Abstract
Inflammation is relevant in the pathogenesis and progression of heart failure (HF). Previous studies have shown that elevated high-sensitivity C-reactive protein (hsCRP) are associated with greater severity and may be associated with adverse outcomes. In this study, we sought to evaluate the prognostic role of hsCRP in a non-selected cohort of patients with acute HF. We prospectively included a multicenter cohort of 3,395 patients following an admission for acute HF. HsCRP levels were evaluated during the first 24 h following admission. Study endpoints were the risks of all-cause mortality, CV-mortality, and total HF readmissions. The mean age was 74.2 ± 11.2 years and 1,826 (53.8%) showed a left ventricular ejection fraction (LVEF) ≥ 50%. Median hsCRP was 12.9 mg/L (5.4–30 mg/L). Over a median follow-up of 1.8 (0.6–4.1) years, 1,574 (46.4%) patients died, and 1,341 (39.5%) patients were readmitted for worsening HF. After multivariable adjustment, hsCRP values were significantly and positively associated with a higher risk of all-cause and CV mortality (p = 0.003 and p = 0.001, respectively), as well as a higher risk of recurrent HF admissions (p < 0.001). These results remained consistent across important subgroups, such as LVEF, sex, age, or renal function. In patients with acute HF, hsCRP levels were independently associated with an increased risk of long-term death and total HF readmissions.
Similar content being viewed by others
Introduction
Inflammation is a relevant mechanism in the pathophysiology of heart failure (HF)1,2. Patients with HF exhibit higher circulating levels of pro-inflammatory cytokines compared with healthy individuals, irrespective of left ventricular ejection fraction (LVEF) status1,2,3,4. Systemic inflammation has been postulated to promote HF, but also may contribute to disease progression, leading to an adverse cardiac remodeling, endothelial dysfunction, oxidative stress and systemic congestion1,2,5,6,7. The activation of systemic inflammation is more pronounced in acute HF, where inflammatory biomarkers are particularly raised8.
C-reactive protein (CRP) is an acute-phase protein widely used in daily clinical practice as an inflammatory biomarker. It is synthesized in the liver in response to the pro-inflammatory interleukin (IL) cascade, thus serving as a proxy marker for the IL-1/IL-6 pathway activity9,10. Several studies have shown that IL-1/IL-6 signaling pathways exert deleterious cardiac and systemic effects, and are linked to disease progression in HF1,2,11. The blockade of the IL-1/IL6 axis is under evaluation as a potential therapeutical strategy in randomized clinical trials in HF, where elevated high-sensitivity CRP (hsCRP) levels serve as a key inclusion criterion12,13.
Inflammatory mediators, particularly hsCRP, are associated with a worse prognosis in HF5,14,15. Most of these studies have found a positive association between hsCRP and a higher risk of death, however, some studies have yielded contradictory results, and only very few studies have focused on the risk of readmissions14,15,16,17. In the present study, we aimed to assess the association between hsCRP levels and morbimortality burden. Specifically, we separately evaluated long-term mortality, and total HF readmissions following an admission for acute HF.
Methods
Study group and protocol
This is a retrospective analysis from an ongoing multicenter prospective registry of 5222 consecutive patients admitted from January 2012 to December 2021 for acute HF in three hospitals in Spain. We excluded patients without assessment of hsCRP during admission (n = 1179). From the leaving sample (n = 4043), patients with in-hospital death (n = 196) and patients with a clinical diagnosis of infection on admission (n = 452) were excluded. The final study sample included 3395 patients discharged for an acute HF admission (Fig. 1).
A comprehensive dataset of demographics, medical history, standard laboratory, echocardiographic parameters, and treatments at discharge was routinely recorded using pre-established registry questionnaires during the index hospitalization. Either patients with new-onset or worsening HF were enrolled in the registry. Acute HF was defined according to the running European Society of Cardiology Clinical Practice Guidelines though the study timeline18,19. Treatment strategies were individualized following established guidelines operating when patients were included in the registry18,19. The study was conformed to the principles outlined in the 1975 Declaration of Helsinki and was approved by the institutional, local review ethical committees. All patients gave written informed consent.
C reactive protein measurement
Plasma levels of hsCRP were routinely obtained within the first 24 h after admission, along with other biochemical variables such as plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP), blood cells count, blood urea nitrogen, creatinine, sodium, and potassium.
Follow-up and endpoints
The endpoints of interest were post-discharge mortality (total and cardiovascular (CV)), and total HF readmissions. CV death was considered secondary to worsening HF, acute myocardial infarction, stroke or transient ischemic attack, cardiac arrhythmias, peripheral artery disease or sudden cardiac deaths. For the readmission endpoint, only unplanned readmissions were registered. The assessment of endpoints was performed by verifying the patient's survival status or occurrence of readmission by reviewing electronic medical records of the public health care system in the Valencian Community. This assessment utilized data from the SIA-GAIA and Orion Clinics electronic databases, which comprehensively record all medical interactions occurring in the public healthcare system. Endpoint adjudication was performed by paired investigators who were blinded to hsCPR values.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (P25 to P75), as appropriate. Discrete variables were expressed as percentages. We assessed hsCRP levels both as quartiles and as a continuous variable. The association between hsCRP levels and all-cause and CV mortality was evaluated using a Cox regression analysis, and results were reported as hazard ratios (HR) with 95% confidence intervals (CI). Cox regression estimates for CV death were adjusted for non-CV death as a competing event. For the readmission endpoint, negative binomial regression models were employed to simultaneously analyze the number of HF readmissions (as counts) and all-cause mortality (as a terminal event). Regression estimates for both outcomes were mutually adjusted by means of shared frailty (accounting for the positive correlation between the two outcomes). Risk estimates were expressed as incidence rate ratios (IRR).
The variables included in the multivariable models (for mortality and HF endpoints) were: age, sex, first HF admission, ischemic etiology, last known under stable conditions New York Heart Association (NYHA) class, atrial fibrillation, left bundle branch block, systolic blood pressure at admission, heart rate at admission, Charlson comorbidity index, NT-proBNP, blood urea nitrogen, estimated glomerular filtration rate, antigen carbohydrate 125 and tricuspid annular plane systolic excursion by echocardiography. The model for CV mortality included the prior set of covariables plus non-CV death as a competing event. For both multivariate models, candidate covariates were chosen based on previous medical knowledge; then, a backward stepwise selection was performed. During this selection process, the linearity assumption for all continuous variables was simultaneously tested, and the variable transformed, if appropriate, with fractional polynomials. All variables listed in Table 1 were evaluated as potential covariates in the multivariable models, independently of their p-value.
We conducted subgroups analyses on the risk of recurrent HF admissions in pre-defined subgroups by age (≥ 75 years vs. < 75 years), sex (men vs women), left ventricular ejection fraction (LVEF) status (LVEF < 50% vs. LVEF ≥ 50%), or renal dysfunction (glomerular filtration rate < 60 ml/min vs ≥ 60 ml/min).
A 2-sided P-value of < 0.05 was considered statistically significant for all analyses. All survival analyses were performed using STATA 17.1 (StataCorp. 2018. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP).
Results
The mean age of the cohort was 74.2 ± 11.2 years; 1528 (45%) were women, and 1.075 (31.7%) were previously admitted for HF. The number of patients with LVEF ≥ 50% was 1826 (53.8%). The median (p25 to p75) of hsCRP and NT-proBNP were 12.9 mg/L (5.4–30 mg/L) and 3573 pg/ml (1911–7121), respectively. Baseline characteristics across quartiles of hsCRP in the study cohort are detailed in Table 1. Patients in the upper quartiles of hsCRP were more frequently men, more symptomatic, had suffered more previous HF admissions, a displayed a greater comorbidity burden. When moving from lower to upper quartiles patients had a worse renal function, higher leucocytes and plasma NT-proBNP values, and higher LVEF, but lower haemoglobin values. Likewise, patients in the upper quartiles received higher doses of diuretics and were less likely to receive renin–angiotensin–aldosterone system inhibitors (Table 1).
hsCRP levels and long-term mortality
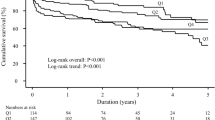
At a median (p25 to p75) follow-up of 1.8 (0.6–4.1) years, we recorded 1,574 (46.4%) deaths (15.6 per 100 person-years). Of them, 708 (45% of deaths) were CV related (10.3 per 100 person-yeas). Kaplan–Meier plots showed a higher risk of all-cause mortality with increasing hsCRP levels (log-rank test; p = 0.002), with a higher risk of death when moving from the lower to the upper quartiles. These differences were clearly patent during the first four years (Fig. 2). Afterwards, the main differences were found between the lowest quartile and others (Fig. 2).
For CV death, we also found a higher risk of the endpoint with increasing hsCRP levels (Gray´s test, p < 0.001), with a stepwise increase in the risk from the lower to the upper quartiles for this endpoint (Fig. 3).
In multivariable survival analysis, the continuum of hsCRP was positively and significantly associated with the risk of all-cause and CV mortality (p = 0.003 and p = 0.001; respectively) displaying a sigmoid-shaped curve (Fig. 4). Below 20 mg/L, each increase in hsCRP was positively and sharply associated with an increased risk. Above 20–25 mg/L, further increases in hsCRP did not translate into a meaningful increase of risk (Fig. 4A). A similar but even more marked pattern of risk was found for CV mortality (Fig. 4B).
hsCRP and recurrent HF hospitalizations
Along the follow up we registered 2721 HF-rehospitalizations in 1341 patients (39.5%). The number of patients with 1, 2, 3, 4, and 5 or more hospitalizations was 699, 306, 154, 82, and 60, respectively.
The rates (per 100 person-years) of HF hospitalizations increased when moving from the lowest to the upper quartiles of hsCRP [36.4, 40.0, 43.8, and 48.0, respectively (p-value = 0.006)]. Multivariate risk estimates, also accounting for the risk of death, showed that higher hsCRP remained independently related to an increased risk of total HF rehospitalizations (Fig. 5). The gradient of HF hospitalizations risk attributable to hsCRP was also non-linear, with a sharp risk increase until 20 mg/L, followed by a risk plateau (Fig. 4). Compared to patients in the lowest quartile (< 5.4 mg/L), patients in the second and third quartiles showed a significantly higher risk of recurrent admissions (IRR = 1.22, CI 95%:1.04 to 1.44) and IRR = 1.18, CI 95%: 1.01 to 1.39, respectively), but not those in the upper quartile (IRR = 1.14, CI 95%: 0.97 to 1.34).
We conducted sensitivity analyses across important pre-defined subgroups, based on age, gender, LVEF status, or renal function. Overall, there was no evidence of risk disparities among the different subgroups (p for interaction = 0.677, 0.492, 0.336, and 0.785; respectively), as it is shown in supplementary Fig. 1.
Discussion
In this study, we assessed the prognostic value of hsCRP in the whole spectrum of patients following an episode of acute HF in clinical daily practice. Our analysis revealed that hsCRP was independently associated with a higher risk of mortality and total HF hospitalizations during follow-up.
These results underscore the prognostic value of this biomarker in HF, notably concerning HF readmission risk, an aspect that has shown inconsistent findings in prior studies, and highlight the importance of systemic inflammation in the pathogenesis and progression of HF.
Inflammation in HF
Recent evidence accentuates the role of inflammatory biomarkers in HF, contributing to its pathophysiology across the entire spectrum of the disease1. Prior studies have established an independent link between CRP levels and an increased risk of developing HF5. Chronic inflammation leads to endothelial dysfunction, renin-angiotensin and sympathetic systems activation, reduced myocardial contractility, and interstitial fibrosis, among other detrimental effects, promoting HF1,2. In established HF, inflammation, through a series of cytokines and other humoral and cellular mediators, also contributes to the disease progression, directly affecting systolic and diastolic functions, promoting adhesion molecule expression, interstitial collagen deposition, sodium retention, and oxidative stress, thereby contributing to HF progression and multi-organ dysfunction1,2. While various mediators and pro-inflammatory cytokines have been identified as elevated in HF, much of this evidence is derived from studies on CRP5,8,14,15. CRP is produced in the liver as a response to inflammation under IL-1/IL-6 pathway activation and is widely available in clinical practice9,10. Patients with HF often exhibit raised hsCRP levels, indicative of systemic inflammation, particularly during acute or worsening HF episodes, where levels are particularly increased1. Our study's median hsCRP level was 14.9 mg/L, aligning with previous studies like the ASCEND-HF trial in which the median value was 12.6 mg/L8. Following an episode of worsening HF, hsCRP levels generally decrease but remain higher than the general population, indicative of HF as a chronic systemic inflammation state1,2,14.
There is growing data from randomized clinical trials and observational studies showing an association between elevated hsCRP levels and the risk of adverse outcomes in HF1,2,8,14,15,20. In chronic HF, a post-hoc analysis from Val-HEFT trial showed that elevated CRP levels were independently associated with adverse events in patients with HF with reduced ejection fraction (HFrEF)21. Likewise, in patients with HFpEF, hsCRP was also found to be related to a higher all-cause or CV mortality risk22. Similarly, in an observational study on 4423 patients with chronic HF, elevated CRP levels were also a powerful independent predictor of mortality14. In acute HF, although few studies have been published, higher CRP is also linked to a higher risk of death. In the ATTEND registry, values over 10 mg/L were associated with a poorer survival23. Also, the dynamic evolution of hsCRP seems to be important. Patients who do no decrease or even rise hsCRP levels following discharge experience the highest mortality risk8,15. However, some studies have shown contradictory results. For instance, analysis from the CORONA or the RED-HF trials have found that CRP was not an independent predictor of mortality, or did not improve risk stratification models beyond natriuretic peptides15,16,17. Additionally, the association between CRP and morbidity burden has been less extensively examined, and some studies have failed to show an association between this biomarker and the risk of readmission8,9,10,11,12,13,14,15,16,17,18.
Our study expands and confirms previous data on the prognostic role of hsCRP in a large and representative cohort of patients with acute HF, showing that hsCRP was strongly associated with mortality but also with total HF admissions using recurrent events methodology. Interestingly, we found a positive association but not a progressive increase of risk at very high hsCRP values. Despite the exclusion of patients with clinical evidence of infection, we postulate that extreme values of hsCRP may identify those with active subclinical of subtle infections as a stressors of acute HF. Thus, these acute very high values may not accurately reflect HF-related inflammation and may not provide additional risk stratification value.
Different mechanisms may be behind this association between inflammation and a higher risk of readmissions in HF. A positive correlation between volume overload and markers of systemic inflammation, such as CRP or IL-6, have been described in previous studies24,25,26. In experimental models, venous congestion is a trigger for the activation of a maladaptive inflammatory cascade27,28. Inflammation exerts deleterious cardiac and systemic effects, leading to endothelial dysfunction, an increase in vascular permeability, triggering fluid extravasation. As a result, congestion and inflammation may reciprocally worsen each other, leading to episodes of worsening HF29,30.
Given the role of inflammation in promoting endothelial dysfunction, microvascular disease and increased arterial stiffness, hsCRP may be a more important feature in HFpEF than in HFrEF. In our data, patients with hsCRP in the upper quartiles showed a higher median LVEF, and we recently described that hsCRP and LVEF are positively correlated in HF, with higher levels in patients in the upper extreme of LVEF31. In contrast, CRP is an independent risk factor for both incident HFrEF or HFpEF5, and in our study this biomarker was associated with outcomes regardless of LVEF status, as it has also been observed in other studies15. So, despite the pathophysiological differences between HFrEF and HFpEF, CRP is a useful biomarker for risk stratification in both phenotypes, reflecting the importance of inflammation across the entire spectrum of LVEF in HF.
Recently, novel inmunoassays analyses can measure upstream pro-inflammatory cytokines, such as IL-6, that it is the primary stimulus to produce CRP in the liver. Consequently, this biomarker is positively correlated to CRP concentrations in patients with HF20,32. Recent studies have postulated IL-6 as prognostic biomarker in HF20,32,33. Interestingly, the association of IL-6 to the risk of HF readmissions is also not consistent in prior studies, as it has been previously described with CRP20,32. Although IL-6 may become a promising biomarker in the future, it is seldom available in clinical daily practice. Alternatively, hsCRP (more available, longer half-life, and widely available) emerges as a reliable proxy of IL-6 inflammation pathway.
Clinical implications
First, we concur that a high hsCRP during hospital admission may help identify patients with a higher morbidity risk in the long-term follow-up. Identifying such patients may be important in transitional care and surveillance after hospital discharge, aiming to reduce the high morbidity burden in HF34,35.
Second, hsCRP may help in identifying patients who may benefit from specific anti-inflammatory therapies. In the CANTOS trial, targeted anti-cytokine therapy with a monoclonal antibody against IL-1β resulted in improved HF outcomes in patients with myocardial infarction with or without established HF36. The REDHART 2 trial assesses whether the IL-1 blockade with anakinra can improve cardiorespiratory fitness in patients with a recent decompensation of HF13. The ongoing HERMES trial (NCT05636176) is randomizing > 5000 patients with HF and LVEF > 40% to the IL-6 antagonist ziltivekimab vs. placebo, and will help to elucidate if the blockade of the innate immune system may reduce outcomes in HF. Of note, in all these trials, an elevated hsCRP (> 2 mg/L) is one the key inclusion criteria. Thus, hsCRP may aid in risk stratification in HF and identify patients with an inflammatory phenotype who may be candidates to specific anti-inflammatory therapies.
Limitations
First, this is an observational retrospective study, and therefore, residual confounders may be playing a role. Second, we did not assess other upstream inflammatory cytokines (such as IL-1β, TNF-alfa, or IL-6); thus, we could not perform a prognostic comparison among inflammatory markers Third, we did not evaluate the kinetics of hsCRP, precluding to test the utility of hsCRP for monitoring the course of the disease and its prognostic implications. Fourth, the study lasted for almost a decade and medical treatment of HF has been improving over the years. This may introduce a bias in our results. However, the impact of contemporary HF therapies, such as SGLT2 inhibitors, on inflammation is still inconsistent37. Finally, pathophysiological mechanisms underlying our findings are out of the scope of our study.
Conclusions
In patients with acute HF, hsCRP levels were independently associated with an increased risk of post-discharge death and total HF readmissions. Future studies are warranted to confirm these results and to evaluate the clinical implications of these findings.
Data availability
Data is available through a request directed to the corresponding author upon reasonable request.
References
Murphy, S. P., Kakkar, R., McMarthy, C. P. & Januzzi, J. L. Inflammation in heart failure. J. Am. Coll. Cardiol. 75, 1324–1340 (2020).
Adamo, L., Rocha-Resende, C., Prabhu, S. D. & Mann, D. L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 17, 269–286 (2020).
Redfield, M. M. et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 309, 1267–1277 (2013).
Pfisterer, M. et al. BNP-guided vs symptom-guided heart failure therapy: The trial of intensified vs. standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA 301, 383–392 (2009).
Burger, P. M. et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J. Am. Coll. Cardiol. 82, 414–426 (2023).
Dick, S. A. & Epelman, S. Chronic heart failure and inflammation: What do we really know?. Circ. Res. 119, 159–171 (2016).
Van Tassell, B. W. et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. Plos One 7, e33438 (2012).
Kalogeropoulos, A. P. et al. High-sensitivity C-reactive protein in acute heart failure: Insights from the ASCEND-HF trial. J. Cardiac Fail. 20, 319–326 (2014).
Ridker, P. M. C-reactive protein: Eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin. Chem. 55, 209–215 (2009).
Ridker, P. M. From CRP to IL-6 and IL-1: Moving upstream to identify novel targets for atheroprotection. Circ. Res. 118, 145–156 (2016).
Golino, M., Moroni, F. & Abbate, A. Connecting the dots: Inflammatory burden and outcomes in heart failure. J. Am. Heart Assoc. 12(19), e031786 (2023).
van Tasell, B. W. et al. Interleukin-1 blockade in recently decompensated systolic heart failure: Results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 10, e004373 (2017).
Van Tasell, B. et al. Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): A randomized, double blind, placebo controlled, single center, phase 2 study. J. Transl. Med. 20, 270 (2022).
Pellicori, P. et al. High-sensitivity C-reactive protein in chronic heart failure: Patient characteristics, phenotypes, and mode of death. Cardiovasc. Res. 116, 91–100 (2020).
Zhang, L. et al. Long-term cumulative high-sensitivity C-reactive protein and mortality among patients with acute heart failure. J. Am. Heart Assoc. 12, e029386 (2023).
Wedel, H. et al. Predictors of fatal and non-fatal outcomes in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): Incremental value of apolipoprotein A-1, high-sensitivity C-reactive peptide and N-terminal pro B-type natriuretic peptide. Eur. J. Heart Fail. 11, 281–291 (2009).
Welsh, P. et al. Prognostic importance of emerging cardiac, inflammatory, and renal biomarkers in chronic heart failure patients with reduced ejection fraction and anaemia: RED-HF study. Eur. J. Heart Fail. 20, 268–277 (2018).
McMurray, J.J.V. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2020 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33, 1787–1847 (2012).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2020 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200 (2016).
Mooney, L. et al. Adverse outcomes associated with interleukin-6 in patients recently hospitalized for heart failure with preserved ejection fraction. Circ. Heart Fail. 16e010051 (2023).
Anand, I. S. et al. C-Reactive protein in heart failure. Circulation. 112, 1428–1514 (2005).
Lakhani, I. et al. Diagnostic and prognostic value of C-reactive protein in heart failure with preserved ejection fraction. Heart Fail Rev. 26, 1151–1150 (2021).
Minami, Y. et al. C-reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur. Heart J. Qual. Care Clin. Outcomes 3, 148–156 (2017).
Nuñez, J. et al. Clinical role of CA125 in worsening heart failure. A BIOSTAT-CHF study subanalysis. J. Am. Coll. Cardiol. HF 8, 386–397 (2020).
Núñez, J. et al. Antigen carbohydrate 125 as a biomarker in heart failure. A narrative review. Eur. J. Heart Fail. 23, 1445–1457 (2021).
Pandhi, P. et al. Pathophysiologic processes and novel biomarkers associated with congestion in heart failure. J. Am. Coll. Cardiol. HF 10, 623–632 (2022).
Colombo, P. C. et al. Peripheral venous congestion causes inflammation, neuhormonal, and endothelial cell activation. Eur. Heart J. 35, 448–454 (2014).
Colombo, P. C. et al. Experimentally induced peripheral venous congestion exacerbates inflammation, oxidative stress, and neuhormonal and endothelial cell activation in patients with systolic heart failure. J. Card Fail. 23, S1071-9164 (2023).
van Linthout, S. & Tshope, C. Inflammation: Cause or consequence of heart failure or both?. Curr. Heart Fail. Rep. 14, 251–265 (2017).
Kittipibul, V., Fudim, M. & Sobotka, P. A. Congestion and inflammation in heart failure: Beyond the chicken or the egg. J. Card Fail. 30, 592–595 (2023).
Villar, S. et al. C-reactive protein in patients with acute heart failure and preserved ejection fraction. Rev. Esp. Cardiol. 77, 430–433 (2024).
Michou, E. et al. Quantifying inflammation using interleukin-6 for improved phenotyping and risk stratification in acute heart failure. Eur. J. Heart Fail. 25, 174–184 (2023).
Markousis-Mavrogenis, G. et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 21, 965–973 (2019).
Dunlay, S. M. et al. Hospitalizations after heart failure diagnosis: A community perspective. J. Am. Coll. Cardiol. 54, 1695–1702 (2009).
Santas, E. et al. Burden of recurrent hospitalizations following an admission for acute heart failure: Preserved versus reduced ejection fraction. Rev. Esp. Cardiol. (Engl. Ed.) 70, 239–246 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 139, 1289–1299 (2019).
Buttice, L. et al The effect of sodium-glucose cotransporter-2 inhibitors on inflammatory biomarkers. A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 26, 2706–2721 (2024)
Funding
CIBER Cardiovascular (grant numbers 16/11/00420 and 16/11/00403).
Author information
Authors and Affiliations
Contributions
E.S, S.V., and J.N designed the overall study, E.S. S.V. collected data, completed the data analysis and manuscript preparation. P.P., R.D.E., P.L., G.M., M.L., and G.N. collected the data and supervised data collection; A.C., and E.R.collected and supervised laboratory analysis, J.J.G., A.B.G, and J.S. provided scientific advice and revised the study and manuscript. J.N. designed the study, conduct the statistical analysis and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
J. Núñez received board speaker fees and travel expenses from Novartis, Roche Diagnostics, Abbott, Rovi, Vifor Pharma, Daiichi Sankyo, Boehringer Ingelheim, and Astra Zeneca (modest). J. Sanchis reveived speaker fees from Astra Zeneca, Abbott, and Edwards Lifesciences (modest). A. Bayés-Genís received board membership fees and travel expenses from Novartis, Roche Diagnostics, Vifor Pharma, and Critical Diagnostics (modest). JL Górriz, received consultancy fees from Astellas, GSK, CSL VIFOR, and speaker fees from AstraZeneca, Boehringer Ingelheim, Esteve, Bayer, Lilly, Astellas, and Novonordisk (modest). The remaining authors have no disclosures to report.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Santas, E., Villar, S., Palau, P. et al. High-sensitivity C-reactive protein and risk of clinical outcomes in patients with acute heart failure. Sci Rep 14, 21672 (2024). https://doi.org/10.1038/s41598-024-72137-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72137-0
- Springer Nature Limited