoms on social support and quality of life among rural older Chinese. Health Qual. Life Outcomes 18(1), 1–242 (2020).
Kahn-Greene, E. T. et al. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 8(3), 215–221 (2006).
Almeida, O. P. & Pfaff, J. J. Sleep complaints among older general practice patients: Association with depression. Br. J. Gen. Pract. 55(520), 864–866 (2005).
Daniela, T. et al. Lack of sleep affects the evaluation of emotional stimuli. Brain Res. Bull. 82(1), 104–108 (2010).
Tranter, R. et al. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci. 27(4), 241–247 (2002).
Fang, H. et al. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 23(4), 2324–2332 (2019).
Jaussent, I. et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep 34(8), 1103–1110 (2011).
Lauer, C. J. et al. In quest of identifying vulnerability markers for psychiatric disorders by all-night polysomnography. Arch. Gen. Psychiatry 52(2), 145–153 (1995).
Liao, F. et al. Longitudinal cohort study of the relationship between sleep duration and depressive symptoms in older people in China. Sichuan da xue xue bao. Yi xue ban J. Sichuan Univ. Med. Sci. Edn. 53(1), 109–113 (2022).
Hyong, J. C. et al. Sleep disturbance and depression recurrence in community-dwelling older adults: A prospective study. Am. J. Psychiatry 165(12), 1543–1550 (2008).
Miller, W. R. & Seligman, M. E. Depression and learned helplessness in man. J. Abnorm. Psychol. 84(3), 228–238 (1975).
Ismail, Z. et al. Prevalence of depression in patients with mild cognitive impairment: A systematic review and meta-analysis. JAMA Psychiatry 74(1), 58–67 (2017).
Johnson, L. A. et al. Cognitive differences among depressed and non-depressed MCI participants: A project FRONTIER study. Int. J. Geriatr. Psychiatry 28(4), 377–382 (2013).
Yatawara, C. et al. Depressive symptoms influence global cognitive impairment indirectly by reducing memory and executive function in patients with mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 87(12), 1375–1383 (2016).
Pusswald, G. et al. The impact of depressive symptoms on health-related quality of life in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease. Int. Psychogeriatr. 28(12), 2045–2054 (2016).
Zhou, Y. The effects of emotional states on executive functioning. Adv. Psychol. Sci. 21(7), 1186 (2013).
Nolenhoeksema, S. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 100(4), 569–582 (1991).
Koster, E. H. W. et al. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin. Psychol. Rev. 31(1), 138–145 (2011).
Davis, R. N. & Nolen-Hoeksema, S. Cognitive inflexibility among ruminators and nonruminators. Cogn. Ther. Res. 24(6), 699–711 (2000).
Hester, R. & Garavan, H. Working memory and executive function: The influence of content and load on the control of attention. Mem. Cogn. 33(2), 221–233 (2005).
Watkins, E. & Brown, R. G. Rumination and executive function in depression: An experimental study. J. Neurol. Neurosurg. Psychiatry 72(3), 400–402 (2002).
Tan, J.-P. et al. Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. J. Alzheimer Dis. 43(4), 1403–1412 (2015).
Jin, H. et al. The utility of Beijing Version Montreal Cognitive Assessment in ischemic cerebrovascular disease patients of Changsha area and the development of Changsha Version Montreal Cognitive Assessment. Chin. J. Nervous Dis. 37(6), 349–353 (2011).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53(4), 695–699 (2005).
Morris, J. C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 9(S1), 173–176 (1997).
Grace, J. B. Structural Equation Modeling and Natural Systems (Cambridge University Press, 2006).
Spitzer, R. L. et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 272(22), 1749 (1994).
Li, Z. et al. Use of patient health questionnaire-9(PHQ-9) among Chinese rural elderly. Chin. J. Clin. Psychol. 19(2), 171–174 (2011).
Haddad, M. et al. Detecting depression in patients with coronary heart disease: A diagnostic evaluation of the PHQ-9 and HADS-D in primary care, findings from the UPBEAT-UK study. Plos One 8(10), e78493 (2013).
Buysse, D. J. et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28(2), 193–213 (1989).
Bentler, P. M. & Bonett, D. G. Significance tests and goodness of fit in the analysis of covariance structures. Psychol. Bull. 88(3), 588–606 (1980).
Chen, F. et al. An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Soc. Methods Res. 36(4), 462–494 (2008).
Chen, F. F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct. Equ. Model. 14(3), 464–504 (2007).
Dahl, R. E. & Lewin, D. S. Pathways to adolescent health sleep regulation and behavior. J. Adolescent Health 31(6), 175–184 (2002).
Walker, M. P. et al. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 10(3), 385–392 (2007).
Butters, M. A. et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 10(3), 345–357 (2008).
Hermida, A. P. et al. The association between late-life depression, mild cognitive impairment and dementia: Is inflammation the missing link?. Expert Rev. Neurother. 12(11), 1339–1350 (2012).
Rapp, M. A. et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch. Gen. Psychiatry 63(2), 161–167 (2006).
Alexopoulos, G. S. Vascular disease, depression, and dementia. J. Am. Geriatr. Soc. (JAGS) 51(8), 1178–1180 (2003).
Lara, E. et al. The impact of depression on the development of mild cognitive impairment over 3 years of follow-up: A population-based study. Dement. Geriatr. Cogn. Disord. 43(3–4), 155–169 (2017).
Acknowledgements
We thank all the participants in the study, and we are also grateful to research assistants who participated in the study for their assistance in research coordination and data collection.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (2023A1515110169), and the Humanities and Social Sciences Youth Foundation, Ministry of Education of the People's Republic of China (22YJCZH209) and the Research Start-Up Funds of Guangdong Medical University (Grant Number: 4SG24300G).
Author information
Authors and Affiliations
Contributions
Jiayu Wang Investigation, Data curation, Writing-Original draft preparation; Shulin Chen Conceptualization, Methodology, Supervision; Jiang Xue Conceptualization, Methodology, Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Chen, S. & Xue, J. Depressive symptoms mediate the longitudinal relationships between sleep quality and cognitive functions among older adults with mild cognitive impairment: A cross-lagged modeling analysis. Sci Rep 14, 21242 (2024). https://doi.org/10.1038/s41598-024-72159-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72159-8
- Springer Nature Limited