Abstract
Skeletal muscle is a highly heterogeneous tissue, and its contractile proteins are composed of different isoforms, forming various types of muscle fiber, each of which has its own metabolic characteristics. It has been demonstrated that endurance exercise induces the transition of muscle fibers from fast-twitch to slow-twitch muscle fiber type. Herein, we discover a novel epigenetic mechanism for muscle contractile property tightly coupled to its metabolic capacity during muscle fiber type transition with exercise training. Our results show that an 8-week endurance exercise induces histone methylation remodeling of PGC-1α and myosin heavy chain (MHC) isoforms in the rat gastrocnemius muscle, accompanied by increased mitochondrial biogenesis and an elevated ratio of slow-twitch to fast-twitch fibers. Furthermore, to verify the roles of reactive oxygen species (ROS) and AMPK in exercise-regulated epigenetic modifications and muscle fiber type transitions, mouse C2C12 myotubes were used. It was shown that rotenone activates ROS/AMPK pathway and histone methylation enzymes, which then promote mitochondrial biogenesis and MHC slow isoform expression. Mitoquinone (MitoQ) partially blocking rotenone-treated model confirms the role of ROS in coupling mitochondrial biogenesis with muscle fiber type. In conclusion, endurance exercise couples mitochondrial biogenesis with MHC slow isoform by remodeling histone methylation, which in turn promotes the transition of fast-twitch to slow-twitch muscle fibers. The ROS/AMPK pathway may be involved in the regulation of histone methylation enzymes by endurance exercise.
Similar content being viewed by others
Introduction
Skeletal muscle is a heterogeneous organ composed of different types of muscle fibers with different structural and functional characteristics. According to the speed of contraction, skeletal muscle fibers are divided into fast-twitch fibers and slow-twitch fibers. According to the differences in myosin heavy chain isoforms, slow-twitch muscle fibers are called type I muscle fibers, which express myosin heavy chain (MHC) seven protein, and fast-twitch muscle fibers are subdivided into type IIa muscle fibers expressing MYH2 protein, type IId/x muscle fibers expressing MYH1 protein, and type IId/x muscle fibers expressing MYH4 protein type IIb muscle fibers1. Notably, rodents have type IIb fibers, whereas humans do not. Furthermore, compared to fast-twitch fibers, slow-twitch fibers exhibit higher mitochondrial content and faster mitochondrial turnover rates2. Additionally, there are differences in mitochondrial biogenesis3, mitochondrial dynamics4, and mitophagy5 among different muscle fiber types. Skeletal muscle fibers are highly plastic, which is characterized mainly by plasticity in gene expression patterns such as those of MHC isoforms and plasticity in skeletal muscle metabolic types6. Previous studies have shown that various pathological and physiological challenges can induce structural and functional changes in skeletal muscle. For example, aging leads to a reduction in the proportion of type II muscle fibers, resulting in decreased power and strength in elderly individuals7,8. Conditions such as diabetes9, heart failure10, weightlessness11 and disuse12,13 accompanied by impaired mitochondrial respiratory function, ultimately resulting in reduced muscle mass and strength1014. Endurance exercise promotes the transition of fast-twitch fibers to slow-twitch fibers15. Therefore, the mechanisms regulating muscle fiber type transition may serve as potential targets for disease intervention or health improvement.
Significant progress has been made in understanding the mechanisms of muscle fiber type transition, and several molecular pathways have been proposed. Research has shown that calcium ions (Ca2+)16, AMP-activated protein kinase (AMPK)17, and mitogen-activated protein kinases (MAPKs)18 can induce muscle fiber type switching. Sustained elevation of Ca2+ concentration leads to the activation of the calmodulin-calcineurin complex, resulting in the dephosphorylation and translocation of nuclear factor of activated T-cells (NFAT)c into the nucleus. This process activates myocyte enhancer factor 2 (MEF2), promoting the expression of slow-twitch fiber-associated genes19,20. Additionally, Ca2+ can phosphorylate histone deacetylase (HDAC) 4 via CaMK, causing its translocation out of the nucleus, which alleviates HDAC-mediated repression of MEF2 genes and enhances MEF2 expression, further promoting slow-twitch fiber gene expression21. AMPK facilitates the transition of fast-twitch fibers to slow-twitch fibers by upregulating PGC-1α expression [Arginine promotes skeletal muscle fiber type transition from fast-twitch to slow-twitch via Sirt1/AMPK pathway]. Similarly, P38 MAPK can increase the promoter activity of PGC-1α, promoting the expression of PGC-1α protein22, thereby upregulating the expression of slow MHC isoform proteins. Thus, muscle fiber type transition is a complex and critical physiological process involving multiple signaling pathways and molecular mechanisms.
Currently, muscle fiber type transitions are considered to be based on changes in MHC isoforms and their corresponding metabolic profiles23. MHC is an important component of the skeletal muscle contraction mechanism24. Skeletal muscle contraction is achieved by adenosine 5'-triphosphate (ATP) hydrolysis-driven cyclic interactions of myosin cross-bridges with myosin, and the sequence of amino acids in the heavy chain myosin cross-bridges influences the activity of ATPase, which may be an influencing factor in the differences in muscle fiber dynamics25. Thus, MHC protein isoforms may determine the contractile characteristics of skeletal muscle fibers. Type I muscle fibers contract slowly and have strong fatigue resistance, relying primarily on the slow and sustained energy supply via mitochondrial oxidative phosphorylation (OXPHOS), while type II muscle fibers contract quickly and have poor fatigue resistance, primarily depending on glycolysis for rapid and short-term energy supply2,26,27. Furthermore, numerous studies have observed that changes in myosin heavy chain (MHC) during muscle fiber type transition are accompanied by alterations in mitochondrial content and metabolic type17,18,28. Chemello et al.23. demonstrated that miRNAs regulate muscle fiber type transitions by modulating the metabolic profiles of muscle fibers. Further research found that miR-499 can couple MHC isoform expression with mitochondrial OXPHOS function, indicating that changes in muscle fiber type and metabolic mode are coordinated29.
Epigenetic mechanisms are the molecular mechanisms that regulate gene expression without changing the DNA sequence. Studies have demonstrated that histone methylation plays an important role in the regulation of muscle fiber types. For example, Pandorf et al.30 found that H3K4me3 modification is associated with muscle fiber type transition, that slow-twitch to fast-twitch muscle fiber transition occurs after skeletal muscle unloading, and that this transition is accompanied by increased H3K4me3 enrichment in type IIx and IIb MHC genes. Krämer et al.31 reviewed the regulation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a key mitochondrial regulatory gene, by histone methylation. We have recently reviewed how histone methylation can regulate mitochondrial homeostasis32. We also explored the potential mechanisms by which exercise-mediated epigenetic modifications couple mitochondrial function with skeletal muscle fiber type33. Exercise can regulate various histone methylation states34. For example, exercise increases the levels of H3K4me3 in the PGC-1α promoter in skeletal muscle, which upregulates PGC-1α protein expression and increases mitochondrial biogenesis35,36. Recent studies have also demonstrated that exercise-induced changes in H3K27me3 modification are associated with the transition of type IIb to type IIa myofibers37. The above implies that histone methylation may be a key mechanism in exercise-induced muscle fiber type switching.
The histone methylation in a gene promoter is regulated by many methylases and demethylases. Studies have shown that adenosine 5′-monophosphate-activated protein kinase (AMPK) is involved in the regulation of various methylases and demethylases38.AMPK can phosphorylate enhancer of zeste homolog (EZH) 2 at T311, disrupting the interaction between EZH2 and SUZ12, resulting in a decrease in the enrichment rate of H3K27me339. Other studies have shown that COMPASS, a key complex that regulates H3K4me3 modification, is also regulated by AMPK and that activation of AMPK targets components of the COMPASS complex, effectively blocking histone methyltransferases and ultimately inhibiting transcription elongation40. AMPK also activates the demethyltransferases lysine demethylase (KDM) 5 and lysine-specific histone demethylase-1 (LSD1), thus reducing H3K4me3 levels41. AMPK may also inhibit fumarase through phosphorylation, increase the level of fumarate, and then inhibit KDM2A42. In addition, AMPK is a key factor in many events regulated by exercise, including mitochondrial homeostasis remodeling43 and skeletal muscle fiber type transition44. Additionally, endurance exercise promotes health by activating AMPK through ROS45. In summary, the aim of this study was to investigate whether endurance exercise can promote the transition of fast-twitch to slow-twitch fibers by regulating the corresponding histone methylation and synergistically regulating MHC isoforms and mitochondrial function. We also explored the role of ROS/AMPK and its downstream histone methylation-related enzymes.
Materials and methods
Animals and treatment
Sixteen 8-week-old male Wistar rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All rats were fed a standard laboratory chow diet (12.8% fat, 21.6% protein, 65.6% carbohydrate; HFK Bioscience, Beijing, China) and supplied with water ad libitum at a temperature of 22 ± 2 °C with a 12 h light and dark cycle and a relative humidity of 50 ± 20%. All rats underwent adaptive feeding for one week before use in the experiment. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All experimental procedures were approved by the Animal Ethics Commission of Tianjin University of Sport (Tianjin, China).
All rats were randomly divided into two groups: a control (C) group and an endurance exercise (E) group. After one week of adaptive rearing, the E group was subjected to moderate-intensity aerobic endurance training. Moreillon et al.46 have shown that moderate-intensity endurance exercise training induces the transition of fast-twitch to slow-twitch fibers. The exercise training protocol for this study was modified from a previously published moderate-intensity endurance training program47. Adaptive treadmill training was performed in the first week. On the first day, training was performed at a speed of 12 m/min for 20 min. Then, the speed was increased by 2 m/min and the exercise time was increased by 10 min every day until the fifth day, at which time the speed was 20 m/min and the duration was 60 min. Training was then performed at this exercise intensity for 8 weeks, 5 days per week. Twenty-four hours after the last training session, the mice were anesthetized with tribromoethanol, and the gastrocnemius muscle was rapidly collected. A portion of fresh tissue was used to isolate mitochondria to measure the reactive oxygen species (ROS) production rate, mitochondrial membrane potential (MMP) and mitochondrial respiratory function. A part of the tissue was snap-frozen in liquid nitrogen and stored at − 80 °C for RNA, protein and histone methylation analyses. Another part of the tissue was embedded within optimum cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan, # 4583) for immunofluorescence staining.
Cell culture and treatment
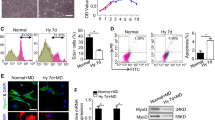
To verify the roles of ROS and AMPK in exercise-regulated epigenetic modifications and muscle fiber type transitions, mouse C2C12 myotubes were used. C2C12 myoblasts (Pricella, China, #CL-0044) were incubated at 37 °C in a humidified 5% CO2 incubator, and the complete medium consisted of high-glucose DMEM (Corning, USA, #10-013-CV), 10% fetal bovine serum (BI, USA, #04-001-1ACS), and 1% penicillin (100 U/mL) and streptomycin (0.1 mg/mL) (Gibco, USA, #15,140,122). Cells were passaged every 48 h. When the cell density reached 70–80%, the cells were placed in a differentiation medium consisting of high-glucose DMEM, 2% horse serum (GIBCO, Grand Island, NE, USA), and 1% penicillin/streptomycin to induce differentiation. The medium was refreshed every other day. After four days of differentiation, the cells transformed into myotubes, and their morphology and growth status were observed under a microscope, as shown in Fig. 1. Cell experiments were divided into three groups: the control (DMSO) group, the rotenone (ROT) group, and the rotenone + MitoQ (R + M) group. Myotubes were treated with rotenone, a mitochondrial complex I inhibitor, and mitoquinone (MitoQ), a mitochondria-targeting antioxidant. Rotenone and MitoQ were serially diluted with DMSO and then dissolved in the culture medium at 0.2% DMSO. MitoQ was purchased from MedChemExpress (USA, #HY-100116A), and rotenone was purchased from Sigma (USA, #R8875). The concentration of rotenone was 20 nM48, and the concentration of MitoQ was 60 nM49,50. The actual doses of drugs used were based on the references with appropriate modifications. After four days of differentiation, the medium containing MitoQ or rotenone was replaced, and the cells were collected after 48 h of treatment for subsequent experiments. A portion of the live cells was used for ROS and MMP measurement. Another portion of the cells was stored at − 80 °C for RNA, protein and histone methylation measurement.
C2C12 myotube differentiation process. Cell morphology and growth status were observed under a microscope. As differentiation progressed, C2C12 myotubes gradually fused and differentiated into myotubes. On day 1, there was minimal change in cell morphology. By day 2, a few myotubes were observed, and their numbers increased progressively. By day 4, a substantial number of well-aligned myotubes were evident. Therefore, interventions were initiated on day 4. Scale bar: 180 μm.
Mitochondrial isolation from skeletal muscles
Freshly dissected gastrocnemius muscle were rinsed in 4℃ PBS to remove residual blood, and then blotted dry with sterile absorbent paper. Approximately 100 mg of gastrocnemius muscle tissue was finely minced in a dish placed on ice. The tissue fragments were then transferred to a Dounce homogenizer pre-rinsed with cold mitochondrial isolation medium. The mitochondrial isolation medium consisted of 120 mM KCl (Sigma, USA, #P9333), 5 mM MgCl2.6H2O (Sigma, USA, #M2670), 20 mM HEPES (Sigma, USA, # H3375), 1 mM EGTA(Sigma, USA, # 4100-OP), and 0.5% BSA(Solarbio, China, #9048-46-8), mixed in large batches and adjusted to pH 7.4. Add an appropriate volume of mitochondrial isolation medium, then homogenize by gently lifting and pressing the pestle 6–8 times. Transfer the homogenate to a centrifuge tube, repeating this process about 3 times until no visible solid tissue remained. The homogenate was centrifuged at 1000 g, 4℃, for 10 min, and the supernatant was transferred to a new centrifuge tube. The supernatant was then centrifuged at 10,000 g, 4℃, for 10 min, and the resulting pellet was collected as the mitochondrial fraction. The pellet was resuspended in an appropriate amount of storage medium to obtain the mitochondrial solution. The storage medium for mitochondria contained 300 mM sucrose (Solarbio, China, #57-50-1), 20 mM HEPES, and 0.1 mM EGTA, mixed in large batches and adjusted to pH 7.4.
Measurement of ROS
When skeletal muscle was used as the experimental object, an Oxygraph-2 k (O2k, OROBOROS Instruments, Innsbruck, Austria) was used for measurements of respiration and combined with a Fluorescence-Sensor Green O2k-Fluo LED2-Module for H2O2 measurement51. Amplex Ultra Red (Thermo Scientific, USA, #A36006) (10 μM), horseradish peroxidase (Sigma, USA, #P8375) (HRP; 1 U/mL), and superoxide dismutase (SOD; 5 U/mL) were titrated into the chambers. The fluorescence signal was calibrated using known amounts of H2O2(Millipore, USA, #88,597). Mitochondria (0.8 mg/ml) were added to the buffer; malate (2 mM) (Solarbio, China, # S9750, glutamate (5 mM) (Sigma, USA, #1,446,600), and ADP (5 mM) (Sigma, USA, #A5285) were added; and fluorescence was detected. Approximately 3 min was allowed after each drug was added for the system to reach a steady state, followed by data collection. The rate of ROS generation from ADP-stimulated mitochondrial maximal respiration were recorded and analysed by Datlab 7.3.0.3 software (Oroboros Instruments, Innsbruck, Austria; https://wiki.oroboros.at/index.php/DatLab).
When C2C12 myotubes were used as experimental objects, intracellular ROS levels were measured based on ROS-mediated oxidation of nonfluorescent dichlorofluorescein (DCFH) (Beyotime,China, # S0033S). DCFH-DA passively diffuses into the cell, and esterases deacetylate DCFH-DA, leading to the formation of nonfluorescent DCFH. When ROS are present, DCFH reacts with ROS, leading to the production of fluorescent DCF, which cannot leave the cell. Thus, the level of fluorescence is related to the level of ROS in the cell. C2C12 myoblasts were inoculated into 24-well plates. When the cell density reached 70–80%, the medium was replaced with differentiation medium to induce differentiation. After four days of differentiation, the medium was changed to differentiation medium containing either MitoQ or rotenone. Following a 48-h drug treatment period, the medium was removed. C2C12 myotubes were then incubated with 2´, 7´-dichlorofluorescein–diacetate (DCFH-DA) dissolved in serum-free DMEM (1:1000) in the dark for 30 min at 37 °C.The fluorescence intensity of the cells in each well was measured by using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm after 3 washes with serum-free medium.
Measurement of MMP
When muscles were used as the experimental objects, samples were taken 24 h after the end of the last exercise. The gastrocnemius muscles were rapidly separated on ice, and 100 mg of muscle belly was cut off for purification of mitochondria. Mitochondrial purification and MMP assays were performed according to our previous work52. Gastrocnemius mitochondria were purified by differential centrifugation, and then the mitochondrial concentration was determined by the Bradford method. The fluorescent probe JC-1 was used to label the MMP of the purified mitochondria, and a microplate reader was used to detect red fluorescence (excitation wavelength 535 nm, emission wavelength 590 nm) and green fluorescence (excitation wavelength 485 nm, emission wavelength 520 nm). The ratio of the red and green fluorescence intensities was calculated as the membrane potential. Mitochondrial depolarization (i.e., loss of MMP) was indicated by a decrease in the red/green fluorescence ratio.
When C2C12 myotubes were used as experimental subjects, C2C12 myoblasts were seeded into 24-well plates. When the cell density reached 70–80%, the medium was replaced with differentiation medium to induce differentiation. After four days, the medium was changed to differentiation medium containing MitoQ or rotenone. Following 48 h of drug treatment, the medium was removed. Then, the myotubes were incubated in JC-I working solution for 20 min in the dark at 37 °C. After removing the supernatant, wash twice with JC-1 dying buffer (1×), add 1 mL of cell culture medium, and imaged with a inverted fluorescence microscope (Revolve, Echo Laboratories, USA). Red fluorescence represents J-aggregates, whereas green fluorescence represents the monomeric form of JC-1.
Measurement of mitochondrial respiratory function
To measure mitochondrial OXPHOS function, an Oxygraph-2 k (O2k, OROBOROS Instruments, Innsbruck, Austria) was used to measure gastrocnemius mitochondrial respiration according to a previous experimental protocol with some modifications53. Medium from a MiR05-Kit (Oroboros Instruments, 60,101-01) was used as the mitochondrial respiration medium. Two milliliters of medium were initially injected into the system, and an O2 calibration procedure with sufficient O2 dissolution was immediately performed. After O2 calibration, 100 µg of mitochondria were added to the reaction system. When the O2 consumption curve became stable, a mixture of malic acid (final concentration 2 mM) and glutamic acid (final concentration 10 mM) was added to the system to determine mitochondrial basal respiration in State 2. ADP (5 mM) was then added to induce the transition to the State 3 respiration rate. The oxygen consumption rate decreased when ADP was depleted and eventually reached the oxygen consumption rate plateau, which was the State 4 respiration rate. The oxygen consumption rate was allowed to plateau after each reagent was added before the next step was performed. The respiratory control ratio (RCR) was calculated by dividing the oxygen consumption rate of State 3 by the oxygen consumption rate of State 4.
Immunofluorescence staining
Immunofluorescence staining was routinely performed54. Muscle tissues were dehydrated in isopentane precooled in liquid nitrogen and embedded with OCT embedding agent, and frozen tissues were cut to 10 μm using a cryostat (Leica, Germany, #CM3050 S). The sections were rinsed five times with PBS, each for 2 min and then permeabilized with 0.3% Triton X-100 for 10 min at room temperature. The sections were subsequently rinsed five times with PBS, each for 2 min and blocked in PBS containing 10% normal goat serum for 1 h at room temperature. Each section was subsequently incubated with primary antibodies (MYH7 and MYH4) at 4 °C overnight. The following primary antibodies were used: anti-MYH7 (1:50, Santa, USA, #sc-53089) and anti-MYH4 (1:50, Proteintech, USA, #20,140–1-AP). The next day, the sections were rinsed with PBS for 1 h and then incubated with the corresponding fluorescent secondary antibody (1:150) (Zhongshan Jinqiao, ZF-0511, Alexa Fluor® 488-labeled goat anti-rabbit IgG; Zhongshan Jinqiao Jinqiao, ZF-0513, Alexa Fluor® 594-labeled goat anti-mouse IgG.) for 1 h at room temperature. The tissue sections were rinsed five times with PBS, each for 2 min. Subsequently, 200 μl of DAPI solution was added to the tissue sections to stain the nuclei, and incubation was carried out at room temperature for 5 min. The sections were then rinsed again with PBS five times, each for 2 min. An anti-fade mounting medium was carefully applied to the tissue, and a coverslip was gently placed on top using tweezers. The stained sections were visualized and photographed with a multispectral panoramic tissue scanning microscope (Tissue FAXS Plus S, Tissue Gnostics, Austria).
Quantitative real-time polymerase chain reaction (qRT–PCR)
qRT‒PCR was used to assess the relative mRNA expression levels of PGC-1α, MYH7, and MYH4. Total RNA from rat gastrocnemius tissues and C2C12 myotubes was extracted using TRIzol Reagent (Beyotime, Shanghai, China), and the mRNA concentration of all samples was diluted to 800 ng/μl.The mRNA was reverse-transcribed into cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA, # K1622). In brief, 1 μL of Oligo (dT) primer and 1.6 μg of total RNA were mixed, then RNase-free water was added to reach 12 μL, which was then incubated at 65 °C for 5 min. Afterward, 4 μL of 5 × Reaction buffer, 1 μL of RiboLock RNase Inhibitor (20 U/µL), and 2 μL 10 mM dNTP Mix, 1μL RevertAid M-MuLV RT (200 U/µL) were added to the tube. The mixture was incubated at 42 °C for 60 min and then incubated at 70 °C for 5 min. The cDNA was stored at − 20 °C for later use. The cDNA, primers for the target genes (Table 1), and PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Waltham, MA, USA, # A25918) were mixed, and qRT–PCR assays were performed in a Model 7500 Real-Time Fluorescence Quantitative PCR System (Applied Biosystems, Waltham, MA, USA). All procedures were performed according to the manufacturer's instructions. Each reaction mixture contained 10 μl of master mix, 6 μl of ultrapure water, 1 μl(10 μM) each primer, and 2 μl of cDNA template.。The thermal run consisted of an enzyme activation step for 2 min at 95 °C and 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min, followed by a final extension at 60 °C for 1 min and melt curve analysis from 60 °C to 98 °C in 0.5 °C steps for 15 s. Standard curves for testing primer efficiency were made using dilution series of pooled cDNA as a template. The amplification efficiency and linearity of cDNA amplification was determined and only primer pairs with an amplification efficiency of > 90% were used. The relative expression of the target genes was calculated using the 2 −ΔΔCT method.
Western blotting
In this study, Western blotting was used to assess protein expression. Western blot experiments were performed according to standard procedures55. One hundred milligrams of muscle was cut into small pieces and transferred to a Dounce homogenization tube with 1 mL of RIPA lysis buffer (Beyotime, P0013B) containing 10 μl of protease and phosphatase inhibitor cocktail (Thermo Scientific, USA, #78,445). The tissue was homogenized thoroughly and transferred to a 1.5 mL EP tube. For C2C12 myotube samples, RIPA lysis buffer containing protease and phosphatase inhibitors was added directly to the cell culture dish, and the cells were collected with a cell spatula and transferred to a 1.5 mL EP tube. The supernatant was collected after centrifugation. The protein concentration was determined by the BCA method (Solarbio, China, #A25918). Follow the directions provided by the supplier of the reagent to the letter. After protein quantification, we added 5X loading buffer (Solarbio, China, #P1040) for protein denaturation. Protein denaturation was performed at 100 ℃ for 10 min. Equal amounts of proteins (30 μg prot) were separated by SDS‒PAGE electrophoresis and then transferred to PVDF membranes (Millipore, Billerica, USA, #ISEQ00010). The membranes were incubated with 5% skim milk at room temperature for 2 h. The membranes were incubated with primary antibodies overnight at 4 °C. Primary antibodies against the following proteins were used: AMPK (1:1000, Cell Signaling Technology, USA, #5831), phosphorylated AMPK at threonine 172 (p-AMPKThr172) (1:1000, Cell Signaling Technology, USA, #2535), PGC-1α (1:1000, Cell Signaling Technology, USA, #ab191838), COX IV (1:1000, Abcam, USA, #ab209727), MYH7 (1:1000, Santa Cruz, USA, #sc-53089), MYH4 (1:1000, Proteintech, USA, #20,140–1-AP), JMJD3 (1:500, MyBioSource, USA, #MBS150629), EZH2 (1:1000, Cell Signaling Technology, USA, #5246), JARID1B (1:1000, Cell Signaling Technology, USA, #15,327) and MLL (1:1000, Santa, USA, #sc-374392), with β-actin (1:1000, Abcam, USA, #ab8226) or GAPDH (1:2000, Cell Signaling Technology, USA, #5174S) as internal reference protein. The role of AMPK is manifested through the regulation of nuclear translocation of GAPDH, but it does not directly participate in the modulation of GAPDH protein expression56. We extracted whole-cell proteins, and the variance in the cellular localization of GAPDH does not impact the overall protein quantity. Therefore, we utilized GAPDH as an internal reference internal reference protein. The samples were incubated with an HRP-labeled rabbit secondary antibody (1:20,000, Zhongshan Jinqiao, China, #ZB-2301) or an HRP-labeled murine secondary antibody (1:20,000, Zhongshan Jinqiao, China, #ZB-2305) for 1 h at room temperature, and staining was then visualized with Immobilon Western Chemiluminescent HRP Substrate (Millipore, USA, #WBKLS0500) for development and X-ray film for recording. The grayscale value of each protein blot on the film was analyzed using ImageJ 1.53t software (ImageJ, RRID: SCR_003070; https://imagej.net), and the relative protein expression was calculated.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using a SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology, USA, #9005). The ChIP assay was carried out according to the manufacturer’s protocol (Cell Signaling Technology, USA, #9005) with a slight modification. A standard ChIP (Chromatin Immunoprecipitation) reaction requires approximately 4 × 106 cells or 25 mg of skeletal muscle tissue, yielding a total chromatin mass of about 5 μg. Following cross-linking with formaldehyde at a final concentration of 1%, the samples are incubated with micrococcal nuclease for 20 min to digest the DNA into fragments ranging from approximately 150 to 900 bp in length. The samples are then subjected to sonication multiple times to disrupt the nuclear membrane using a 3 mm probe at a power setting of 40 Hz for 20 s each time, for a total of three sonication cycles. For each reaction, 5 μg of the chromatin fragments are used, and 2 μg of the primary antibody is added for the immunoprecipitation step, with the mixture incubated overnight at 4 °C.Anti-H3K4me3 (Cell Signaling Technology, USA, #9751), anti-H3K27me3 (Cell Signaling Technology, USA, #9733), and IgG control antibodies (Cell Signaling Technology, USA) were used. Chromatin fragments were isolated using Protein G magnetic beads and a magnetic stand, followed by DNA fragment purification. Following ChIP, qRT–PCR was carried out using primers designed against the promoter regions of the target genes (Table 2). qRT–PCR was performed using a 7500 Real-Time PCR System and a StepOnePlus machine (Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 10 μl of master mix, 6 μl of ultrapure water, 1 μl of each primer (10 μM), and 2 μl of purified DNA fragments. This part of the experiment also used primer pairs with amplification efficiencies greater than 90%. The primers were designed to target the promoter regions of various genes. Initially, the gene promoter sequences were obtained from the NCBI gene website. Primer design was performed using Primer Premier 6.0 software (Premier Biosoft, Palo Alto, CA, USA; https://www.premierbiosoft.com/primerdesign/index.html). It is required that the primer length be between 18–24 base pairs and that the primer Tm value be between 50°C–65 °C. Then we can calculate the IP efficiency manually using the Percent Input Method and the equation shown below.
Statistical analysis
The results of this study were analyzed using SPSS version 21.0 (IBM Company, Chicago, USA). The distribution of the data was determined by the Shapiro–Wilk statistic and the results showed a normal distribution at a 95% confidence interval. The in vivo experiments were divided into two groups, and the means were compared by a two-tailed independent samples t-test. The in vitro experiments were divided into three groups, and the means were compared by one-way analysis of variance (one-way ANOVA) followed by an LSD post hoc test. The results were visualized with GraphPad Prism 8.0.2 software (GraphPad Software Inc., La Jolla, CA; https://www.graphpad.com/). All data are presented as the mean ± standard deviation (SD). p < 0.05 or p < 0.01 was considered to indicate a statistically significant difference.
Result
Endurance exercise induces fast-twitch to slow -twitch muscle fiber transition
Endurance exercise induces adaptive changes in skeletal muscle fiber types33. To explore the muscle fiber type transition induced by endurance exercise, we analyzed the effect of endurance exercise on muscle fiber type. Compared with the control regimen, endurance exercise increased the proportion of slow-twitch fibers and reduced the proportion of fast-twitch fibers, suggesting that endurance exercise may promote the transition of fast-twitch fibers to slow-twitch fibers (p < 0.05, p = 0.00067) (Fig. 2a). We also examined changes in the transcriptional and translational levels of MHC protein isoform MYH7 expressed in slow-twitch muscle fibers and MHC protein MYH4 expressed in fast-twitch muscle fibers. We found that endurance exercise significantly increased MYH7 gene transcription (p < 0.01, p = 0.0003) (Fig. 2b) and protein expression (p < 0.05, p = 0.031) (Fig. 2c). Whereas endurance exercise downregulated MYH4 mRNA expression (p < 0.01, p = 0.0002) (Fig. 2d), there was no significant difference in MYH4 protein expression (Fig. 2e). These results suggest that endurance exercise promotes the transition of fast-twitch muscle fibers to slow-twitch muscle fibers.
Endurance exercise induces slow-twitch-to-fast-twitch muscle fiber transition. (a) MYH7 and MYH4 immunofluorescence; representative images are shown. Anti-MYH7 was detected by red fluorescence (indicated by white arrows), whereas anti-MYH4 was detected by green fluorescence (indicated by yellow arrows). 4’,6-diamidino-2-phenylindole (DAPI)-stained nuclei. Scale bar: 130 μm. (b) Endurance exercise-induced changes in MYH7 gene mRNA content (RT‒qPCR) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 4). (d) Endurance exercise-induced changes in MYH4 gene mRNA content (RT‒qPCR) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 4). (c) Endurance exercise-induced changes in MYH7 protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (e). Endurance exercise-induced changes in MYH4 protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). Data information: All values represent the mean ± SD. The p value was determined using a two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01.
Endurance exercise enhances mitochondrial biogenesis
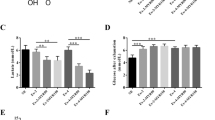
Endurance exercise induced adaptive changes in skeletal muscle fiber metabolism, an increase in mitochondrial biogenesis, and enhancement of mitochondrial oxidative metabolism57. To investigate how endurance exercise induced adaptive changes in mitochondrial oxidative metabolism, we first examined changes in mitochondrial oxidative metabolism capacity after endurance exercise in rats. Consistent with the findings of previous studies, endurance exercise increased the MMP (p < 0.05, p = 0.015) (Fig. 3a). Purified mitochondria were then assayed for the mitochondrial respiratory control rate (RCR) with malate and glutamate as substrates. It was found that endurance exercise significantly increased the mitochondrial RCR (p < 0.05, p = 0.046) (Fig. 3b), mainly by increasing State 3 respiration (p < 0.05, p = 0.035) (Fig. 3c). This result suggests that endurance exercise increases ADP-stimulated maximal mitochondrial respiration and improves mitochondrial oxidative metabolism.
Endurance exercise increases mitochondrial biogenesis. (a) The mitochondrial membrane potential (MMP) was measured using a fluorescent probe, JC-1. The ratio of red (JC-1 polymer) to green (JC-1 monomer) fluorescence was calculated (n = 6). (b–d) Mitochondrial respiratory function of isolated mitochondria from gastrocnemius muscle. Basal (State 4) respiration (d) was measured first with glutamate (5 mM) and malate (2 mM). ADP (5 mM) was added to determine oxidative phosphorylation capacity (State 3) (c). The respiratory control ratio (RCR) (c) was the ratio of State 3 to State 4 respiration rate (the blue bar represents C group, and the red bar represent E group) (n = 6). (e) Endurance exercise-induced changes in PGC-1α mRNA expression (qRT‒PCR) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 4). (f). Endurance exercise-induced changes in PGC-1α and protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (g) Endurance exercise-induced changes in COX IV protein expression (Western blotting) in gastrocnemius muscle (n = 6). Data information: All values represent the mean ± SD. The p value was determined using a two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01.
To investigate the reasons for the endurance exercise-induced increase in mitochondrial oxidative metabolism capacity, we examined changes in the transcription and translation of PGC-1α, a key gene related to mitochondrial biogenesis. Consistent with the findings of previous studies58, endurance exercise significantly upregulated PGC-1α mRNA expression (p < 0.01, p = 0.001) (Fig. 3e) and PGC-1α protein expression (p < 0.01, p = 0.002) (Fig. 3f). COX IV, a key subunit of the mitochondrial electron transport chain complex IV (cytochrome c oxidase), plays a crucial role in oxidative phosphorylation. The expression level of COX IV is typically used to reflect changes in mitochondrial quantity59. We also found that endurance exercise upregulated the protein expression of COX IV (p < 0.01, p = 0.001) (Fig. 3g). The findings suggest that endurance exercise promotes mitochondrial biogenesis by upregulating the expression of PGC-1α in skeletal muscle.
Endurance exercise-induced changes in histone methylation
The previous experiments demonstrated that endurance exercise increased mitochondrial biogenesis through upregulation of PGC-1α protein expression and promoted the transition of fast-twitch to slow-twitch fibers through upregulation of MYH7 protein expression60. The results indicate that the transition of muscle fiber types induced by endurance exercise is accompanied by mitochondrial biogenesis. Next, we sought to explore the common mechanisms by which endurance exercise induced the coupling of mitochondrial biogenesis and muscle fiber type. Histone methylation is a modification that can regulate the expression of different genes. Previous studies have demonstrated that different sites and types of histone methylation play different roles in regulating gene transcription; for example, H3K4me3 at gene promoters activates transcription, while H3K27me3 represses transcription61,62,63. Several studies have confirmed that exercise induces a variety of histone methylation changes in the genome35,37. We found that endurance exercise induced a significant increase in H3K4me3 (p < 0.05, p = 0.01) and a significant decrease in H3K27me3 (p < 0.05, p = 0.043) at the PGC-1α gene promoter (Fig. 4a,b). Similar changes occurred at the MYH7 gene promoter, where H3K4me3 was significantly increased (p < 0.05, p = 0.034) (Fig. 4c). A significant increase in H3K27me3 enrichment at the MYH4 gene promoter was also found (p < 0.01, p = 0.009) (Fig. 4f), but no significant change in H3K4me3 enrichment was observed. The above findings indicated that endurance exercise induced increases in PGC-1α and MYH7 gene expression and a decrease in MYH4 mRNA expression and that the H3K4me3 and/or H3K27me3 levels of different gene promoters changed accordingly. These results suggest that histone methylation plays a key role in the coupling of increased mitochondrial oxidative metabolism and muscle fiber type transition induced by endurance exercise.
Endurance exercise-induced changes in histone methylation. (a) ChIP-qPCR assay showing the enrichment levels of H3K4me3 on the PGC-1α gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). (b) ChIP-qPCR assay showing the enrichment levels of H3K27me3 on the PGC-1α gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). (c) ChIP-qPCR assay showing the enrichment levels of H3K4me3 on the MYH7 gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). (d) ChIP-qPCR assay showing the enrichment levels of H3K27me3 on the MYH7 gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). (e) ChIP-qPCR assay showing the enrichment levels of H3K4me3 on the MYH4 gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). (f) ChIP-qPCR assay showing the enrichment levels of H3K27me3 on the MYH4 gene promoter in the gastrocnemius muscles of C group (blue bar) and E group (red bar). Data information: All values represent the mean ± SD. The p value was determined using a two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01.
Endurance exercise activates the ROS/AMPK pathway to regulate histone methylase expression
The increase in mitochondrial biogenesis and the transition of muscle fiber type are two physiological processes whose coupling mechanisms are worthy of attention. The reactive oxygen species (ROS)/AMPK pathway plays a key role in exercise-induced health benefits, and activation of AMPK increases mitochondrial biogenesis and promotes the transition of fast-twitch fibers to slow-twitch fibers64,65. Therefore, we hypothesized that the ROS/AMPK pathway plays an important role in the coupling of mitochondrial biogenesis and muscle fiber type transition. We measured the mitochondrial ROS production rate and AMPK activation in the gastrocnemius muscle and found that endurance exercise increased the mitochondrial ROS production rate (p < 0.05, p = 0.019) (Fig. 5a) and significantly increased AMPK activation (p < 0.01, p = 0.001) (Fig. 5b,c).
Endurance exercise activates the ROS/AMPK pathway to regulate histone methylase expression. (a) Endurance exercise-induced changes in the mitochondrial ROS generation rate in gastrocnemius muscle as measured by Amplex Ultra Red assay (the blue bar represents C group, and the red bar represent E group) (n = 6). (b, c) Endurance exercise-induced changes in p-AMPKThr172/AMPK protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (d) Endurance exercise-induced changes in JARID1B protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (e) Endurance exercise-induced changes in MLL protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (f) Endurance exercise-induced changes in JMJD3 protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). (g) Endurance exercise-induced changes in EZH2 protein expression (Western blotting) in gastrocnemius muscle (the blue bar represents C group, and the red bar represent E group) (n = 6). Data information: All values represent the mean ± SD. The p value was determined using a two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01.
p-AMPKThr172 can regulate various histone methylation enzymes and histone demethylation enzymes through direct or indirect means38,39,66. To investigate the mechanism by which endurance exercise induces alterations in histone methylation, we examined histone methyltransferases and histone demethylases of H3K4me3 and H3K27me3. Jumonji AT-rich interactive domain 1B (JARID1B, also known as KDM5B) is an H3K4me3-specific demethylase, and mixed lineage leukemia (MLL) is a key subunit of the H3K4me3-specific methylase complex COMPASS. Jumonji domain containing-3 (JMJD3, also known as KDM6B) is an H3K27me3-specific demethylase, and EZH2 is a key subunit of the H3K27me3-specific methylase polycomb repressive complex 2 (PRC2) complex67. Therefore, we examined the expression of several methylation-modifying enzymes. We found that JARID1B(p < 0.05, p = 0.015) and EZH2(p < 0.01, p = 0.0006) protein expression was significantly decreased (Fig. 5d,g), while MLL(p < 0.05, p = 0.013) and JMJD3(p < 0.01, p = 0.001) protein expression was significantly increased (Fig. 5e,f), in the endurance exercise group compared with the control group. Given the above results, we speculate that endurance exercise can reduce the enrichment of H3K27me3 in the gene promoter by activating the ROS/AMPK pathway and then by upregulating JMJD3 and downregulating EZH2. Gene promoter H3K4me3 enrichment can also be increased by downregulation of JARID1B and upregulation of MLL. The alterations in histone methylation described above promote gene transcription and protein expression.
Rotenone activates the ROS/AMPK pathway to enhance mitochondrial biogenesis and increase MHC slow isoform expression
To further verify that the ROS/AMPK pathway is involved in the regulation of histone methylation in mitochondria and MHC-related genes, we treated C2C12 myotubes with rotenone, an inhibitor of mitochondrial complex I, to activate the ROS/AMPK pathway. MitoQ, a mitochondrion-targeted oxidant that scavenges ROS and inhibits activation of the ROS/AMPK pathway, was also used to explore the role of the ROS/AMPK pathway in the coupling of mitochondrial oxidative capacity and muscle fiber type.
Compared with that in the DMSO group, the ROS content in the rotenone group was significantly elevated (p < 0.01, p = 0.004) (Fig. 6b), and MitoQ was able to inhibit the rotenone-induced ROS elevation (p < 0.01, p = 0.001) (Fig. 6b). Similarly, regarding AMPK activation, rotenone increased the p-AMPKThr172/AMPK ratio (p < 0.01, p = 0.002), while MitoQ inhibited AMPK activation (p < 0.05, p = 0.017) (Fig. 6c,d). These results suggest that rotenone activates AMPK by phosphorylating Thr172 of the α subunit via ROS, whereas MitoQ inhibits the activation of the ROS/AMPK pathway. These findings are consistent with the results of the in vivo experimental part of this study as well as with the results of previous studies68.
Rotenone activates the ROS/AMPK pathway to enhance mitochondrial biogenesis and increase MHC slow isoform expression. (a) Rotenone or rotenone + MitoQ combined treatment induced changes in mitochondrial membrane potential in C2C12 myotubes . JC-1 labels mitochondria with a high membrane potential in red (JC-1 aggregates) and mitochondria with a low membrane potential in green (JC-1 monomers). Scale bar: 60 μm (b) Rotenone or rotenone + mitoquinone (MitoQ) combined treatment induced changes in ROS levels in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 7). (c, d, f, g) Rotenone or rotenone + MitoQ combined treatment induced changes in p-AMPKThr172/AMPK/PGC-1α/COX IV protein expression (Western blotting) in C2C12 myotubes (n = 4). (c, d) Rotenone or rotenone + mitoquinone combined treatment induced changes in p-AMPKThr172/AMPK protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (e) Rotenone or rotenone + mitoquinone combined treatment induced changes in PGC-1α gene mRNA content (qRT‒PCR) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (f) Rotenone or rotenone + mitoquinone combined treatment induced changes in p- PGC-1α protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (g) Rotenone or rotenone + mitoquinone combined treatment induced changes in COX IV protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (h) Rotenone or rotenone + MitoQ combined treatment induced changes in MYH7 gene mRNA content (qRT‒PCR) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (i) Rotenone or rotenone + MitoQ combined treatment induced changes in MYH4 gene mRNA content (qRT‒PCR) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). Data information: All values represent the mean ± SD. The p value was determined using one-way ANOVA coupled to Fisher’s least-significant difference (LSD) post hoc test. *p < 0.05, **p < 0.01.
To explore the influence of the ROS/AMPK pathway on mitochondrial biogenesis, we used JC-1 to measure the MMP and used a fluorescence microscope to obtain fluorescence images. We found that rotenone increased the MMP (p < 0.01, p = 0.001) (Fig. 6a) and significantly upregulated the expression of PGC-1α (p < 0.01, p = 0.0002), while MitoQ inhibited the rotenone-induced upregulation of the MMP (p < 0.01, p = 0.005) and PGC-1α expression (p < 0.05, p = 0.037) (Fig. 6e,f). It was also found that rotenone upregulated the protein expression of mitochondrial COX IV (p < 0.01, p = 0.002), while MitoQ inhibited the upregulation of COX IV (p < 0.05, p = 0.015) (Fig. 6g). This suggests that rotenone can increase the mitochondrial biogenesis by activating the ROS/AMPK pathway. Meanwhile, rotenone significantly upregulated MYH7 mRNA expression (p < 0.05, p = 0.00049), while MYH4 did not undergo significant changes (Fig. 6h,i). The above results suggest that the ROS/AMPK pathway may be a common pathway for the increase in mitochondrial biogenesis and muscle fiber type transition induced by endurance exercise.
Rotenone regulates histone methylation by modulating the corresponding catalytic enzymes
We further demonstrated that the ROS/AMPK signaling pathway couples mitochondrial oxidative metabolic capacity and muscle fiber type transition through histone methylation. We measured the expression of four histone methylation-related enzymes that regulate H3K4me3 and H3K27me modifications in vitro. The results were consistent with those of the animal experiments. Compared with those in the control group, the protein expression levels of JARID1B(p < 0.01, p = 0.001) and EZH2(p < 0.05, p = 0.03) in the rotenone intervention group were significantly lower (Fig. 7b,c), while the protein expression levels of MLL(p < 0.01, p = 0.003) and JMJD3(p < 0.05, p = 0.034) were significantly higher (Fig. 7d,e). Compared with rotenone alone, the combined treatment of rotenone and MitoQ decreased the protein expression of MLL (p < 0.01, p = 0.006) and JMJD3 (p < 0.01, p = 0.003) (Fig. 7c,d), while increasing the protein expression of EZH2 (p < 0.01, p = 0.00033). The above results suggest that endurance exercise regulates the expression of histone methylases by activating the ROS/AMPK pathway.
Rotenone regulates histone methylation by modulating the corresponding catalytic enzymes. (a,b) Rotenone or rotenone + mitoquinone combined treatment induced changes in JARID1B protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (c) Rotenone or rotenone + mitoquinone combined treatment induced changes in MLL protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (d) Rotenone or rotenone + mitoquinone combined treatment induced changes in EZH2 protein expression (western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). e. Rotenone or rotenone + mitoquinone combined treatment induced changes in JMJD3 protein expression (Western blotting) in C2C12 myotubes (the blue bar represents DMSO group, the red bar represents ROT group, and the green bar represent R + M group) (n = 4). (f) ChIP-qPCR assay showing the enrichment levels of H3K4me3 at the PGC-1α gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). (g) ChIP-qPCR assay showing the enrichment levels of H3K27me3 at the PGC-1α gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). (h) ChIP-qPCR assay showing the enrichment levels of H3K4me3 at the MYH7 gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). (i) ChIP-qPCR assay showing the enrichment levels of H3K27me3 at the MYH7 gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). (j) ChIP-qPCR assay showing the enrichment levels of H3K4me3 at the MYH4 gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). )(k) ChIP-qPCR assay showing the enrichment levels of H3K27me3 at the MYH4 gene promoter in cells treated with DMSO (blue bars), ROT (red bars), and R + M (green). Data information: All values represent the mean ± SD. The p value in a–f was determined using a two-tailed unpaired Student’s t test; the value in G–L was determined using one-way ANOVA coupled to Fisher’s least-significant difference (LSD) post hoc test. *p < 0.05, **p < 0.01.
We also assessed histone methylation at the promoters of the PGC-1α, MYH7, and MYH4 genes. We found that H3K4me3 at the promoter of the PGC-1α gene was significantly increased after rotenone treatment (p < 0.05, p = 0.02); however, MitoQ inhibited the effect of rotenone and reduced the enrichment level of H3K4me3 (p < 0.05, p = 0.03) (Fig. 7f). Following rotenone treatment, H3K4me3 levels on the MYH7 gene promoter were significantly elevated (p < 0.05, p = 0.047), while H3K27me3 modifications on the MYH7 gene promoter decreased (p < 0.05, p = 0.049). MitoQ inhibited the biological effects of rotenone and reversed the rotenone-induced H3K4me3 elevation (p < 0.05, p = 0.041) (Fig. 7h). MitoQ also increased H3K27me3 modification at the promoter of the MYH7 gene (p < 0.01. p = 0.00028) (Fig. 7i). Following rotenone treatment, H3K4me3 levels on the MYH4 gene promoter significantly decreased (p < 0.05, p = 0.018), while H3K27me3 levels significantly increased (p < 0.01, p = 0.004). MitoQ inhibited the rotenone-induced changes in both H3K4me3 (p < 0.05, p = 0.048) and H3K27me3 (p < 0.05, p = 0.031) (Fig. 7j,k). These results demonstrate that the ROS/AMPK pathway plays a key role in regulating histone methylation at the promoters of genes related to mitochondrial oxidative metabolism and MHC isoforms.
The above research results suggest that endurance exercise regulates the histone methylation of the PGC-1α, MYH7 and MYH4 gene promoters by activating the ROS/AMPK pathway and then couples mitochondrial oxidative metabolism and muscle fiber MHC isoforms to promote fast-twitch-to-slow-twitch muscle fiber transition.
Discussion
In this study, we found that endurance exercise promotes mitochondrial biogenesis and mitochondrial OXPHOS by regulating the histone methylation of the promoter of the mitochondrial biogenesis-related gene PGC-1α. In addition, it upregulates MYH7 gene and protein expression and downregulates MYH4 gene expression by regulating histone methylation of the MHC isomer gene promoters. However, MYH4 protein expression did not change, possibly because endurance exercise primarily increases MYH7 protein expression, potentially exerting a limited effect on MYH4. This result is consistent with the findings of Mok et al.69, who reported that activation of nuclear receptor subfamily 4 group A member 2 (NR4A2) led to an increase in oxidative muscle fibers, with MYH7 protein levels increasing 2.6-fold in mice and 4.6-fold in human myotubes, while MYH4 protein expression remained unchanged. Another study similarly observed that interventions aimed at inducing an increase in slow muscle fibers upregulated MYH7 protein expression, with no change in MYH4 protein expression70. In a C2C12 myotube model, we showed that rotenone-induced mitochondrial ROS triggered histone methylation changes in the promoters of mitochondria-related genes and MHC isomers similar to endurance exercise, while MitoQ, a mitochondrion-targeted antioxidant, partially blocked this change. Our results further confirm the epigenetic mechanism of muscle fiber type transition induced by endurance exercise.
Mitochondrial OXPHOS is the main energy supply method for the contraction of slow-twitch muscle fibers. PGC-1α is an important mitochondrial regulatory gene. Our study found that H3K4me3 was increased and that H3K27me3 was decreased in the PGC-1α gene promoter in the skeletal muscles of rats subjected to endurance exercise. H3K27me3 is associated with transcriptionally repressed genes, whereas H3K4me3 activates transcription71. Consistent with our findings, Lochmann et al.35 demonstrated that quadriceps PGC-1α promoter H3K4me3 levels were increased 2- to fourfold and that PGC-1α mRNA levels were increased in an acute exercise model in mice. Shimizu et al.37 similarly found that H3K4me3 of the PGC-1α gene was significantly increased after acute endurance exercise but that H3K27me3 was increased at 2 h and decreased at 24 h after exercise. In our study, we obtained samples 24 h after the last exercise session; therefore, our results are comparable to those of the prior study.
Exercise induced an increase in H3K4me3 and a decrease in H3K27me3 at the PGC-1α gene promoter, resulting in upregulation of PGC-1α protein expression. Moreover, the expression of COX IV, an indicator related to mitochondrial content, was increased. This result is consistent with previous studies showing that endurance exercise can increase mitochondrial biogenesis72.This study confirmed that endurance exercise can increase MMP and enhance mitochondrial function. Moreover, endurance exercise can significantly increase mitochondrial maximal respiration (State 3) and the RCR. The results also showed that endurance exercise increases mitochondrial OXPHOS ability, consistent with the results of previous studies72,73. Research has shown that exercise can also induce epigenetic changes in humans, affecting pathways related to energy metabolism, insulin sensitivity, and mitochondrial biogenesis, thereby promoting physiological adaptations74. In conclusion, endurance exercise increases mitochondrial biogenesis and enhances mitochondrial OXPHOS function by altering PGC-1α gene promoter histone methylation (H3K4me3 and/ or H3K27me3).
In humans, the plasticity of muscle fiber composition has been well-documented, with aerobic and mixed exercises leading to a transition from fast-twitch to slow-twitch muscle fibers75. A case study by Bathgate et al.76 examined twins, one of whom was predominantly sedentary while the other engaged in recreational endurance exercise for decades. The trained twin had a vastus lateralis muscle composition predominantly of slow-twitch fibers (95% MHC I), which was 55% more than the untrained twin. This study indicates that long-term endurance training can induce a shift from fast to slow muscle fibers. However, it remains unclear how established regulatory factors of fiber type conversion, such as PGC-1 and NFATC1, interact with MHC promoter and enhancer dynamics to facilitate activity-dependent fiber type transitions77. Epigenetics may offer an answer to this question. Muscle fiber types are usually classified according to their specific MHC isomers, and the head of the MHC is an important component of the muscle force production mechanism78. The expression of MHC isomers is regulated by histone methylation30. In this study, we found that endurance exercise upregulated MYH7 mRNA and protein expression and downregulated MYH4 mRNA expression. The MYH7 gene promoter H3K4me3 level was increased. While the MYH4 gene promoter H3K27me3 was increased, H3K4me3 was unchanged. Pandorf et al.30 compared the H3K4me3 modification of the MHC isomer gene of fast-twitch muscle fiber-dominated plantar muscle and slow-twitch muscle fiber-dominated soleus muscle. They found that compared with those in plantar muscle, soleus muscle MYH7 gene H3K4me3 levels were higher and MYH4 gene H3K4me3 levels were lower. However, after 7 days of hind limb suspension, H3K4me3 levels at the MYH4 gene promoter were increased in soleus muscle, and MYH4 mRNA levels were similarly increased. This result is consistent with the current view that hind limb suspension induces histone methylation modifications in genes associated with muscle fiber types. Our research supports the view that exercise induces the transition of fast-twitch muscle fibers to slow-twitch muscle fibers by modulating histone methylation. Therefore, our finding that H3K4me3 in the MYH7 gene increased was consistent with expectations. In addition, Shimizu et al.37 demonstrated that the levels of H3K27me3 and H3K4me3 at target loci in mice were increased after a single bout of exercise. Chronic intervention with the EZH2 inhibitor GSK343 during exercise further increased the level of H3K27me3 and promoted the transition of type IIb to type IIa muscle fibers. Therefore, endurance exercise may regulate the levels of H3K27me3 and H3K4me3 in the MHC gene and regulate the differential expression of MHC isomers. This study suggests that histone methylation is involved in the exercise-induced transition of fast-twitch muscle fibers to slow-twitch muscle fibers.
Skeletal muscle adaptability to exercise is reflected in different ways. Both an in vivo exercise model and an in vitro cell electrical stimulation model have revealed that the upregulation of MHC (MYH7 and MYH2) expression related to slow-twitch muscle fibers, accompanied by the upregulation of PGC-1α expression related to mitochondrial biogenesis60. Gan et al.79 demonstrated that the estrogen receptor-related receptor gamma (ERRγ)/miR-499 pathway is involved in the coordinated control of muscle energy metabolism and muscle fiber types. The team also demonstrated that inhibition of Fnip1 by miR-499 activated the AMPK-PGC-1α signaling pathway and enhanced the mitochondrial oxidative metabolism in skeletal muscle; furthermore, overexpression of miR-499 resulted in a significant increase in the proportion of type I muscle fibers in gastrocnemius muscle29. This study demonstrated that miR-499 plays a key role in coordinating the transition of muscle fibers to type I muscle fibers and regulating mitochondrial metabolism. The findings suggest that epigenetic modification may be the mechanism coupling the different physiological reactions. Recent studies have shown that epigenetic modification plays an important role in the extensive regulation of gene expression induced by exercise34. Furthermore, our recently published paper elucidates that the coupling of mitochondrial function with skeletal muscle fiber types through exercise may be achieved by regulating epigenetic modifications33. In this study, we found that endurance exercise induced remodeling of PGC-1α, MTH7, and MYH4 histone methylation (H3K4me3 and H3K27me3). We speculate that endurance exercise may induce the transition of skeletal muscle fibers to slow-twitch muscle fibers by regulating histone methylation of mitochondria-related genes and MHC isoform genes, which then synergistically regulate the expression of MHC slow isoforms and the energy demand of skeletal muscle fibers.
Endurance exercise has previously been shown to regulate muscle fiber type transition, possibly by remodeling histone methylation. However, how exercise regulates different types of histone methylation has remained unclear. Previous studies have demonstrated that different kinds of histone methylation require different types of histone-modifying enzymes80. JMJD3 is an H3K27me3 demethylase, and EZH2 is a key component of the PRC2 methyltransferase81. In this study, we found that endurance exercise resulted in decreased EZH2 expression and increased JMJD3 expression. Consistent with our JMJD3 results, Ziemann et al.82 found that JMJD3 mRNA expression was significantly upregulated in the vastus lateralis muscle after exercise in healthy men. Several studies have confirmed that the EZH2 content in the soleus muscle is lower than that in fast-twitch muscles, such as the plantaris muscle, gastrocnemius muscle, and vastus lateralis muscle83,84. The low EZH2 content in the slow muscle is also consistent with our finding that exercise induces the transition of muscle fibers to slow-twitch muscle fibers. However, Gulri84 found in their study that chronic exercise induced upregulation of EZH2 protein expression in gastrocnemius muscle. This is inconsistent with our finding of EZH2 downregulation. The possible reason may be different movement patterns and sampling times. We obtained samples 24 h after the last exercise session, while Gulri obtained samples 72 h after the last exercise session.
JARID1B is a demethylase of H3K4me3, and MLL is a critical component of the COMPASS complex of histone methyltransferase85. We found that endurance exercise resulted in decreased JARID1B expression and increased MLL expression. Similar to our study, a previous study has revealed that running wheel exercise in rats increases the levels of genomic H3K4me3 and improves mitochondrial function by increasing the expression of hemoglobin β (HBB)86. JARID1B is known to require oxygen molecules to exert its catalytic activity, which is sensitive to changes in oxygen concentration87. HBB represses the activity of JARID1B by sequestering the oxygen required for JARID1B to upregulate H3K4me3 levels86. Thus, exercise may alter gene H3K4me3 modification by regulating JARID1B. Thus far, no data have been published on MLL protein expression after endurance exercise, but MLL4, an MLL family member, has been shown to be enriched in slow-twitch muscle fibers and plays a critical role in controlling muscle fiber type and skeletal muscle function. MLL4 knockdown in mouse skeletal muscle suppresses H3K4me1 levels, induces downregulation of the slow-twitch muscle fiber gene program, and reduces the number of type I muscle fibers and mitochondrial respiratory function, which in turn leads to decreased muscle fatty acid utilization and muscle endurance during exercise83. We believe that MLL, as a histone methyltransferase whose main function is to increase H3K4me3 levels in gene promoters, may play the same important role as MLL4. Therefore, it is necessary to expand research on MLL in sports and exercise science.
At present, the mechanism by which endurance exercise induces changes in histone methylation remains unclear. The published data of our research group show that ROS are necessary for endurance exercise to promote health. Ristow et al.88 found that antioxidant supplements actually prevent the health-promoting effects of physical exercise. We have previously demonstrated that endurance exercise increases skeletal muscle OXPHOS efficiency by activating mitochondrial inner membrane uncoupling proteins via ROS52,89. We have recently shown that antioxidants block the activation of the mitochondrial quality control system of skeletal muscle by endurance training. In addition, we recently reported that exercise-induced ROS can regulate multiple epigenetic modifications32. Exercise-induced ROS in skeletal muscle can directly activate AMPK through S-glutathionylation of cysteine on the α and β subunits of AMPK90,91,92. Human exercise can also enhance muscle function through the activation of AMPK by ROS45,93. Activated AMPK is a key regulator of epigenetic modification38. In this study, the ROS production rate and p-AMPKThr172/AMPK ratio in the skeletal muscle of rats subjected to endurance exercise were increased. However, it was unclear whether the ROS/AMPK pathway was involved in regulating histone methylation in mitochondrial and MHC isoform genes.
To further verify whether the ROS/AMPK pathway is involved in the regulation of histone methylation-modifying enzymes (JARID1B, EZH2, MLL, and JMJD3), we used rotenone, which induces an increase in mitochondrial ROS production, to establish a ROS/AMPK activation model in C2C12 myotubes. Rotenone is a specific inhibitor of mitochondrial respiratory chain complex I, which blocks complex I ubiquinone binding sites to increase ROS levels94,95. Rotenone, like metformin, activates AMPK by inhibiting mitochondrial respiratory chain complex I96,97. Thus, treatment of C2C12 myotubes with rotenone induces mitochondrial ROS production and AMPK activation68. Rotenone not only activates AMPK via ROS-induced S-glutathiolation on cysteines but also activates AMPK by inhibiting COX I, leading to a reduction in the ATP/AMP or ATP/ADP ratio98. Wu et al.99 demonstrated that ROS produced due to exercise may directly promote AMPK phosphorylation (activation) independent of AMP upregulation. Therefore, we focused on the role of ROS-induced AMPK activation in the regulation of muscle fiber type transition in this study.
The main application of rotenone-treated cell models is to mimic neurodegenerative pathological processes such as those in Parkinson's disease100,101,102. Treatment of cells with rotenone appears to result in dose-dependent effects103,104. Rotenone induces oxidative stress and other pathological reactions at high doses and does not appear to cause pathological reactions at low doses105. Sotzny et al.106 treated SH-SY5Y human neuroblastoma cells with 100 nM rotenone for 24 h and found that the activation of caspase-3/7 activity did not increase. Therefore, the authors concluded that 100 nM rotenone is not toxic to cells. Forkink et al.107 treated HEK293 cells with 100 nM rotenone, which resulted in a significant increase in MMP. In addition, Hou et al.108 found that rotenone ameliorated hyperglycemia and improved insulin sensitivity in a diabetic mouse model and that rotenone induced glucose consumption and glycolysis and reduced hepatic glucose output. That study also confirmed that rotenone concentrations in the range of 20–200 nM not only did not produce toxic effects but also activated AMPK in HepG2 and C2C12 myotubes. Therefore, in this study, we used 20 nM rotenone to treat C2C12 myotubes.
We found that rotenone treatment increased cellular ROS levels and promoted AMPK phosphorylation, confirming that rotenone activated the AMPK pathway. This is consistent with previous research68. In this cell model, MLL and JMJD3 protein expression was upregulated, and JARID1B and EZH2 protein expression was downregulated. PGC-1α gene promoter H3K4me3 increased, promoting PGC-1α and COXIV protein expression and resulting in enhanced mitochondrial biogenesis. We also found that MYH7 gene promoter H3K4me3 was increased, which promoted MYH7 transcription. Activation of the AMPK pathway may remodel histone methylation by regulating the expression of histone methylation-modifying enzymes. Histone methylation promotes both mitochondrial biogenesis and MHC slow isoform expression. The coupling of the two promotes the transition of muscle fibers into slow-twitch muscle fibers. In addition to the ROS/p-AMPKThr172 signaling pathway, the metabolic byproduct lactate can also regulate gene transcription through histone modifications. Lactate serves as a precursor for histone lysine lactylation, exerting epigenetic regulatory effects109. In both human and mouse cells, 28 lysine lactylation sites have been identified on core histones110. Although no specific reports have been published on this mechanism, given that exercise significantly increases lactate production in skeletal muscle111, it can be hypothesized that histone lysine lactylation may also play a role in regulating muscle fiber type transition. In addition, studies indicate that AMPK can also modify epigenetic marks by phosphorylating DNMT1, RBBP7, and HAT1, leading to increased expression of nuclear genes involved in mitochondrial biogenesis and function, such as PGC-1α, transcription factor A (Tfam), and uncoupling proteins 2 and 3 (UCP2 and UCP3)112. Furthermore, research has shown that the calcium-calcineurin signaling pathway is subject to epigenetic regulation. The combined treatment with DNMT inhibitors and EZH2 inhibitors transcriptionally upregulated the calcium-calcineurin signaling pathway113. Additionally, calcineurin expression has been found to be regulated by HDAC4114. Thus, we hypothesize that ROS/AMPK-mediated epigenetic modifications may also regulate the expression of calcineurin and genes related to mitochondrial biogenesis. This represents a significant area for further scientific investigation.
To further confirm the role of ROS, we added MitoQ to the rotenone-treated model and found that MitoQ inhibited rotenone-induced mitochondrial ROS production and AMPK activation. This result demonstrated that rotenone activated AMPK by increasing mitochondrial ROS levels, while MitoQ was able to inhibit activation of the ROS/AMPK pathway. MitoQ also inhibited the rotenone-induced increase in MLL and JMJD3 protein expression. We found that a reduction in H3K4me3 in the PGC-1α gene promoter inhibited the expression of the PGC-1α protein. In addition, we found that MitoQ reduced H3K4me3 and increased H3K27me3 at the MYH7 gene promoter and increased H3K4me3 and reduced H3K27me3 at the MYH4 gene promoter. However, we did not observe changes in MYH4 gene transcription. This is in agreement with the results of our experiments in vivo. We observed that histone methylation in the MYH4 gene did not match changes in histone-modifying enzymes. We suggest that modulation of histone methylation at the MYH4 gene promoter may be regulated by other histone-modifying enzymes, such as EZH180. Shimizu et al.37 found that GSK343, an inhibitor of EZH2, further increases H3K27me3 levels. In contrast, valemetostat, a dual EZH1/2 inhibitor, reduces the levels of H3K27me3 and H3K4me3 following acute exercise. This suggests that EZH1 may be a histone-modifying enzyme involved in exercise-induced H3K27me3 in skeletal muscle. In conclusion, our study demonstrates that MitoQ partially blocks rotenone-induced enhancement of mitochondrial biogenesis and alteration of MHC isoform expression. Endurance exercise activates the ROS/AMPK pathway, which promotes the transition of fast-twitch muscle fibers to slow-twitch muscle fibers through histone methylation coupling regulation of the expression of MHC7 with enhancement of mitochondrial biogenesis.
Conclusion
The present study demonstrates a novel epigenetic mechanism for muscle contractile property tightly coupled to its metabolic capacity during muscle fiber type transition with exercise training. Endurance exercise couples mitochondrial biogenesis with MHC slow isoform by remodeling histone methylation, which in turn promotes the transition of fast-twitch to slow-twitch muscle fibers. The ROS/AMPK pathway may be involved in the regulation of histone methylation enzymes by endurance exercise (Fig. 8). However, future work applying genetic or pharmacological approaches should further uncover the relationship between mitochondrial function and myofiber typing.
A model of endurance exercise-induced muscle fiber type transition. The schematic depicts that the ROS/AMPK pathway mediates endurance exercise remodeling histone methylation to couple mitochondrial biogenesis and the expression of MHC slow isoform. ROS: reactive oxygen species; AMPK: AMP-activated protein kinase; JARID1B: jumonji AT-rich interactive domain 1B; MLL: mixed lineage leukemia; JMJD3: jumonji domain containing-3; EZH2: enhancer of zeste homolog 2; H3K4me3: histone-3 lysine-4 trimethylation; H3K27me3: histone-3 lysine-27 trimethylation; PGC-1α: peroxisome proliferator-activated receptor γ coactivators 1α; MYH7: myosin heavy chain 7.
Data availability
Data will be made available upon reasonable request. If someone wants to request the data from this study, he should contact Hai Bo (bohaixd@126.com).
References
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531. https://doi.org/10.1152/physrev.00031.2010 (2011).
Crupi, A. N. et al. Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging. Aging (Albany NY) 10, 3327–3352. https://doi.org/10.18632/aging.101643 (2018).
Lin, J. et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801. https://doi.org/10.1038/nature00904 (2002).
Mishra, P., Varuzhanyan, G., Pham, A. H. & Chan, D. C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 22, 1033–1044. https://doi.org/10.1016/j.cmet.2015.09.027 (2015).
Rahman, F. A. & Quadrilatero, J. Emerging role of mitophagy in myoblast differentiation and skeletal muscle remodeling. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2021.11.026 (2021).
Deshmukh, A. S. et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 12, 304. https://doi.org/10.1038/s41467-020-20556-8 (2021).
Deschenes, M. R. Effects of aging on muscle fibre type and size. Sports Med. 34, 809–824. https://doi.org/10.2165/00007256-200434120-00002 (2004).
Purves-Smith, F. M., Sgarioto, N. & Hepple, R. T. Fiber typing in aging muscle. Exerc. Sport Sci. Rev. 42, 45–52. https://doi.org/10.1249/JES.0000000000000012 (2014).
Vesentini, G. et al. Alterations in the structural characteristics of rectus abdominis muscles caused by diabetes and pregnancy: A comparative study of the rat model and women. PLoS One 15, e0231096. https://doi.org/10.1371/journal.pone.0231096 (2020).
Tucker, W. J., Haykowsky, M. J., Seo, Y., Stehling, E. & Forman, D. E. Impaired exercise tolerance in heart failure: Role of skeletal muscle morphology and function. Curr. Heart Fail. Rep. 15, 323–331. https://doi.org/10.1007/s11897-018-0408-6 (2018).
Shenkman, B. S. From slow to fast: Hypogravity-induced remodeling of muscle fiber myosin phenotype. Acta Nat. 8, 47–59 (2016).
Yeon, M., Choi, H., Chun, K. H., Lee, J. H. & Jun, H. S. Gomisin G improves muscle strength by enhancing mitochondrial biogenesis and function in disuse muscle atrophic mice. Biomed. Pharmacother 153, 113406. https://doi.org/10.1016/j.biopha.2022.113406 (2022).
Gallagher, P. et al. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol. Scand. 185, 61–69. https://doi.org/10.1111/j.1365-201X.2005.01457.x (2005).
Drexler, H. Skeletal muscle failure in heart failure. Circulation 85, 1621–1623. https://doi.org/10.1161/01.cir.85.4.1621 (1992).
Wilson, J. M. et al. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 26, 1724–1729. https://doi.org/10.1519/JSC.0b013e318234eb6f (2012).
Sinha, S., Elbaz-Alon, Y. & Avinoam, O. Ca(2+) as a coordinator of skeletal muscle differentiation, fusion and contraction. FEBS J. 289, 6531–6542. https://doi.org/10.1111/febs.16552 (2022).
Suwa, M., Nakano, H. & Kumagai, S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 95, 960–968. https://doi.org/10.1152/japplphysiol.00349.2003 (1985).
Lawan, A. et al. Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes 67, 624–635. https://doi.org/10.2337/db17-0826 (2018).
Villota-Narvaez, Y., Ramírez-Martínez, A., Garzón-Alvarado, D. & Röhrle, O. A dynamical model for the calcineurin-NFATc signaling pathway and muscle fiber shifting. Pamm https://doi.org/10.1002/pamm.202000274 (2021).
Ehlers, M. L., Celona, B. & Black, B. L. NFATc1 controls skeletal muscle fiber type and is a negative regulator of MyoD activity. Cell Rep. 8, 1639–1648. https://doi.org/10.1016/j.celrep.2014.08.035 (2014).
Cohen, T. J. et al. HDAC4 regulates muscle fiber type-specific gene expression programs. Mol. Cells 38, 343–348. https://doi.org/10.14348/molcells.2015.2278 (2015).
Akimoto, T. et al. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 280, 19587–19593. https://doi.org/10.1074/jbc.M408862200 (2005).
Chemello, F. et al. Transcriptomic analysis of single isolated myofibers identifies miR-27a-3p and miR-142-3p as regulators of metabolism in skeletal muscle. Cell Rep. 26(3784–3797), e8. https://doi.org/10.1016/j.celrep.2019.02.105 (2019).
Neunhauserer, D. et al. Human skeletal muscle: Transition between fast and slow fibre types. Pflugers Arch. 461, 537–543. https://doi.org/10.1007/s00424-011-0943-4 (2011).
Andruchov, O., Andruchova, O., Wang, Y. & Galler, S. Kinetic properties of myosin heavy chain isoforms in mouse skeletal muscle: Comparison with rat, rabbit, and human and correlation with amino acid sequence. Am. J. Physiol. Cell Physiol. 287, C1725–C1732. https://doi.org/10.1152/ajpcell.00255.2004 (2004).
Yan, Z., Okutsu, M., Akhtar, Y. N. & Lira, V. A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 110, 264–274. https://doi.org/10.1152/japplphysiol.00993.2010 (2011).
Westerblad, H., Bruton, J. D. & Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 316, 3093–3099. https://doi.org/10.1016/j.yexcr.2010.05.019 (2010).
Lee, S. H. & Kim, B. J. Park DR and Kim UH (2018) Exercise induces muscle fiber type switching via transient receptor potential melastatin 2-dependent Ca(2+) signaling. J. Appl. Physiol. 124, 364–373. https://doi.org/10.1152/japplphysiol.00687.2017 (1985).
Liu, J. et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol. Med. 8, 1212–1228. https://doi.org/10.15252/emmm.201606372 (2016).
Pandorf, C. E., Haddad, F., Wright, C., Bodell, P. W. & Baldwin, K. M. Differential epigenetic modifications of histones at the myosin heavy chain genes in fast and slow skeletal muscle fibers and in response to muscle unloading. Am. J. Physiol. Cell Physiol. 297, C6-16. https://doi.org/10.1152/ajpcell.00075.2009 (2009).
Kramer, A. I. & Handschin, C. How epigenetic modifications drive the expression and mediate the action of PGC-1alpha in the regulation of metabolism. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20215449 (2019).
Li, J. et al. Impact of exercise and aging on mitochondrial homeostasis in skeletal muscle: roles of ROS and epigenetics. Cells https://doi.org/10.3390/cells11132086 (2022).
Li, J., Zhang, Z., Bo, H. & Zhang, Y. Exercise couples mitochondrial function with skeletal muscle fiber type via ROS-mediated epigenetic modification. Free Radic. Biol. Med. 213, 409–425. https://doi.org/10.1016/j.freeradbiomed.2024.01.036 (2024).
Widmann, M., Niess, A. M. & Munz, B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 49, 509–523. https://doi.org/10.1007/s40279-019-01070-4 (2019).
Lochmann, T. L., Thomas, R. R., Bennett, J. P. Jr. & Taylor, S. M. Epigenetic modifications of the PGC-1alpha promoter during exercise induced expression in mice. PLoS One 10, e0129647. https://doi.org/10.1371/journal.pone.0129647 (2015).
Barres, R. et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15, 405–411. https://doi.org/10.1016/j.cmet.2012.01.001 (2012).
Shimizu, J. & Kawano, F. Exercise-induced histone H3 trimethylation at lysine 27 facilitates the adaptation of skeletal muscle to exercise in mice. J. Physiol. 600, 3331–3353. https://doi.org/10.1113/JP282917 (2022).
Gongol, B., Sari, I., Bryant, T., Rosete, G. & Marin, T. AMPK: an epigenetic landscape modulator. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19103238 (2018).
Wan, L. et al. Phosphorylation of EZH2 by AMPK suppresses PRC2 methyltransferase activity and oncogenic function. Mol. Cell 69(279–291), e5. https://doi.org/10.1016/j.molcel.2017.12.024 (2018).
Demoinet, E., Li, S. & Roy, R. AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A 114, E2689–E2698. https://doi.org/10.1073/pnas.1616171114 (2017).
Eissenberg, J. C. & Shilatifard, A. Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev. Biol. 339, 240–249. https://doi.org/10.1016/j.ydbio.2009.08.017 (2010).
Wang, T. et al. O-GlcNAcylation of fumarase maintains tumour growth under glucose deficiency. Nat. Cell Biol. 19, 833–843. https://doi.org/10.1038/ncb3562 (2017).
Weir, H. J. et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 26(884–896), e5. https://doi.org/10.1016/j.cmet.2017.09.024 (2017).
Wen, W. et al. Lycopene increases the proportion of slow-twitch muscle fiber by AMPK signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 94, 108750. https://doi.org/10.1016/j.jnutbio.2021.108750 (2021).
Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP and Louzada RA (2021) Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants (Basel) 10. https://doi.org/10.3390/antiox10040537
Moreillon, M. et al. Hybrid fiber alterations in exercising seniors suggest contribution to fast-to-slow muscle fiber shift. J. Cachexia Sarcopenia Muscle 10, 687–695. https://doi.org/10.1002/jcsm.12410 (2019).
Ye, F., Wu, Y., Chen, Y., Xiao, D. & Shi, L. Impact of moderate- and high-intensity exercise on the endothelial ultrastructure and function in mesenteric arteries from hypertensive rats. Life Sci. 222, 36–45. https://doi.org/10.1016/j.lfs.2019.01.058 (2019).
Wyart, E. et al. Iron supplementation is sufficient to rescue skeletal muscle mass and function in cancer cachexia. EMBO Rep. 23, e53746. https://doi.org/10.15252/embr.202153746 (2022).
Al-Zubaidi, U. et al. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum. Reprod. 36, 771–784. https://doi.org/10.1093/humrep/deaa300 (2021).
Schroeder, P. et al. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic. Biol. Med. 43, 128–135. https://doi.org/10.1016/j.freeradbiomed.2007.04.002 (2007).
Yardeni, T. et al. An mtDNA mutant mouse demonstrates that mitochondrial deficiency can result in autism endophenotypes. Proc. Natl. Acad. Sci. U S A https://doi.org/10.1073/pnas.2021429118 (2021).
Bo, H. et al. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: Role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic. Biol. Med. 44, 1373–1381. https://doi.org/10.1016/j.freeradbiomed.2007.12.033 (2008).
Napa, K. et al. LPS from P. gingivalis negatively alters gingival cell mitochondrial bioenergetics. Int. J. Dent. https://doi.org/10.1155/2017/2697210 (2017).
Zhang, Y. et al. Activation of sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: implications for blood-brain barrier integrity in stroke. Transl. Stroke Res. 11, 267–287. https://doi.org/10.1007/s12975-019-00711-0 (2020).
Yan, X. et al. Nrf2 contributes to the benefits of exercise interventions on age-related skeletal muscle disorder via regulating Drp1 stability and mitochondrial fission. Free Radic. Biol. Med. 178, 59–75. https://doi.org/10.1016/j.freeradbiomed.2021.11.030 (2022).
Chang, C. et al. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell 60, 930–940. https://doi.org/10.1016/j.molcel.2015.10.037 (2015).
Hargreaves, M. & Spriet, L. L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2, 817–828. https://doi.org/10.1038/s42255-020-0251-4 (2020).
Jung, S. & Kim, K. Exercise-induced PGC-1alpha transcriptional factors in skeletal muscle. Integr. Med. Res. 3, 155–160. https://doi.org/10.1016/j.imr.2014.09.004 (2014).
Luo, Y., Zhang, L., Su, N., Liu, L. & Zhao, T. YME1L-mediated mitophagy protects renal tubular cells against cellular senescence under diabetic conditions. Biol. Res. 57, 10. https://doi.org/10.1186/s40659-024-00487-0 (2024).
Son, Y. H. et al. Comparative molecular analysis of endurance exercise in vivo with electrically stimulated in vitro myotube contraction. J. Appl. Physiol. 127, 1742–1753. https://doi.org/10.1152/japplphysiol.00091.2019 (2019).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837. https://doi.org/10.1016/j.cell.2007.05.009 (2007).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560. https://doi.org/10.1038/nature06008 (2007).
Blanco, E., Gonzalez-Ramirez, M., Alcaine-Colet, A., Aranda, S. & Di Croce, L. The bivalent genome: characterization, structure, and regulation. Trends Genet. 36, 118–131. https://doi.org/10.1016/j.tig.2019.11.004 (2020).
Rabinovitch, R. C. et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell. Rep. 21, 1–9. https://doi.org/10.1016/j.celrep.2017.09.026 (2017).
Lantier, L. et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 28, 3211–3224. https://doi.org/10.1096/fj.14-250449 (2014).
Aloia, L., Di Stefano, B. & Di Croce, L. Polycomb complexes in stem cells and embryonic development. Development 140, 2525–2534. https://doi.org/10.1242/dev.091553 (2013).
Chi, P., Allis, C. D. & Wang, G. G. Covalent histone modifications–miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. Cancer 10, 457–469. https://doi.org/10.1038/nrc2876 (2010).
Tang, H. et al. Down-regulation of HSP60 suppresses the proliferation of glioblastoma cells via the ROS/AMPK/mTOR pathway. Sci. Rep. 6, 28388. https://doi.org/10.1038/srep28388 (2016).
Mok, J., Park, J. H., Yeom, S. C. & Park, J. PROKR1-CREB-NR4A2 axis for oxidative muscle fiber specification and improvement of metabolic function. Proc. Natl. Acad. Sci. U S A 121, e2308960121. https://doi.org/10.1073/pnas.2308960121 (2024).
Ahn, J. S. et al. Ectopic overexpression of porcine MYH1 increased in slow muscle fibers and enhanced endurance exercise in transgenic mice. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19102959 (2018).
Zeng, Z. et al. Cold stress induces enhanced chromatin accessibility and bivalent histone modifications H3K4me3 and H3K27me3 of active genes in potato. Genome Biol. 20, 123. https://doi.org/10.1186/s13059-019-1731-2 (2019).
Granata, C., Jamnick, N. A. & Bishop, D. J. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 48, 1809–1828. https://doi.org/10.1007/s40279-018-0936-y (2018).
Zoladz, J. A., Koziel, A., Woyda-Ploszczyca, A., Celichowski, J. & Jarmuszkiewicz, W. Endurance training increases the efficiency of rat skeletal muscle mitochondria. Pflugers Arch. 468, 1709–1724. https://doi.org/10.1007/s00424-016-1867-9 (2016).
Barron-Cabrera, E. et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: A systematic review. Lifestyle Genom. 12, 25–44. https://doi.org/10.1159/000503289 (2019).
Ahmetov, I. I., Vinogradova, O. L. & Williams, A. G. Gene polymorphisms and fiber-type composition of human skeletal muscle. Int. J. Sport Nutr. Exerc. Metab. 22, 292–303. https://doi.org/10.1123/ijsnem.22.4.292 (2012).
Bathgate, K. E. et al. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur. J. Appl. Physiol. 118, 2097–2110. https://doi.org/10.1007/s00421-018-3943-7 (2018).
Smith, J. A. B., Murach, K. A., Dyar, K. A. & Zierath, J. R. Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 24, 607–632. https://doi.org/10.1038/s41580-023-00606-x (2023).
Huxley, A. F. Mechanics and models of the myosin motor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 433–440. https://doi.org/10.1098/rstb.2000.0584 (2000).
Gan, Z. et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J. Clin. Invest. 123, 2564–2575. https://doi.org/10.1172/JCI67652 (2013).
Seaborne, R. A. & Sharples, A. P. The interplay between exercise metabolism, epigenetics, and skeletal muscle remodeling. Exerc. Sport Sci. Rev. 48, 188–200. https://doi.org/10.1249/JES.0000000000000227 (2020).
Jiang, Y. et al. Histone H3K27 methyltransferase EZH2 and demethylase JMJD3 regulate hepatic stellate cells activation and liver fibrosis. Theranostics 11, 361–378. https://doi.org/10.7150/thno.46360 (2021).
Ziemann, M., Hargreaves, M., Harikrishnan, K., Khurana, I., Kaspi, A., Rafehi, H., Flores-Opazo, M., Garnham, A. & El-Osta, A. Endurance exercise stimulates rapid changes in promoter histone acetylation in human muscle.
Liu, L. et al. Histone methyltransferase MLL4 controls myofiber identity and muscle performance through MEF2 interaction. J, Clin. Invest. 130, 4710–4725. https://doi.org/10.1172/JCI136155 (2020).
Gulri, M.K. (2020). Investigating the role of selected epigenetic silencing marks in determining mouse skeletal muscle oxidative phenotype.
Hughes, A. L., Kelley, J. R. & Klose, R. J. Understanding the interplay between CpG island-associated gene promoters and H3K4 methylation. Biochim. Biophys. Acta Gene Regul. Mech. 1863, 194567. https://doi.org/10.1016/j.bbagrm.2020.194567 (2020).
Weaver, A. E. The Beneficial Impact of Exercise on Mechanisms of Neurodegeneration: Potential Therapeutic Approach for Multiple Sclerosis (Kent State University, Kent, 2021).
Hancock, R. L., Masson, N., Dunne, K., Flashman, E. & Kawamura, A. The Activity of JmjC histone lysine demethylase KDM4A is highly sensitive to oxygen concentrations. ACS Chem. Biol. 12, 1011–1019. https://doi.org/10.1021/acschembio.6b00958 (2017).
Ristow, M. et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U S A 106, 8665–8670. https://doi.org/10.1073/pnas.0903485106 (2009).
Jiang, N. et al. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: A potential antioxidant function. Free Radic. Biol. Med 46, 138–145. https://doi.org/10.1016/j.freeradbiomed.2008.09.026 (2009).
Thirupathi, A., Pinho, R. A. & Chang, Y. Z. Physical exercise: An inducer of positive oxidative stress in skeletal muscle aging. Life Sci. 252, 117630. https://doi.org/10.1016/j.lfs.2020.117630 (2020).
Emerling, B. M. et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 46, 1386–1391. https://doi.org/10.1016/j.freeradbiomed.2009.02.019 (2009).
Zmijewski, J. W. et al. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 285, 33154–33164. https://doi.org/10.1074/jbc.M110.143685 (2010).
Campos, J. C. et al. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc. Natl. Acad. Sci. U S A 120, e2204750120. https://doi.org/10.1073/pnas.2204750120 (2023).
Shuvo, S. R., Wiens, L. M., Subramaniam, S., Treberg, J. R. & Court, D. A. Increased reactive oxygen species production and maintenance of membrane potential in VDAC-less Neurospora crassa mitochondria. J. Bioenerg. Biomembr. 51, 341–354. https://doi.org/10.1007/s10863-019-09807-6 (2019).
Li, N. et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525. https://doi.org/10.1074/jbc.M210432200 (2003).
Agius, L., Ford, B. E. & Chachra, S. S. The Metformin mechanism on gluconeogenesis and AMPK activation: the metabolite perspective. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21093240 (2020).
Stephenne, X. et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54, 3101–3110. https://doi.org/10.1007/s00125-011-2311-5 (2011).
Hinchy, E. C. et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 293, 17208–17217. https://doi.org/10.1074/jbc.RA118.002579 (2018).
Wu, M. et al. Hepatic AMPK signaling activation in response to dynamic REDOX balance is a biomarker of exercise to improve blood glucose control. Elife 11, e79939 (2022).
Abdelkader, N. F., Farid, H. A., Youness, E. R., Abdel-Salam, O. M. E. & Zaki, H. F. The role of KATP channel blockade and activation in the protection against neurodegeneration in the rotenone model of Parkinson’s disease. Life Sci. 257, 118070. https://doi.org/10.1016/j.lfs.2020.118070 (2020).
Landau, R. et al. The rat rotenone model reproduces the abnormal pattern of central catecholamine metabolism found in Parkinson’s disease. Dis. Model Mech. https://doi.org/10.1242/dmm.049082 (2022).
Goldstein, D. S. et al. Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson’s disease. J. Neurochem. 133, 14–25. https://doi.org/10.1111/jnc.13042 (2015).
Fleming, S. M. et al. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp. Neurol. 187, 418–429. https://doi.org/10.1016/j.expneurol.2004.01.023 (2004).
Richter, F., Hamann, M. & Richter, A. Chronic rotenone treatment induces behavioral effects but no pathological signs of Parkinsonism in mice. J. Neurosci. Res. 85, 681–691. https://doi.org/10.1002/jnr.21159 (2007).
Shaikh, S. B. & Nicholson, L. F. Effects of chronic low dose rotenone treatment on human microglial cells. Mol. Neurodegener. 4, 55. https://doi.org/10.1186/1750-1326-4-55 (2009).
Sotzny, F. et al. TCF11/Nrf1-mediated induction of proteasome expression prevents cytotoxicity by Rotenone. Antioxid. Redox Signal 25, 870–885. https://doi.org/10.1089/ars.2015.6539 (2016).
Forkink, M. et al. Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochim. Biophys. Acta 1837, 1247–1256. https://doi.org/10.1016/j.bbabio.2014.04.008 (2014).
Hou, W. L. et al. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell Mol. Med. 22, 1316–1328. https://doi.org/10.1111/jcmm.13432 (2018).
Lee, T. Y. Lactate: a multifunctional signaling molecule. Yeungnam Univ. J. Med. 38, 183–193. https://doi.org/10.12701/yujm.2020.00892 (2021).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. https://doi.org/10.1038/s41586-019-1678-1 (2019).
Brooks, G. A. Cell-cell and intracellular lactate shuttles. J. Physiol. 587, 5591–5600. https://doi.org/10.1113/jphysiol.2009.178350 (2009).
Marin, T. L. et al. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci. Signal https://doi.org/10.1126/scisignal.aaf7478 (2017).
Chomiak, A. A. et al. Select EZH2 inhibitors enhance viral mimicry effects of DNMT inhibition through a mechanism involving NFAT:AP-1 signaling. Sci. Adv. 10, eadk4423. https://doi.org/10.1126/sciadv.adk4423 (2024).
Shi, W. et al. Histone deacetylase 4 mediates high glucose-induced podocyte apoptosis via upregulation of calcineurin. Biochem. Biophys. Res. Commun. 533, 1061–1068. https://doi.org/10.1016/j.bbrc.2020.09.121 (2020).
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32071177, 31771320, and 31571224), the Tianjin Scientific Research Foundation (23JCYBJC00870), the Military Scientific Research Foundation (WJBWS23J2005), and the Tianjin Research Innovation Project for Postgraduate Students (No. 2021YJSB375).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jialin Li, Ziyi Zhang, Sheng Zhang, Can Li, Xiaoxia Zhang and Yuhui Shan. The first draft of the manuscript was written by Jialin Li, Hai Bo and Yong Zhang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All the authors have read the manuscript and consented for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal study was reviewed and approved by Ethics Committee of Tianjin University of Sport (TJUS 2022–30).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Zhang, S., Li, C. et al. Endurance exercise-induced histone methylation modification involved in skeletal muscle fiber type transition and mitochondrial biogenesis. Sci Rep 14, 21154 (2024). https://doi.org/10.1038/s41598-024-72088-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72088-6
- Springer Nature Limited