Abstract
The objective of this research was to explore the potential association between lipid accumulation product (LAP) and chronic kidney disease (CKD) among adult population of United States (US). Using cross-sectional data from the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES), we explored the association of LAP with CKD, low estimated glomerular filtration rate (eGFR), and albuminuria. This analysis encompassed multivariate logistic regression analyses, smoothed curve fitting, subgroup analyses, and interaction tests. We found a significant positive association between higher ln-transformed LAP (LAP was transformed using a natural logarithm) and the prevalence of CKD, low-eGFR and albuminuria. Notably, this association of ln-transformed LAP with CKD and albuminuria was significantly influenced by diabetes status and sex (P for interaction < 0.05), while no significant interaction was observed regarding the association with low-eGFR (P for interaction > 0.05). Additionally, in model 3 (adjusted for all included covariates except eGFR and urinary albumin-creatinine ratio (UACR)), a nonlinear relationship was identified between ln-transformed LAP and the presence of both CKD and albuminuria, with inflection points of 4.57 and 4.49, respectively. This indicates that this correlation is more pronounced on the right of the inflection point. In conclusion, the findings indicate a significant association between LAP and the prevalence of CKD in US adults.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD), a prevalent non-communicable disease, represents a significant contributor to the global mortality rate. Frequently designated as the “silent killer”, CKD is distinguished by its high prevalence, low awareness, and low cure rate1,2,3. It is estimated that more than 15% of the US population is affected by CKD, which equates to approximately 37 million individuals4. This condition significantly increases the probability of cardiovascular disease acquiring and augments the overall risk for mortality4. Therefore, it is of paramount importance to actively investigate novel biomarkers to facilitate the early recognition and intervention of CKD.
A growing body of evidence highlights the impact of obesity on the progression of CKD5,6,7. This relationship between obesity and CKD is mediated by a complex biological mechanism, including insulin resistance, lipotoxicity, and the dysregulation of adipokines5,7,8,9. It is, however, important to note that not all adipose tissue has a deleterious effect on renal health. In fact, visceral fat accumulation has the most profound adverse effects on renal function10. A cross-sectional study of the Korean population revealed a significantly higher prevalence of obesity among individuals with CKD compared to those without the condition. What’s more, the high occurrence of obesity was accompanied by an increase in visceral obesity11. The lipid accumulation product (LAP) initially proposed by Kahn, may be a more effective measure indicator of visceral fat accumulation compared to BMI, waist-to-hip ratio (WC), waist-to-hip ratio (WHR) and other indicators12,13. It is calculated by combining waist circumference (WC) and serum triglyceride (TG) levels, offering a convenience advantage over costly and complex imaging techniques such as magnetic resonance imaging (MRI) and/or computed tomography (CT)14. This approach is more suitable for large-scale health screening and disease risk assessment. The association between LAP and CKD has been evaluated in various regions and populations14,15,16,17,18. However, there are fewer reports on the relationship between LAP and CKD in the overall US population. Only the study by Bullen et al. showed an association between LAP and the occurrence of CKD, as well as a gradual deterioration in kidney function19. Hence, the primary objective of this study is to explore the connection between LAP and CKD among adult population of US.
Methods
Study design and participants
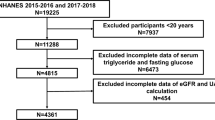
For this study, we used data from the public database of National Health and Nutrition Examination Survey (NHANES), which was conducted between 2013 and 2018 (https://www.cdc.gov/nchs/nhanes/index.html). NHANES assesses the health and nutritional status of the population of adults and children residing in the US using a mix of stratified, multistage, and probability sampling techniques20. All studies were subjected to a comprehensive review and received approval from the National Center for Health Statistics (NCHS) Research Ethics Review Board. Prior to participation, all participants provided written informed consent. Figure 1 illustrates the inclusion of 6,741 participants in the study after excluding those younger than 20 years, those with absences of data on CKD, estimated glomerular filtration rate (eGFR), urine albumin/creatinine ratio (UACR) and LAP, and pregnant individuals.
Variables
Within this investigation, LAP was regarded as an exposure variable and was determined by the subsequent formula: [WC (cm)—65] × [TG (mmol/L)] (males), and [WC (cm)—58] × [TG (mmol/L)] (females)21. Due to its skewed distribution, the LAP index was transformed using a natural logarithm (ln-transformed LAP).
CKD was regarded as outcome variable, along with low-eGFR and albuminuria, which are also analyzed as outcome variables. We used the CKD-EPI2021 equation recommended by the National Kidney Foundation to calculate eGFR22 (detailed information in supplementary material 1). CKD was characterized by UACR ≥ 30 mg/g or eGFR < 60 ml/min/1.73 m2 23. The criterion for low-eGFR was specifically designated as eGFR < 60 ml/min/1.73 m2, while albuminuria was designated as UACR ≥ 30 mg/g.
In our study, we incorporated a range of demographic and clinical variables as covariates to ensure the robustness of our analysis. These included age, sex, race, poverty-to-income ratio (PIR), education level, and lifestyle factors such as drinking and smoking status. Anthropometric and biochemical measures were also considered, encompassing BMI, weight, high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), blood urea nitrogen (BUN), albumin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT). Furthermore, the potential influence of hypertension and diabetes was considered. The NHANES website provides a comprehensive information about the interpretation, and measurement, along with the calculation of every variable. The definitions of the variables involved are given in supplementary material 2.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables were represented as percentages. The demographic characteristics of the participants were evaluated based on the classification of CKD using weighted chi-square (categorical variables) and weighted linear regression model (continuous variables). To examine the association of ln-transformed LAP with CKD, low-eGFR, and albuminuria, multifactorial linear regression analysis was conducted, controlling for potential confounding variables. Model 1 was not adjusted for covariates. Model 2 was adjusted for age, sex, and race. Model 3 incorporated additional variables, including education, PIR, weight, BMI, HDL-cholesterol, LDL-cholesterol, ALP, ALT, AST, BUN, albumin, alcohol use, cigarette use, as well as hypertension and diabetes status. Furthermore, generalized additive models and smoothed curve fitting were employed to examine the nonlinear association between ln-transformed LAP with outcome variables. To further explore the relationship between them, stratified analyses were performed according to sex, age, BMI, as well as hypertension and diabetes status. All statistical analyses were performed using R (version 4.2.3) and Empowerstats (version 2.0). We considered statistical significance as P < 0.05.
Results
Baseline characteristics
A total of 6,741 participants were enrolled in the study, with a mean age of 48.33 ± 16.82 years. The population consisted of 49.53% males and 50.47% females. A total of 1,142 individuals were diagnosed with CKD (weighted percentage 13.24). As detailed in in Table 1, individuals with CKD were more likely to be of advanced age, female sex, and non-Hispanic Black ethnicity. Additionally, they exhibited a higher prevalence of diabetes and higher levels of BMI, BUN, ALP, AST, UACR, and ln-transformed LAP. In contrast, these individuals exhibited lower levels of education, PIR, LDL-C, albumin, eGFR. Furthermore, they were less likely to engage in smoking and alcohol consumption.
Association of ln-transformed LAP with CKD, low-eGFR and albuminuria
Results showed a significant and positive association of ln-transformed LAP with CKD (Table 2). The positive association still existed after accounting for potential confounders. Specifically, there was a 52% higher odds of CKD prevalence when the ln-transformed LAP increased by one unit in Model 1 (OR:1.52, 95% CI 1.40, 1.64). Similarly, the odds increased by 35% in Model 3 (OR:1.35, 95% CI 1.18,1.55). Notably, when ln-transformed LAP was categorized into quartiles, individuals in the highest quartile exhibited a 57% increased odds of CKD prevalence compared to those in the lowest quartile in Model 3 (OR:1.57, 95% CI 1.15, 2.15; P for trend = 0.005).
Similarly, a positive connection was noted between the ln-transformed LAP and both low-eGFR and albuminuria. An analysis of Model 3 data indicated that for each one-unit increase in ln-transformed LAP, there was a 48% higher odds ratio for the prevalence of low-eGFR (OR:1.48, 95% CI 1.14, 1.91) and a 34% higher odds ratio for the prevalence of albuminuria (OR: 1.34, 95% CI 1.16, 1.55). Upon categorizing ln-transformed LAP into quartiles, individuals in the highest quartile of model 3 exhibited 93% increased odds of having low-eGFR compared to those in the lowest quartile, as well as 54% increased odds of having albuminuria (low-eGFR, OR:1.93, 95% CI 1.10, 3.38; P for trend = 0.0196; albuminuria, OR: 1.54, 95% CI 1.10, 2.13; p for trend = 0.0108).
The application of generalized additive models and smoothed curve fitting provided further evidence of correlation between ln-transformed LAP and three outcome variables in Model 3. As illustrated in Fig. 2, a U-shaped nonlinear relationship was identified between ln-transformed LAP and CKD and albuminuria, with inflection points of 4.57 and 4.49, respectively (Table 3). This indicates that this correlation is more pronounced on the right of the inflection point.
Subgroup analysis
To ascertain whether the findings were consistent across different subgroups as they were in the total population, we performed subgroup analyses. Table 4 presents the results, which demonstrate a positive association between ln-transformed LAP and both CKD and albuminuria in subgroups stratified by sex, age, BMI, as well as diabetes and hypertension status. However, this association was not statistically significant in female participants and those with a BMI ≤ 25 kg/m2 (P > 0.05). It is noteworthy that in participants aged 20 to less than 40 years, there was a negative but not statistically significant association between the ln-transformed LAP and low-eGFR (OR: 0.87, 95% CI 0.37, 2.05, P > 0.05). While other subgroups exhibited a positive association, this was not statistically significant in female participants and those with a BMI between 25 and 30 kg/m2. Further interaction tests revealed that sex, age, BMI, and diabetes and hypertension status did not have statistically significant impact on the association between ln-transformed LAP with low-eGFR (P for interaction > 0.05), suggesting that these factors did not have a significant effect on this positive association. In contrast, sex and diabetes status may influence the association between ln-transformed LAP and CKD and albuminuria (P for interaction < 0.05), with the association being more pronounced in male and patients with diabetic.
Discussion
The present study, which included 6741 participants, has research revealed a significant positive correlation between higher levels of ln-transformed LAP and CKD, low-eGFR, and albuminuria among adult population of US. Moreover, the study indicates that the association of ln-transformed LAP with CKD and albuminuria was significantly influenced by sex and diabetes status. It is also noteworthy that the data exhibit a U-shaped nonlinear relationship, with inflection points at 4.57 and 4.49, respectively. This implies that beyond the inflection points, continued elevation of ln-transformed LAP levels would significantly increase the risk of CKD. Understanding these inflection points can help in risk assessment and stratification of individuals.
Various investigations have demonstrated that LAP could function as a promising indicator for a range of health disorders, including metabolic syndrome24,25, osteoarthritis21, diabetic nephropathy26, and metabolic dysfunction-associated fatty liver disease (MAFLD)27. The findings of our results corroborate this notion, revealing a positive correlation between higher ln-transformed LAP and odds of CKD prevalence. In accordance with previous research results, in cross-sectional studies of Korean adults15, a rural population in Northeast China16, and a population in East China17, respectively, it was found that LAP was a good predictor of low-eGFR. In female subjects, LAP was better recognized than conventional indicators (BMI, WC)16. In addition, a cross-sectional investigation by Yan et al. on a Chinese population aged 40 years and older has demonstrated a strong association of elevated LAP with higher odds of CKD in adult females, a correlation that was not observed in males14. Notably, Tang et al. have found a positive correlation between LAP and diabetic kidney disease (DKD) in patients with 2 diabetics26. Another cohort study of non-diabetic Iranian adults found that LAP could not serve as a reliable predictor of decreased eGFR18. Nevertheless, our findings indicate that diabetes status did not alter the association between ln-transformed LAP and low-eGFR. However, sex and diabetes status exerted a significant influence on the association of ln-transformed LAP with CKD. This association was particularly pronounced in males and diabetic patients. It has been demonstrated that the accumulation and distribution of body fat are influenced by sex hormone levels, with males typically exhibiting a higher amount of visceral fat. Additionally, postmenopausal females may have a significant rise in visceral fat due to the decline in estrogen levels28. These hormonal variations may contribute to some of the observed discrepancies in study findings, along with factors such as age, race, covariates adjustment, and study design. Further studies are necessary to gain a more comprehensive understanding of the association between LAP and CKD.
The etiology of the link between obesity and CKD is intricate and multifaceted. On the one hand, it can be understood as a crosstalk between the “adipose-renal axis”, which is primarily characterized by the secretion of adipokines, cytokines, and metabolites from adipose tissue that subsequently affect kidney health29. It is widely recognized that persistent, low-grade chronic inflammation plays a pivotal role in the adverse outcomes observed in individuals with CKD30. Meanwhile, obesity is frequently linked to chronic inflammation of adipose tissue31. Consequently, therapeutic interventions that target inflammation have attracted considerable interest in the context of CKD. It has been demonstrated that in the context of obesity, a notable increased number of macrophages within adipose tissue, accompanied by a polarization shift towards a pro-inflammatory state (M1). This shift enhances the generation of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-12 (IL-12)32, while concurrently reducing the secretion of anti-inflammatory adipokines, such as lipofuscin33. This series of events ultimately leads to renal injury. In contrast, relevant investigations have demonstrated that the generation of uremic toxins in individuals afflicted with CKD contributes to insulin resistance and inflammation in adipose tissue31,34. More importantly, the occurrence of localized inflammation within adipocytes is correlated with reduced insulin sensitivity35. Adipocytes are a significant contributor to the presence of inflammatory cytokines in circulatory systems. Consequently, interventions aimed at adipose tissue inflammation may offer a promising approach to ameliorating renal impairment associated with excessive adiposity. On the other hand, the lipid nephrotoxicity hypothesis also warrants consideration as an important factor. A high-fat diet has been demonstrated to promote lipid accumulation in mice, a process that is thought to occur via the vascular endothelial growth factor-B (VEGF-B) signaling pathway, mitochondrial damage and the subsequent enhancement of insulin resistance, the generation of reactive oxygen species, and endoplasmic reticulum stress, all of which eventually resulted in renal impairment36,37,38. Furthermore, obesity is a recognized risk factor fo the onset of various chronic diseases, including hepatic steatosis, atherosclerosis, hypertension, hyperlipidemia, and others, which also result in the deterioration of renal function.
It is imperative to acknowledge the multitude of strengths in this research. First, the study’s population is nationally representative, thereby enhancing the generalizability of the findings. Secondly, the analysis was adjusted for relevant confounders variables, thus ensuring the reliability of the results. In addition, subgroup analysis was performed. Most importantly, LAP, with its straightforward calculation, emerges as a potentially tool for identifying individuals at increased odds of CKD prevalence. It is nevertheless imperative to recognize the constraints of our study. The cross-sectional nature of our research design precludes the determination of a causal relationship between the exposed and outcome variables. Furthermore, confounding factors that were not accounted for in the analysis may also have an impact. Although previous studies have indicated that the correlation between obesity and CKD is not evident in patients with CKD stage 4/511, our study did not further categorize CKD by stage to investigate this association.
Conclusion
The present study demonstrated a notable positive association of higher ln-transformed LAP with CKD. This finding underscores the potential utility of LAP as a biomarker for identifying individuals odds of CKD prevalence. However, additional comprehensive prospective studies are imperative to substantiate this discovery.
Data availability
All anonymized data were obtained from the NHANES open database (https://www.cdc.gov/nchs/nhanes/index). For further consultations, contact the corresponding author.
References
Shlipak, M. G. et al. The case for early identification and intervention of chronic kidney disease: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 99, 34–47 (2021).
McMahon, G. M., Hwang, S.-J. & Fox, C. S. Residual lifetime risk of chronic kidney disease. Nephrol. Dial. Transpl. 32, gfw253 (2016).
Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 12, 7–11 (2022).
Vassalotti, J. A. & Boucree, S. C. Integrating CKD Into US primary care: Bridging the knowledge and implementation gaps. Kidney Int. Rep. 7, 389–396 (2022).
Nawaz, S. et al. Obesity and chronic kidney disease: A current review. Obes. Sci. Pract. 9, 61–74 (2023).
Ejerblad, E. et al. Obesity and risk for chronic renal failure. J. Am. Soc. Nephrol. 17, 1695–1702 (2006).
Jiang, Z. et al. Obesity and chronic kidney disease. Am. J. Physiol-endoc. M. 324, E24–E41 (2023).
Seng, N. S. H. L., Lohana, P., Chandra, S. & Jim, B. The fatty kidney and beyond: A silent epidemic. Am. J. Med. 136, 965–974 (2023).
Gai, Z. et al. Lipid accumulation and chronic kidney disease. Nutrients 11, 722 (2019).
Stefan, N. et al. Obesity and renal disease: Not all fat is created equal and not all obesity is harmful to the kidneys. Nephrol. Dial. Transpl. 31, 726–730 (2016).
Evangelista, L. S., Cho, W.-K. & Kim, Y. Obesity and chronic kidney disease: A population-based study among South Koreans. PLoS One 13, e0193559 (2018).
Kahn, H. S. The ‘lipid accumulation product’ performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 5, 26 (2005).
Chen, T. & Zhao, C. Relationship between visceral adipose index, lipid accumulation product and type 2 diabetes mellitus. Adv. Clin. Med. 12, 3350 (2022).
Yan, P. et al. Association of lipid accumulation product with chronic kidney disease in Chinese community adults: A report from the REACTION study. Lipids Health Dis. 20, 131 (2021).
Seong, J. M. et al. Gender difference in the association of chronic kidney disease with visceral adiposity index and lipid accumulation product index in Korean adults: Korean National Health and Nutrition Examination Survey. Int. Urol. Nephrol. 53, 1417–1425 (2021).
Dai, D. et al. Visceral adiposity index and lipid accumulation product index: Two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int. J. Environ. Res. Public. Health 13, 1231 (2016).
Zhang, K., Li, Q., Chen, Y., Wang, N. & Lu, Y. Visceral adiposity and renal function: An observational study from SPECT-China. Lipids Health Dis. 16, 205 (2017).
Mousapour, P. et al. Predictive performance of lipid accumulation product and visceral adiposity index for renal function decline in non-diabetic adults, an 8.6-year follow-up. Clin. Exp. Nephrol. 24, 225–234 (2020).
Bullen, A. L. et al. Lipid accumulation product, visceral adiposity index and risk of chronic kidney disease. Bmc Nephrol. 23, 401 (2022).
Chen, W. et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol. Dial. Transplant. 35, 1171–1178 (2020).
Huang, J. et al. Association between lipid accumulation products and osteoarthritis among adults in the United States: A cross-sectional study, NHANES 2017–2020. Prev. Med. 180, 107861 (2024).
Miller, W. G. et al. National kidney foundation laboratory engagement working group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin. Chem. 68, 511–520 (2022).
Chapter 1: Definition and classification of CKD. Kidney Int. Suppl (2011) 3, 19–62 (2013).
Chen, Z.-Y. et al. Lipid accumulation product is a better predictor of metabolic syndrome in Chinese adolescents: A cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1179990 (2023).
Li, Y. et al. Association between four anthropometric indexes and metabolic syndrome in US Adults. Front. Endocrinol. 13, 889785 (2022).
Tang, M. et al. Interrelation between the lipid accumulation product index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front. Endocrinol. 14, 1224889 (2023).
Li, H., Zhang, Y., Luo, H. & Lin, R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front. Endocrinol. (Lausanne) 13, 977625 (2022).
Kataoka, H., Nitta, K. & Hoshino, J. Visceral fat and attribute-based medicine in chronic kidney disease. Front. Endocrinol. 14, 1097596 (2023).
Brennan, E., Kantharidis, P., Cooper, M. E. & Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 17, 725–739 (2021).
Ebert, T. et al. Inflammation and premature ageing in chronic kidney disease. Toxins 12, 227 (2020).
Arabi, T. et al. Obesity-related kidney disease: Beyond hypertension and insulin-resistance. Front. Endocrinol. 13, 1095211 (2023).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: Implications for kidney disease. Nat. Rev. Nephrol. 14, 105–120 (2018).
D’Apolito, M. et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Invest. 120, 203–213 (2010).
Bensussen, A., Torres-Magallanes, J. A. & Roces De Álvarez-Buylla, E. Molecular tracking of insulin resistance and inflammation development on visceral adipose tissue. Front. Immunol. 14, 1014778 (2023).
Lieben, L. Lipid toxicity drives renal disease. Nat. Rev. Nephrol 13, 194–194 (2017).
Aguilar, A. Shielding mitochondria from lipotoxicity prevents renal injury. Nat. Rev. Nephrol. 12, 580–580 (2016).
Izquierdo-Lahuerta, A., Martínez-García, C. & Medina-Gómez, G. Lipotoxicity as a trigger factor of renal disease. J. Nephrol. 29, 603–610 (2016).
Acknowledgements
We express our gratitude to the personnel and individuals involved in the NHANES project for their invaluable contributions.
Funding
The funding was supported by the National Natural Science Foundation of China (82170757), Huai’an City Science and Technology Program Project (HAB202211), Scientific Research Project from Jiangsu Commission of Health of China (H2019062), Project of Science and Technology Development of Affiliated Hospital of Xuzhou Medical University (XYFM202248).
Author information
Authors and Affiliations
Contributions
X.Y., X.P., Y.X. and X.L. designed the research. X.Y., X.P. and Y.X. collected, analyzed the data, and drafed the manuscript. X.Y., X.P., H.L. and D.Z. revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The survey protocol for the NHANES was approved by CDC’s National Center for Health Statistics Institutional Research Ethics Review Board. The protocol descriptions are available at (https://www.cdc.gov/nchs/nhanes/irba98.htm). The methods involved in this study are carried out in accordance with relevant guidelines and regulations (Helsinki Declaration). Prior to their participation in the study, all subjects and/or their legal guardian(s) were written informed consent.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, X., Pu, X., Xi, Y. et al. Association between the lipid accumulation product and chronic kidney disease among adults in the United States. Sci Rep 14, 21423 (2024). https://doi.org/10.1038/s41598-024-71894-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71894-2
- Springer Nature Limited