Abstract
This study aims to evaluate the neutrophil-to-lymphocyte ratio (NLR) as a predictive biomarker for cardiovascular mortality among cancer patients, utilizing data from the National Health and Nutrition Examination Survey (NHANES). From the NHANES dataset (2007–2018), we analyzed 4974 cancer survivors, investigating the prognostic significance of NLR for all-cause, cardiovascular, and cancer-specific mortality. Survival outcomes were analyzed using Cox regression and Kaplan–Meier methods. Optimal NLR cutoffs were identified as 2.61 for differentiating the higher NLR group from lower NLR group. Elevated NLR levels significantly correlated with increased all-cause mortality (HR 1.11, 95% CI 1.07–1.14, P < 0.001) and cardiovascular mortality (HR 1.14, 95% CI 1.08–1.21, P < 0.001) in adjusted models. Subgroup analyses revealed that age, sex, smoking status, and hypertension significantly influence NLR’s association with cardiovascular mortality. Specific cancers including breast, prostate, non-melanoma skin, colon and melanoma experience increased all-cause and cardiovascular mortality in the higher NLR group compared to lower NLR group. Elevated NLR is a significant predictor of increased mortality in cancer patients, particularly for cardiovascular outcomes. These findings support that NLR acts as a pivotal prognostic tool with significant implications for clinical practice in the realm of cardio-oncology.
Similar content being viewed by others
Introduction
The confluence of oncology and cardiology, known as cardio-oncology, has emerged as a critical field of investigation amidst the rising tide of cancer survivorship and the attendant surge in long-term cardiovascular complications1. While advancements in cancer treatments have extended lives, they have also introduced a spectrum of cardiotoxic effects, particularly through chemotherapy and radiation therapy2. Despite significant strides in delineating the cardiovascular risks facing cancer patients, a glaring lacuna persists in the identification of precise predictors or biomarkers of cardiac mortality within this cohort. This gap highlights an urgent need for innovative methodologies to predict and attenuate the cardiovascular impact of cancer therapies3,4.
The neutrophil-to-lymphocyte ratio (NLR), a straightforward and readily accessible inflammatory marker, has garnered attention for its prognostic significance across various clinical settings5,6. Its elevation is consistently associated with poor outcomes in a range of conditions, from cardiovascular diseases to diverse cancers, underscoring its role in systemic inflammation and immune response modulation7,8,9. While the prognostic value of NLR in predicting overall mortality in contexts such as breast cancer and coronary heart disease is established, its potential in forecasting cardiac mortality among cancer patients remains underexplored5,10.
Considering the intricate interplay between inflammation, cancer, and cardiovascular disease, this study posits that NLR could be a pivotal biomarker for identifying cancer patients at an increased risk of cardiac mortality. By harnessing data from the National Health and Nutrition Examination Survey (NHANES), this retrospective cohort study aims to illuminate the association between NLR and cardiac mortality in cancer patients, thus seeking to bridge a critical knowledge gap in cardio-oncology. This endeavor not only furthers our understanding of the biomarkers that could guide clinical decision-making but also heralds a step towards personalized medicine, where indicators like NLR could inform intervention strategies, optimizing outcomes at the nexus of cancer treatment and cardiovascular risk management.
Methods
Study population
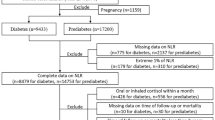
The study utilized data from NHANES, a comprehensive research program conducted by the Centers for Disease Control and Prevention (CDC) aimed at assessing the health and nutritional status of the American population through interviews, examinations, dietary, and laboratory data11,12,13,14,15. The NHANES protocol was approved by the Institutional Review Board of the National Center for Health Statistics, adhering to the Declaration of Helsinki, with informed consent obtained from all participants. Our analysis encompassed NHANES public data files spanning from 1999 to 2018. From an initial dataset of 108,858 participants, 64,956 individuals with available survival data were identified. Specifically focusing on cancer survivors, we selected 5661 participants, excluding those lacking NLR data (n = 687), culminating in a cohort of 4974 cancer survivors for our study (Fig. 1). Cancer diagnoses were self-reported, confirmed by a physician’s diagnosis, with cancer type further categorized based on participant responses. Complete blood count tests, utilizing Beckman Coulter methodology, provided the necessary data for calculating the NLR by dividing the neutrophil count by the lymphocyte count. The linkage of NHANES participants to death certificate records from the National Death Index facilitated follow-up for mortality outcomes, with follow-up duration measured in person-months from the interview date until death, loss to follow-up, or the end of the study period on December 31, 2019. Cause of death was classified using International Statistical Classification of Diseases, 10th Revision (ICD-10) codes for cardiovascular deaths (I00-I09, I11, I13, and I20-I51) and cancer-specific mortality (C00-C97)16.
Covariates
Demographic and clinical information, including age, gender, body mass index (BMI), smoking history, and race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race), were collected. Educational attainment was categorized into less than high school, high school graduate, some college or associate degree, and college graduate. Marital status was classified into married or living with a partner, never married or separated or divorced or widowed. The ratio of family income to poverty level was categorized as < 1, 1 to 3, or > 3. Pre-existing comorbidities, including diabetes mellitus (DM), coronary heart disease (CHD), heart failure (HF), hypertension (HBP), high cholesterol, stroke, chronic bronchitis, and liver diseases, were identified based on physician-diagnosed conditions documented in the NHANES data.
Statistical analysis
We accounted for the complex survey design of NHANES by incorporating sample weights, clustering and stratification. To achieve nationally representative estimates, the original survey weights were adjusted and utilized in the analysis, taking into account the appropriate adjustments17. Data analysis was conducted using R software version 4.3.1. Categorical variables were analyzed using Rao-Scott adjusted Chi-square test and continuous variables were analyzed using weighted mean comparisons. The optimal NLR cutoff point corresponding to the most significant association with survival outcomes was obtained by maximally selected rank statistics based on the ‘maxstat’ package (https://CRAN.R-project.org/package=maxstat)18,19, which were then used to separate participants into higher- and lower-NLR groups. The association of the NLR with all-cause mortality, cardiovascular mortality and cancer-specific mortality among participants was assessed by survey-weighted Cox regression analysis. Two models were constructed to adjust for possible confounding factors. Model 1 was adjusted for gender, age, race, marital status, educational level, family income level, smoke history. Model 2 was additionally adjusted for the presence of comorbidities such as DM, CHD, HF, HBP, high cholesterol, stroke, chronic bronchitis and liver diseases. Restricted cubic spline (RCS) with four knots was adopted to visualize the potentially nonlinear association between the NLR and all-cause mortality, cardiovascular mortality and cancer-specific mortality. The probabilities of survival outcomes were calculated according to the Kaplan–Meier method and compared using the log-rank test. The association of NLR values with mortality was analyzed by using subgroups based on age, sex, smoking status and HBP, and their interactions were explored. A two-tailed p < 0.05 indicated statistical significance. In sensitivity analysis, we excluded participants with a follow-up duration of less than 1 year, as well as those who died due to accidental events. Due to the favorable prognosis of non-melanoma skin cancer, we also removed subjects with non-melanoma skin cancer and re-analyzed the data accordingly.
Results
Characteristics of the study population
In this retrospective cohort study, a total of 4974 cancer survivors were analyzed, representing an extrapolated patient population of 21,256,627 in the United States. Participants were stratified into two groups based on the optimal NLR cutoff value of 2.61, identified through maximally selected rank statistics. Those with NLR > 2.61 comprised the higher NLR group (n = 1836), while those with NLR ≤ 2.61 formed the lower NLR group (n = 3138). The higher NLR group exhibited a greater proportion of older individuals, males and non-Hispanic whites. Additionally, this group showed a higher prevalence of marital status categorized as never married, widowed, divorced, or separated. Comorbidities such as HF, CHD, DM, stroke and HBP were more prevalent in the higher NLR group. No significant differences were observed between the groups in terms of BMI, educational level, smoking history and other comorbidities, including high cholesterol, chronic bronchitis, and liver diseases. Detailed cancer type distribution and additional characteristics are presented in Table 1.
Association of NLR with mortality outcomes
During a median follow-up of 7.66 years (Interquartile Range [IQR] 3.91–12.50 years), 1841 (37.1%) of the 4974 cancer participants died. This included 401 (8.1%) cardiovascular disease deaths and 539 (10.8%) cancer-specific deaths. In the unadjusted model, increased NLR values were significantly associated with higher risks of all-cause mortality (Hazard Ratio [HR] 1.21, 95% Confidence Interval [CI] 1.15–1.25, P < 0.001), cardiovascular mortality (HR 1.23, 95% CI 1.16–1.31, P < 0.001), and cancer-specific mortality (HR 1.15, 95% CI 1.09–1.21, P < 0.001). Multivariate adjustment revealed that each unit increase in NLR was correlated with an 11% increase (Model 1, HR 1.11, 95% CI 1.07–1.14, P < 0.001) and a 10% increase (Model 2, HR 1.10, 95% CI 1.07–1.13, P < 0.001) in the risk of all-cause mortality. Similarly, each unit increase in NLR was associated with a 14% (Model 1, HR 1.14, 95% CI 1.08–1.21, P < 0.001) and 12% (Model 2, HR 1.12, 95% CI 1.06–1.18, P < 0.001) increased risk of cardiovascular mortality. For cancer-specific mortality, the increases were 7% (Model 1, HR 1.07, 95% CI 1.01–1.12, P = 0.014) and 6% (Model 2, HR 1.06, 95% CI 1.01–1.12, P = 0.023), respectively (Table 2).
The higher NLR group showed a significant elevation in the risk for all-cause mortality, from the unadjusted model (HR 2.02, 95% CI 1.73–2.36, P < 0.001) to the adjusted models (Models 1 and 2: HR 1.44, 95% CI 1.27–1.63, P < 0.001; HR 1.38, 95% CI 1.22–1.55, P < 0.001). A similar trend was observed for cardiovascular mortality (unadjusted HR 2.72, 95% CI 2.11–3.52, P < 0.001; Model 1 HR 1.74, 95% CI 1.36–2.22, P < 0.001; Model 2 HR 1.63, 95% CI 1.27–2.09, P < 0.001) and cancer-specific mortality (unadjusted HR 1.92, 95% CI 1.54–2.41, P < 0.001; Model 1 HR 1.48, 95% CI 1.19–1.86, P < 0.001; Model 2 HR 1.45, 95% CI 1.17–1.79, P < 0.001) (Table 2).
Nonlinear associations and survival rates
RCS analysis indicated a nonlinear relationship between NLR and all-cause mortality, cardiovascular mortality, cancer-specific mortality among cancer participants (P for nonlinearity = 0.002, 0.042, and < 0.001, respectively) (Fig. 2). Kaplan–Meier survival analysis demonstrated significantly lower survival rates for all-cause mortality, cardiovascular mortality, and cancer-specific mortality in the higher NLR group compared to the lower NLR group (P < 0.001) (Fig. 3).
The association of NLR with all-cause (A), cardiovascular mortality (B) and cancer-specific mortality (C) among cancer participants visualized by restricted cubic spline (NLR breakpoint:1.42,2.25,1.66). Hazard ratios were adjusted for gender, age, race, marital status, educational level, family income level, smoke history and presence of comorbidities including DM, CHD, HF, HBP, high cholesterol, stroke, chronic bronchitis, liver diseases. HF heart failure, CHD coronary heart disease, HBP hypertension, DM Diabetes.
For breast cancer, all-cause mortality (P < 0.001) and cardiovascular mortality (P < 0.001) were higher in the higher-NLR group than the lower-NLR group, while there is no difference in cancer-specific mortality (P = 0.086) between the higher-NLR group and the lower-NLR group (Fig. S1). For prostate cancer, all-cause mortality (P < 0.001) and cardiovascular mortality (P = 0.003) were higher in the higher-NLR group than the lower-NLR group, while there is no difference in cancer-specific mortality (P = 0.612) between the higher-NLR group the lower-NLR group (Fig. S2). For non-melanoma skin cancer, all-cause mortality (P < 0.001), cardiovascular mortality (P < 0.001) and cancer-specific mortality(P = 0.001) were higher in the higher-NLR group than the lower-NLR group (Fig. S3). For colon cancer, all-cause mortality (P = 0.004) was higher in the higher-NLR group than the lower-NLR group, while there is no difference in cardiovascular mortality (P = 0.271) and cancer-specific mortality (P = 0.108) between the higher-NLR group the lower-NLR group (Fig. S4). For Melanoma, all-cause mortality (P < 0.001), cardiovascular mortality (P < 0.001) and cancer-specific mortality(P = 0.012) were higher in the higher-NLR group than the lower-NLR group (Fig. S5).
Subgroup analyses of the association between NLR and cardiovascular mortality
We also investigated the association of NLR levels with cardiovascular mortality by using subgroup analysis based on age, sex, smoking status and whether had HBP (Table 3). The subgroup analysis demonstrated significant variations in the association between NLR and cardiovascular mortality across various subgroups. A significant association was observed in participants over 70 years (P < 0.001) but not in those under 70 (P = 0.184). Notably, this association was significant in men (P < 0.001) but not in women (P = 0.211). Smoking status, also showed variability, with a significant association in ever smoker (P = 0.003), but not in individuals who never smoke (P = 0.064). The association was significant in participants with HBP (P < 0.001), but not in those without HBP (P = 0.081). There was also no significant interaction between the aforementioned characteristics and the NLR for cardiovascular mortality (P interaction > 0.05).
Sensitivity analysis
In sensitivity analyses, where participants with follow-up time less than 1 year and those who died from accidental causes and non-melanoma skin cancer were excluded, leaving 3677 subjects. The characteristics of these participants are shown in Supplementary table 1. The NLR was still an independent risk factor for all-cause mortality and cardiovascular mortality from the crude model to the adjusted models (Models 1 and 2). The risk for all-cause mortality and cardiovascular mortality also significantly increased in the higher-NLR group compared with lower-NLR group from the crude model to the adjusted models (Models 1 and 2).NLR was still an independent risk factor for cancer-specific mortality in the crude model, but NLR was not associated with increased cancer-specific mortality in the adjusted models (Models 1 and 2), although Cox regression analysis showed that the risk for cancer-specific mortality significantly increased in the higher-NLR group compared with lower-NLR group from the crude model to the adjusted models (Models 1 and 2) (Supplementary Table 2).
Discussion
NLR stands as a critical biomarker, reflecting the balance between inflammatory response and immune function, with elevated NLR indicating systemic inflammation and potential immune suppression. Our study presents compelling evidence that the NLR serves as a significant prognostic marker for cardiovascular mortality among cancer patients, underscoring a novel intersection between oncology and cardiology. By leveraging a comprehensive analysis of data from NHANES, we have elucidated the independent predictive power of elevated NLR levels for increased risks of all-cause mortality, with a special focus on cardiovascular mortality within this demographic. This finding is particularly groundbreaking, as it extends the utility of NLR from its recognized role in predicting overall mortality and cancer-specific outcomes to highlighting its relevance in foreseeing cardiovascular complications in cancer survivors. Despite the well-documented cardiotoxic effects of various cancer treatments, there has been a paucity of reliable biomarkers for predicting cardiovascular outcomes in this vulnerable population. Our study addresses this gap by demonstrating that NLR, a simple and readily available inflammatory marker, can effectively identify cancer participants at higher risk of cardiovascular death. This not only enriches our understanding of the interplay between cancer, inflammation, and cardiovascular disease but also opens new avenues for preventative strategies and interventions aimed at reducing cardiovascular morbidity and mortality among cancer survivors.
Specifically, non-Hispanic White individuals were more prevalent in the higher NLR group, while those who were never married, widowed, divorced, or separated were also more likely to have a higher NLR. These findings suggest potential interplay between sociodemographic factors, inflammation, and cardiovascular risk in cancer survivors. Previous studies have explored the association between race and NLR, with some indicating higher NLR levels in non-Hispanic White populations compared to other racial groups20. Similarly, the association between marital status and NLR has been investigated, with studies suggesting that unmarried individuals, particularly those who are widowed or divorced, may exhibit higher levels of inflammation21. This could be related to psychosocial stressors, reduced social support, and lifestyle factors associated with marital status. Several studies have previously highlighted NLR’s value in predicting overall and disease-specific mortality, particularly within oncology and cardiology disciplines22,23,24,25,26. Consistent with the broader scientific consensus, our findings reaffirm NLR’s significant prognostic utility, notably extending its relevance to the specific context of cardiovascular mortality among cancer patients. This alignment with existing literature underscores the robustness of NLR as a marker of systemic inflammation and its associated adverse outcomes across diverse patient populations. Furthermore, variations in study designs, methodologies, and patient demographics across the existing research landscape have contributed to differing magnitudes of risk associated with NLR. Our study's utilization of the NHANES dataset, known for its diverse and representative sample of the American population, provides a comprehensive backdrop against which the predictive value of NLR for cardiovascular mortality in cancer patients can be more accurately gauged. This approach enhances the generalizability of our findings and offers valuable insights into the potential for NLR to guide clinical decision-making in a broad spectrum of patient care scenarios.
The results from our RCS analysis reveal a nuanced, nonlinear relationship between the NLR and various mortality outcomes (all-cause, cardiovascular, and cancer) among cancer patients. This nonlinearity suggests that the risk associated with NLR levels does not increase in a straightforward, proportional manner across the spectrum of NLR values. Instead, the risk of mortality exhibits distinct phases of acceleration and plateau, implying that the impact of systemic inflammation indicated by NLR on mortality risks is more complex than previously understood. The identified NLR cutoff values-1.42 for all-cause mortality, 2.25 for cardiovascular mortality, and 1.66 for cancer-specific mortality—underscore this complexity, indicating that the level of systemic inflammation predictive of mortality risk varies significantly depending on the type of mortality considered. These cutoffs illuminate critical thresholds beyond which the risk of death sharply increases, providing valuable insights for clinicians. Specifically, they highlight the importance of closely monitoring and potentially intervening in participants who exhibit NLR levels beyond these thresholds, given their heightened risk of death from all causes, cardiovascular events, and cancer. The variance in NLR cutoff values across different mortality outcomes further emphasizes the multifaceted role of inflammation in the pathophysiology of these conditions. It suggests that while systemic inflammation broadly contributes to mortality risk, the extent of its impact and the mechanisms through which it operates may differ significantly between cardiovascular and cancer-related deaths27. The findings from our study highlight NLR not only as a biomarker of systemic inflammation but also as a pivotal prognostic tool with significant implications for clinical practice in the realm of cardio-oncology. The demonstrated predictive value of NLR for cardiovascular mortality among cancer participants underscores the necessity of integrating this simple, cost-effective marker into existing risk assessment models. By doing so, clinicians can more accurately identify cancer participants who are at an elevated risk of cardiovascular complications, enabling the implementation of tailored monitoring and intervention strategies.
Our subgroup analysis investigating the association between NLR and cardiovascular mortality reveals insightful variations across different demographic and clinical subgroups, suggesting that the prognostic value of NLR is influenced by specific patient characteristics28,29. Notably, a significant relationship between elevated NLR and increased cardiovascular mortality risk was observed in participants aged over 70 years, indicating that older individuals may be particularly sensitive to the cardiovascular risks associated with systemic inflammation. Conversely, this association was not evident in the younger cohort (under 70 years), suggesting age-specific mechanisms by which NLR impacts cardiovascular risk. Fest et al. found the NLR’s broad applicability as a risk indicator for mortality in the elderly30. Furthermore, the analysis underscores gender differences in the predictive value of NLR, with a significant association found in men but not in women. This distinction could reflect underlying differences in the pathophysiology of cardiovascular or in the inflammatory profiles between genders. The influence of smoking status on the NLR-cardiovascular mortality relationship further highlights the complex interplay between lifestyle factors, systemic inflammation, and cardiovascular risk, with smokers showing a significant association, possibly due to the additive effects of smoking-induced inflammation. Moreover, the presence of HBP amplifies the association between NLR and cardiovascular mortality, suggesting that systemic inflammation in the context of existing cardiovascular stressors like hypertension substantially increases the risk. This finding emphasizes the importance of monitoring and managing inflammation particularly in HBP participants to mitigate their heightened cardiovascular risk. The lack of significant interaction between these characteristics and NLR for cardiovascular mortality suggests that while NLR is an independent predictor of cardiovascular mortality, its predictive power is modulated by age, sex, smoking status, and the presence of hypertension. These results highlight the necessity for a nuanced approach in evaluating and utilizing NLR as a prognostic tool in clinical practice, tailoring cardiovascular risk assessment and management to the individual patient's demographic and clinical profile.
Our sensitivity analyses, after excluding participants with less than one year of follow-up and those who succumbed to accidental causes or non-melanoma skin cancer, further solidify the NLR as a potent independent risk factor for all-cause and cardiovascular mortality. The refinement of our participant pool to 3677 subjects allowed for a more focused examination of NLR’s predictive validity across different mortality outcomes. Notably, the association between higher NLR values and increased risks for both all-cause and cardiovascular mortality remained robust across both crude and adjusted models, reinforcing the utility of NLR as a critical marker for identifying individuals at elevated risk of cardiovascular mortality. Interestingly, while NLR emerged as an independent risk factor for cancer-specific mortality in the crude analysis, this association was attenuated upon adjustment for potential confounders in the subsequent models. Fest et al. found that higher NLR levels were independently associated with an increased risk of all-cause mortality, cardiovascular mortality, and other mortality, but not cancer mortality30. This nuanced finding suggests that, although elevated NLR levels are indicative of an increased risk of cancer-specific mortality in a broad sense, the relationship may be influenced or masked by other variables in more complex models of analysis.
Our analysis across different cancer types reveals a nuanced view of how NLR interacts with mortality outcomes. Notably, for specific cancers such as breast, prostate, non-melanoma skin, colon, and melanoma, a consistent pattern emerges where higher NLR groups experience increased all-cause and cardiovascular mortality compared to those in the lower NLR group, underscoring NLR’s role as a broad indicator of risk across these diverse conditions. However, the relationship between NLR and cancer-specific mortality presents a more complex picture. In breast and prostate cancer participants, there was no statistically significant difference in cancer-specific mortality between the higher and lower NLR groups, suggesting that while NLR is a potent marker for overall health risk, its predictive value for cancer-specific mortality may be influenced by cancer type or stage. Conversely, for non-melanoma skin cancer and melanoma, elevated NLR was associated with increased cancer-specific mortality, indicating a stronger link between systemic inflammation and cancer progression or fatality in these contexts. Interestingly, in colon cancer patients, we observed an increase in all-cause mortality in the higher NLR group without a corresponding increase in cardiovascular or cancer-specific mortality. This might suggest that other factors, possibly related to systemic inflammation or comorbidities not directly related to cardiovascular health or cancer progression, play a significant role in overall mortality risk in this group. These findings highlight the multifaceted role of NLR as a prognostic marker across different types of cancer. They suggest that while NLR is universally associated with increased all-cause and cardiovascular mortality, its relationship with cancer-specific mortality is more variable and may depend on specific tumor biology, stage at diagnosis, and underlying patient health. This underscores the importance of context when considering NLR as a prognostic tool in oncology, further research into the mechanisms underlying these associations will be crucial for leveraging NLR effectively in clinical practice to improve patient outcomes.
This study has several limitations that should be considered. First, as with all observational studies, inherent limitations exist regarding potential biases and confounders that cannot be fully adjusted for in the analysis. Our retrospective design limits the ability to infer causality between elevated NLR levels and increased cardiovascular mortality in cancer patients. Second, the reliance on self-reported cancer diagnoses may not capture all nuances of disease type, stage, or treatment history. The NHANES dataset lacks detailed information on cancer staging, specific treatment modalities received, and concomitant medication use, all of which could potentially influence the observed associations. Third, due to the cross-sectional design of NHANES, we were unable to ascertain the timing of NLR measurement relative to cancer diagnosis and treatment. Finally, while data on other potentially relevant biomarkers like C-reactive protein, N-terminal pro-B-type natriuretic peptide, and troponin were collected, a significant proportion of these measurements were missing, precluding their inclusion in our analysis. Despite these limitations, our study provides valuable insights into the prognostic significance of NLR in a large, nationally representative cohort of cancer survivors, highlighting its potential as a readily available marker for cardiovascular risk stratification in this population.
Conclusion
Our study underscores the significant prognostic value of NLR in predicting cardiovascular mortality among cancer patients, marking a notable advancement in the intersection of oncology and cardiology. The findings highlight the potential of NLR, a simple and readily available biomarker, to significantly enhance the prognostication and management of cancer patients. By integrating NLR into existing risk assessment models, clinicians could better identify participants at increased risk for cardiovascular complications, guiding more personalized and proactive management strategies. This study not only contributes to the growing body of literature supporting the clinical utility of NLR but also paves the way for future research aimed at optimizing care for cancer survivors facing cardiovascular risks.
Data availability
The code and data generated during the study were available from the corresponding author on reasonable request. And the original data was public on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
References
Lyon, A. R. et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 43, 4229–4361. https://doi.org/10.1093/eurheartj/ehac244 (2022).
Leszek, P. et al. A practical approach to the 2022 ESC cardio-oncology guidelines: Comments by a team of experts—Cardiologists and oncologists. Kardiol. Pol. 81, 1047–1063. https://doi.org/10.33963/v.kp.96840 (2023).
Herrmann, J. et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 43, 280–299. https://doi.org/10.1093/eurheartj/ehab674 (2022).
Rubio-Infante, N. et al. A systematic review of the mechanisms involved in immune checkpoint inhibitors cardiotoxicity and challenges to improve clinical safety. Front. Cell Dev. Biol. 10, 851032. https://doi.org/10.3389/fcell.2022.851032 (2022).
Azab, B. et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann. Surg. Oncol. 19, 217–224. https://doi.org/10.1245/s10434-011-1814-0 (2012).
Regolo, M. et al. Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. J. Clin. Med. 11, 235. https://doi.org/10.3390/jcm11082235 (2022).
He, J., Li, J., Wang, Y., Hao, P. & Hua, Q. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality and adverse-outcomes after ST-segment elevation myocardial infarction in Chinese people. Int. J. Clin. Exp. Pathol. 7, 4045–4056 (2014).
Wang, X. et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: A meta-analysis of observational studies. Atherosclerosis 234, 206–213. https://doi.org/10.1016/j.atherosclerosis.2014.03.003 (2014).
Azab, B. et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 106, 470–476. https://doi.org/10.1016/j.amjcard.2010.03.062 (2010).
Dong, C. H., Wang, Z. M. & Chen, S. Y. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clin. Biochem. 52, 131–136. https://doi.org/10.1016/j.clinbiochem.2017.11.008 (2018).
Curtin, L. R. et al. The National Health and Nutrition Examination Survey: Sample design, 1999–2006. Vital Health Stat. 2 1, 1–39 (2012).
Curtin, L. R. et al. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. 2 1, 1–23 (2013).
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. 2 1, 1–33 (2014).
Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. I. National Health and Nutrition Examination Survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2 1, 1–35 (2020).
Patel, C. J. et al. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci. Data 3, 160096. https://doi.org/10.1038/sdata.2016.96 (2016).
Di, D. et al. Exposure to phenols, chlorophenol pesticides, phthalate and PAHs and mortality risk: A prospective study based on 6 rounds of NHANES. Chemosphere 329, 138650. https://doi.org/10.1016/j.chemosphere.2023.138650 (2023).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2 1, 1–24 (2013).
Zhang, L., Chen, S., Wang, W., Wang, Y. & Liang, Y. Inflammatory and nutritional scoring system for predicting prognosis in patients with newly diagnosed multiple myeloma. J. Inflamm. Res. 16, 7–17. https://doi.org/10.2147/jir.s390279 (2023).
Seckinger, A. et al. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood 120, 1087–1094. https://doi.org/10.1182/blood-2012-03-415588 (2012).
Ranjit, N. et al. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation 116, 2383–2390 (2007).
Robles, T. F. Marital quality and health: Implications for marriage in the 21st century. Curr. Direct. Psychol. Sci. 23, 427–432 (2014).
Vakhshoori, M. et al. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc. Disord. 23, 555. https://doi.org/10.1186/s12872-023-03572-6 (2023).
Mouchli, M., Reddy, S., Gerrard, M., Boardman, L. & Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article. Ann. Hepatol. 22, 100249. https://doi.org/10.1016/j.aohep.2020.08.067 (2021).
Marchioni, M. et al. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: A systematic review. Clin. Genitourin Cancer 14, 473–484. https://doi.org/10.1016/j.clgc.2016.04.008 (2016).
Rossi, S. et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer?. Clin. Colorectal Cancer 16, 264–274. https://doi.org/10.1016/j.clcc.2017.03.015 (2017).
Sonaglioni, A. et al. Charlson comorbidity index, neutrophil-to-lymphocyte ratio and undertreatment with renin-angiotensin-aldosterone system inhibitors predict in-hospital mortality of hospitalized COVID-19 patients during the omicron dominant period. Front. Immunol. 13, 958418. https://doi.org/10.3389/fimmu.2022.958418 (2022).
DiGangi, C. Neutrophil-lymphocyte ratio: Predicting cardiovascular and renal complications in patients with diabetes. J. Am. Assoc. Nurse Pract. 28, 410–414. https://doi.org/10.1002/2327-6924.12366 (2016).
Salciccioli, J. D. et al. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: An observational cohort study. Crit. Care 19, 13. https://doi.org/10.1186/s13054-014-0731-6 (2015).
Chen, Y. et al. Association between neutrophil-lymphocyte ratio and all-cause mortality and cause-specific mortality in US adults, 1999–2014. Int. J. Gen. Med. 14, 10203–10211. https://doi.org/10.2147/ijgm.s339378 (2021).
Fest, J. et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur. J. Epidemiol. 34, 463–470. https://doi.org/10.1007/s10654-018-0472-y (2019).
Acknowledgements
We would like to acknowledge the participants and investigators of National Health and Nutrition Examination Survey. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
C G chose the topic. X L, M W, H K provided methodological support. C G completed the subsequent data analysis and article writing. Y H provided guidance and assistance throughout the process. All authors revised the manuscript for important intellectual content, participated in the decision to submit the manuscript for publication, and approved the final submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research has followed the Tenets of the Declaration of Helsinki. Ethical considerations have been rigorously followed, with all data sourced from the publicly available NHANES, which obtained informed consent from all participants and received ethical approval from the NCHS Research Ethics Review Board.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, Y., Liu, X., Wang, M. et al. Neutrophil-to-lymphocyte ratio as a predictor of cardiovascular mortality in cancer survivors. Sci Rep 14, 20980 (2024). https://doi.org/10.1038/s41598-024-72027-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72027-5
- Springer Nature Limited