Abstract
The warming of the Arctic had lead to a diverse range of impacts on local biota, including northward shifts of some species range. Here, we report past and present distribution and abundance of an Arctic zooplanktivorous seabird, the little auk Alle alle in West Greenland south of 74° N, and examine the changes in sea surface temperature (SST) and sea ice concentration (SIC) in the birds foraging areas in 1850–2007. We estimated the little auk population in the studied region to be 5,200 pairs in the 1930s, 6,000–6,500 pairs in the 1940-1970s and 70–80 pairs by the 2000s. We found that periods with increased SST and reduced SIC, especially in the last few decades, coincided with little auk population declines. Besides, years with little auk presence in breeding sites were characterized by either low SST and low to moderate SIC or higher SST but moderate to high SIC. Observed contraction of the breeding range and a decrease in abundance of the little auk may be attributed to more complex climate-driven changes in the marine ecosystem at finer spatial and temporal scales and/or cannot be easily detected given the coarseness of data used. It is possible that the population in this region has never been very numerous being subjected to local impacts such as disease, bycatch, predation, etc. The climate warming that is currently being observed, along with corresponding shifts in zooplankton communities, may lead to extirpation of the studied little auk populations.
Similar content being viewed by others
Introduction
The northern North Atlantic (north of 60oN) has experienced a large regime shift during the 1920s and 1930s with a marked increase in sea temperature and reduction of sea ice extent1. These changes affected the marine ecosystem resulting in significant northward shifts in distribution of several species of fish, marine mammals and seabirds1,2 and changes in the timing and extent of their migration patterns. Some decades later, in 1980s and 1990s and also in the first decades of the twenty-first century3, another shifts were detected and associated with a range shift of some zooplankton species4,5. However, mechanisms driving all these responses are largely unknown. Now, when we are witnessing unprecedented climate change, particularly pronounced in the Arctic6 it is of prime importance to recognize these mechanisms, to better predict response of Arctic endemic species7,8 for the ongoing changes.
The little auk or dovekie (Alle alle) is a small zooplanktivorous colonial seabird with a breeding range largely restricted to the High Arctic9. At present, the largest breeding aggregations of the little auk are located in Northwest and East Greenland, as well as Svalbard, with a few small colonies located in other parts of the Atlantic Low Arctic zone (Fig. 1). With a global population estimated at 37–40 million pairs10, it is considered to be the most numerous seabird in the North Atlantic zone. With a life history dependent on both marine and terrestrial habitats, the species transports enormous amounts of organic matter from the sea to the land11,12,13,14. As such, the little auk plays a significant role in the functioning of High Arctic ecosystems. The little auk’s diet during the breeding season is dominated by zooplankton associated with cold water masses, mainly copepod species from the genus Calanus15,16,17,18,19, supplemented by other energy-rich crustaceans such as amphipods, euphausiids and larval fish20,21,22. As a result of the species’ dietary preferences, the little auk is sensitive to changes in environmental conditions, such as an increase in sea temperature. Studies from the High Arctic colonies of little auks on Svalbard have indicated a negative effect of increase in sea surface temperature in foraging areas upon adult survival23, chick growth24,25 and chick survival24,26, as well as population size27. Also presence of the sea ice in the range of foraging trips may affect foraging of little auks modifying chick diet composition28,29,30,31,32 and time budget16,17,25 securing good quality food when foraging condition in closer foraging areas are suboptimal25,33,34,35.
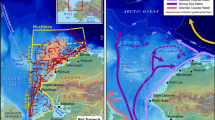
Locations of little auk breeding sites in global scale (red circles) (a) and in West Greenland south of 74° N (violet triangles) (b) with estimated size of other little auk colonies [after ref 10,101]. Numbers in both maps refer to the breeding sites listed in Table 1. Dashed blue lines represent boundaries of Arctic zones according to 40. Maps were produced in ArcMap 10.3.1 (Redlands, CA: Environmental Systems Research Institute). Base map source: Natural Earth. Free vector and raster map data @ naturalearthdata.com. Photo of the little auk by DJ.
Some studies2,11,36,37 have suggested that a shift in sea currents and zooplankton distribution after the end of the “Little Ice Age” (after ~ 1850) resulted in a collapse of the little auk breeding population in Iceland and South Greenland. A recent study investigating reasons for the little auk extirpation in Iceland has revealed that, indeed, during that period the number of breeding little auks declined substantially, coinciding with changes in oceanographic regimes around the breeding colonies, but the population decline was also affected by severe egg harvest38. Dynamics of little auk populations in West Greenland south of 74° N have not so far been investigated.
In this study we therefore investigated dynamics of the little auk population in West Greenland south of 74° N and potential mechanisms of the changes in birds demography. Specifically, we examined whether observed changes in environmental conditions (sea surface temperature, hereafter SST, and sea ice concentration, hereafter SIC) in potential foraging areas of little auks could be linked to changes in size of the local populations. We studied environmental conditions during the chick-rearing period as that is known to affect breeding performance of little auks in the current year [e.g.24,25] and survival of adults23, and all that, in turn, can affect the size of local populations given a high breeding site fidelity in the species11. To this end, we summarized available historical data on the distribution and sizes of local populations of little auks. Then, to investigate whether the changes were similar or different across the range of the studied area we performed retrospective analyses in respect to the breeding site location. Finally, to characterize foraging habitat niches of little auks in the past, we investigated a relationship between important environmental variables (SST and SIC) determining presence/abundance of their main prey (cold water copepods)5,39 in foraging areas. We did it for breeding sites in years with presence of little auks. We expected that in conditions of high mean SST in the foraging areas little auks would search for the regions with lower SST covered with ice. Such a situation has been observed on Northwest Spitsbergen where little auks in conditions of a relatively high SST in close foraging grounds explored regularly cold water marginal ice zone located 100–150 km from the colony33,34,35.
Methods
Study area
We considered breeding sites and potential foraging areas of little auks in West Greenland across latitudes between 59º N and 74º N (Fig. 1) representing mainly the Low Arctic zone (according to Circumpolar Arctic Vegetation Map 40; use of this division into Arctic zones is justified as both phenology of vegetation and breeding of little auks are dependent of snow melting in land 41,42,43,44). The waters in the studied area include Baffin Bay, Davis Strait and northern part of the Labrador Sea. They are dominated by water masses advected into the region from outside: in the surface layer of the coastal zone over the shelf, cold and low saline Polar Water originates from the East Greenland Current while water masses below and to the west of the Polar Water derive from the warmer North Atlantic Current45.
This area is characterized by the presence of three copepod species of the genus Calanus: C. hyperboreus (the largest, deep water species), C. glacialis (medium size, cold water, shelf species), and C. finmarchicus (the smallest, warmer water species)5, and all those have been reported to coexist in Arctic waters and being present in the little auk diet46,47.
Breeding sites: distribution and abundance
We searched published reports and papers as well as unpublished materials to find information about little auk breeding locations and population estimates in West Greenland south of 74ºN (i.e. in the Low Arctic zone; Figs. 1, 2). We searched the Scopus and Google Scholar databases for documents of all types using the words “seabirds”, “birds”, “little auk”, “dovekie”, “Alle alle” or “Plautus alle” and “W Greenland” or “SW Greenland” in the title, abstract or keywords. Then we tried to find all appropriate references cited in published sources that had been located. DB and PE, performing various studies in the area, sought unpublished data from other researchers. In total, we used 11 sources, including nine publications between 1950 and 2014, as well as unpublished data of one of the coauthors (DB) and one personal communication from a researcher conducting a study in the area in the last several decades (see details in Table 1).
Distribution of the little auk breeding locations in West Greenland south of 74° N. Boxes on map represent potential foraging areas from which sea surface temperature (SST) and sea ice concentration (SIC) data were extracted. See Table 1 for breeding sites codes. Maps were created in the ggOceanMaps package 107 in R software version 4.2.2 58. Base map source: Natural Earth. Free vector and raster map data @ naturalearthdata.com.
Environmental conditions in potential foraging areas
We chose to analyze sea surface temperature (SST) and sea ice concentration (SIC) since foraging little auks while chick rearing mainly utilize cold water zone34,48,49,50. Some populations regularly forage in the marginal ice zone34,35 and at coastal glacier melt-water fronts51. The chosen environmental factors are also considered to be important determinants of abundance and biomass of copepods associated with cold Arctic-origin water masses, Calanus glacialis and C. hyperboreus5,39,47,52, serving as the main diet components of little auks during the breeding period across the species range11,15. Both of the aforementioned copepod species prefer cold waters with SST up to 6–7 ℃ and are larger, and more energy-rich than its counterpart, C. finmarchicus53, associated with warmer water masses with SST of up to 13 ºC (with a peak at 9 ºC) and SIC with a range up to 24.2% (Supplementary Materials 1 Table S1).
In a study of the breeding biology and feeding ecology of little auks at Horse Head island (1. APPE in Table 1), dropped diet items collected consisted mainly of warmer water-associated Calanus finmarchicus, making up 94% of prey items 21. However, a recent study from Svalbard has revealed that the diet of little auks consisted almost entirely of Calanus glacialis even in areas with higher SST54. Genetic identification has shown that that C. glacialis individuals of reduced body sizes may be wrongly identified as C. finmarchicus based only on morphometric criteria54. Thus, little auk diet at Horse Head, as well as in other studied areas, may have been dominated by C. glacialis.
To reconstruct past sea surface temperature (SST) in potential foraging areas of little auks breeding in West Greenland south of 74° N (delineated based on foraging trip ranges reported from other colonies, see details below), we extracted monthly data from 1871–2003 from a publicly available dataset CARTON-GIESE SODA 2.2.4, 1871–2008 Assimilation Run: Oceanic and air-sea interface data from the UMD Simple Ocean Data Assimilation Reanalysis (IRI/LDEO Climate Data Library) for 5 m depth with spatial resolution of 0.5 × 0.5 degree55. A 5 m depth of SST data reflects the range of little auk dives (mean ± SD maximum depth of dives for little auks from colonies in East Greenland and on Spitsbergen 9.9 ± 6.9 m48 and on Spitsbergen 9.0 ± 6.6 m56).
To reconstruct sea ice concentration (SIC) in the past in potential foraging grounds of little auks breeding in West Greenland south of 74° N, we extracted monthly data from 1850–2017 from Gridded Monthly Sea Ice Extent and Concentration, 1850 Onward, Version 2 with a spatial resolution of 0.25 × 0.25 degree57. This dataset is combination of remote sensed data and historical sea ice information (in many forms: ship observations, compilations by naval oceanographers, analyses by national ice services, and others) and is dedicated to studies of sea ice variability over time periods that pre-date the satellite era57. We chose this dataset as it covers longer time series compared to other available ones57 being aware of obvious limitations of incompleteness and heterogeneity of data prior to the satellite era.
We delineated potential foraging areas for particular breeding sites based on foraging trip ranges reported from other colonies. Results of GPS-tracking from colonies in Thule district in Northwest Greenland revealed that little auks foraged up to 100 km from the colony while the main feeding areas during chick rearing were located about 10 km from the colony14. At-sea observations of foraging individuals in the Horse Head colony suggested foraging at 2.5—32 km from the colony21. In other regions GPS-tracked little auks foraged during the chick rearing period at the distance up to 150 km from the colony34 in Svalbard and up to 108 km in East Greenland31. Given these findings, we analysed SST and SIC dynamics with a buffer of ~ 100 km radius from the breeding site representing both close and distant foraging areas.
Considering the fact that geographical locations of specific breeding sites relative to latitude impact the distance between meridians, we extracted data from differently sized rectangles (Fig. 2). To this end, we considered the following distances (in degrees) measured from the centre point, i.e. the breeding site:
(A) for latitudes 72-73º N – 1 degree (112 km) of latitude north and south, and 3 degrees of longitude (98–104 km) west and east;
(B) for latitudes 66-69º N—1 degree (112 km) of latitude north and south, and 2.5 degrees of longitude (100–114 km) west and east;
(C) for latitudes 59-65º N latitudes: 1 degree (112 km) of latitude north and south, and 2 degrees of longitude (94–115 km) west and east.
We extracted monthly SST data for July and August for each year from 1871 to 2003 and monthly SIC data for July and August for each year from 1850 to 2017. For each breeding site we derived one datapoint per year representing averaged values from raw 0.5 × 0.5 degree grids for July and August. In the case of SIC, we also had one datapoint for site and year representing maximal values per two months (July and August) and per box representing the foraging area.
Given the little auk breeding phenology reported for the breeding colonies in the studied area (Horse Head Island21) and north of it (in Northwest Greenland within Thule district14), July represents incubation and early chick rearing phases, and August the mid and late phases of chick-rearing, and the post-fledging period. Thus, values of environmental variables for July–August cover the whole breeding period of little auks.
Statistical analyses
We performed all statistical analyses in R software 4.2.258.
As SST in West Greenland followed alternate patterns of colder and warmer periods45,59, we expected a non-linear relationship for SST temporal changes. Thus, we investigated SST dynamics in potential foraging areas of little auks using a model-based recursive partitioning with a generalized additive model (GAM), using the gamtree package60. Model-based recursive partitioning estimates local models on subsets of data that are trained by recursive partitioning. It works in the following steps: (1) fitting a parametric model to a dataset, (2) testing for parameter instability over a set of partitioning variables, (3) in the case of some overall parameter instability, splitting the model with respect to the variable associated with the highest instability, and (4) repeating the procedure in each of the resulting subsamples61,62. We use GAM-based recursive partitioning instead of regular GAM, as we expected spatial variation in SST dynamics. We modelled SST (response variable) with s(Years) as the node specific (local) GAM smoother (regression spline), and latitude and longitude as the potential partitioning variables. To indicate that the individual observations (in this case, breeding sites) in the dataset are not independent, but nested according to latitude and/or longitude (i.e. some breeding colonies share the same latitude and/or longitude) we specified breeding sites as a cluster argument in the analysis60. We implemented the same GAM-based recursive partitioning for SIC analyses. We visualized result of the analyses using the ggparty package63.
To characterize foraging suitability of environmental conditions for little auks in nodes distinguished by recursive partitioning we calculated the proportion of years with mean SST values in July–August in potential foraging areas that exceed a physiological upper threshold for Calanus glacialis presence and functioning in Svalbard, i.e. 6 ℃39,64.
Finally, for all breeding sites for years with documented presence of little auks we studied the relationship between SST and SIC in July–August (averaged, thus one data point per year per breeding site) using GAM with formula SST ~ s(SIC) using function gam in mgcv package65. Given uncertainty of presence of little auks in the case of sites with only one record or records of only singular individuals we performed three separate analyses: 1) for all sites, 2) for sites with more than one record, and 3) sites with at least once noted > 2 pairs (or 4 individuals). For two first cases we used models with breeding site as random effect (as for some sites we had few records). For the third case we used model without random effect due to the smallest sample size.
Results
Size and distribution of the little auk population
In total, we found data for 22 sites in West Greenland south of 74° N, where breeding of little auks was reported in the time range from the 1780s to the 2010s (Table 1). In that in eight sites (36.4%) little auks were recorded more than once. Also in nine sites (40.9%) at least once more than 2 pairs were recoded. Seventeen (77.3%) of sites were active in the twentieth century but only six (27.3%) at the beginning of the twenty-first century. Available data indicate that little auks were not numerous (from single pairs to 50 pairs) at the majority of sites. Only in the northernmost site (Horse Head, APPE) numbers were higher (up to 6,000 pairs). We estimated total population size in West Greenland south of 74° N at 5,250 pairs in the 1930s, 6,500 pairs in 1936–196566 and 6,050 pairs in the 1960-1970s. In the following decades, the number of breeding pairs decreased reaching levels of only ~ 1,000 pairs in the 1990s, and 70–80 pairs in the 2000-2010s (Table 1). The range of breeding sites contracted from much of West Greenland south of 74° N at the beginning of the twentieth century to only the northern (APPE and UIGO in the Upernavik region), and central (GRON, ROTT and AKUN in the Disko Bay area) parts of the studied region by the beginning of twenty-first century (Fig. 2).
Environmental conditions in potential foraging areas of little auks
A GAM-based recursive partitioning revealed some geographic patterns in multi-year dynamics in July–August SST and SIC (Table 2, Figs. 3,4). Temporal patterns of these variables in foraging areas differed significantly with regard to latitude and longitude (SST) or only latitude (SIC) (Figs. 3,4). In the case of SST, the study area was divided into three nodes representing geographical zones covering six northern breeding sites (Northern Zone / Node 5), eight western sites (Western Zone / Node 3) and eight southern sites (Southern Zone/ Node 4) (Figs. 3). In the case of SIC, the study area was also divided into three zones: Northern Zone (Node 5) with twelve sites, Western Zone (Node 4) with five sites, and Southern Zone (Node 3) with five sites (Fig. 4).
(a) Generalized Additive Model (GAM)-based recursive partitioning characterizing factors affecting multi-year (1871–2008) mean sea surface temperature (SST) dynamics in July–August in potential foraging areas of little auks breeding in West Greenland south of 74o N. Longitude and latitude were used as the potential partitioning variables. Encircled variables have the strongest association to the local GAM smoother. The p values listed at each encircled node represent the test of independence between the listed variable and the local GAM smoother. Terminal nodes indicate variable levels characterizing the relationship between the response variable (SST) and predictor (Year) smoothed with GAM fit (lines), N indicates the number of SST sampling points corresponding to specific predictor levels. See Table 1 for breeding site codes; (b) SST dynamics (GAM fit) in potential foraging areas of little auks from nodes distinguished by recursive partitioning in July–August 1871–2008. Black dashed line indicates a physiological upper threshold for Calanus glacialis presence and functioning in Svalbard 39,64; (c) Map showing localization of geographical zones reflecting nodes distinguished by recursive partitioning and all studied breeding sites of little auks. A map was created in the ggOceanMaps package 107 in R software version 4.2.2 58. Base map source: Natural Earth. Free vector and raster map data @ naturalearthdata.com.
(a) Generalized Additive Model (GAM)-based recursive partitioning characterizing factors affecting multi-year (1850–2017) maximal sea ice concentration (SIC) dynamics in July–August in potential foraging areas of little auks breeding in West Greenland south of 74o N. Longitude and latitude were used as the potential partitioning variables. Encircled variables have the strongest association to the local GAM smoother. The p values listed at each encircled node represent the test of independence between the listed variable and the local GAM smoother. Terminal nodes indicate variable levels characterizing the relationship between the response variable (SIC) and predictor (Year) smoothed with GAM fit (lines), N indicates the number of SIC sampling points corresponding to specific predictor levels. See Table 1 for breeding site codes; (b) SIC dynamics (GAM fit) in potential foraging areas of little auks from nodes distinguished by recursive partitioning in July–August 1850–2017; (c) Map showing localization of geographical zones reflecting nodes distinguished by recursive partitioning and all studied breeding sites of little auks. A map was created in the ggOceanMaps package 107 in R software version 4.2.2 58. Base map source: Natural Earth. Free vector and raster map data @ naturalearthdata.com.
The range of recorded SST values differed between the three Zones / Nodes. Western SST Zone (Node 3) had the highest temperatures during the entire study period. Southern and Northern Zones (Nodes 4 and 5) were characterized by the lowest SST values among all SST Zones (Figs. 3 and Table 3). The amplitude of changes in SST (i.e. max–min difference) was the smallest in the Southern Zone (Table 3). From 1870 to the beginning of the twentieth century, SST in the Western Zone was stable while it was increasing in the remaining Zones. The first two decades of the twentieth century were characterized by a rapid decrease in temperature in all Zones. After the 1920s, an abrupt increase in temperature was observed in Northern and Western Zones while it was less pronounced in the Southern Zone. Stabilization of SST dynamics was observed from the 1940s (Southern and Northern Zones). The period from the 1950s to the 1990s was characterized by a slight temperature decrease (Southern Zone), increase (Western Zone) or some fluctuations (Northern Zone). In all zones a rapid increase in SST started from the 1990s (Figs. 3).
The SIC differed between the three zones, with the Northern Zone (Node 5) having a higher range of values than Southern and Western Zones (Nodes 3 and 4) during the entire study period (except for the last years in the 2010s) (Table 3, Fig. 4). Also, the pattern of SIC changes differed between the zones with a more stable pattern from the 1850s until the 1970s in Northern (Node 5) and Western (Node 4) Zones compared to a more fluctuating pattern during the same period in the Southern Zone (Node 3) The period 1970s–2000s was characterized by an increase in SIC in the Northern (Node 5) and decrease in the Southern Zone (Node 3) and fluctuations in the Western Zone (Node 4). Starting from the 2000s, a decrease in SIC was observed in all SIC zones, with a more rapid change in the Northern Zone (Node 5) (Fig. 4).
The proportion of years with mean SST values in July–August above the potential critical threshold of presence of C. glacialis (i.e. representing a less attractive foraging habitat for little auks) was the highest (46.5%) during the study period in the Western SST Zone (Node 5). Years with suboptimal mean SST values were much less frequent in other zones—4.7% in the Southern Zone (Node 4) and 8.9% in the Northern Zone (Node 4) (Fig. 3).
The last presence of little auks in SST Northern and Western Zones was documented in the beginning of the twenty-first century (in that only in two sites more > 2 pairs were detected). In the Southern SST Zone, in two sites the last documented presence was recorded at the end of the twentieth century (in that only in two sites more > 2 pairs were detected) (Fig. 5). In the case of SIC zones, all records of little auks in the twenty-first century were documented in the Northern Zone (Fig. 5).
Records of little auks in particular sites assigned to SST (A) and SIC zones/nodes (B) distinguished by recursive partitioning. Grey dashed vertical lines divide the timeline into centuries. Black dots indicate presence of little auks, black crosses no presence during the survey. records Larger dots and triangles indicate only one and more than one documented presence per colony, respectively. Colors of the dots and triangles represent number/estimate of individuals recorded: green—more than 2 pairs, black – up to 2 pairs. Colony codes – see Table 1. Colors of graph background and colored names represent particular SST and SIC zones/nodes.
Relationship between environmental conditions in years with documented presence of little auk at breeding sites
We found a significant relationship between mean SST and max SIC in July–August for years with presence of little auks in all sites [GAM, significant effect of smoothed term (SIC), p < 0.001, N = 36] and in sites with more than one record (p < 0.001, N = 24). In the case of sites with recorded more than 2 pairs during at least one visit the relationship only tended to be significant (p = 0.099, N = 17) (Table 4). GAM fits for all sites and sites with at least two records were characterized by predominant increase in max SIC with increasing mean SST. A steep increasing trend was observed in the range of SST up to 6 ℃ and SIC until ~ 20% SIC. Then, until ~ 6.8 ℃ and ~ 62% SIC increase trend was slighter. After reaching these values a slight decreasing trend was detected (Fig. 6). GAM fit for more than 2 pairs during at least one visit was characterized by two increasing (for low max SIC values up to ~ 14% and then for max SIC values starting from ~ 55% SIC) and one decreasing (for intermediate values of max SIC) parts of the trend (Fig. 6). Summing up, in the years with little auks presence, their foraging areas were generally characterized by either low mean SST in the foraging areas accompanied with no sea ice (SIC = 0%) or moderate to high SIC (12–78%). High mean SST (beyond the threshold for cold water zooplankton occurrence) were associated with moderate (10–20%) or high (60–90%) max SIC values (Fig. 6) suggesting possibility of exploring of marginal ice zone by little auks in conditions of high mean SST (especially in the closer foraging areas).
A relationship between sea ice concentration (SIC) and sea surface temperature (SST) in July and August in all breeding sites in West Greenland south of 74o N in years with documented presence of little auks. Lines represent GAM fits for all sites (grey solid), sites with > 1 records (green solid), and > 2 pairs (blue dashed). Solid lines represent significant GAM fits (p > 0.001). Shape of points represents number of recorded little auks presence (triangle—only once, circle – more than once). Color of the points represents number/estimate of individuals recorded: blue—> 2 pairs, red – 1–2 pairs.
Discussion
We documented 22 sites with possible breeding of little auks in West Greenland south of 74° N in the time range from the 1780s to the 2010s. Of that, breeding was documented for more than one time in eight sites (36.4%). Estimated population of the little auk in the study area at the turn of the nineteenth and twentieth centuries was at the level of few to several dozen pairs at the majority of locations. Only the northernmost colony on Horse Head (APPE) was more numerous (up to 6,000 pairs). At that time, breeding sites were scattered along the coast of West Greenland south of 74° N. At the beginning of the twenty-first century, after a few decades of population decline, little auks were present only in some locations in the northern (APPE, UIGO and ASSI in the Upernavik region) and west (GRON, ROTT and AKUN in the Disko Bay area) parts of the study area (all representing the Northern SIC Zone).
We found significant fluctuations in SST from the end of the 19th to the beginning of the twenty-first century in the study area, and the dynamics of changes were site dependent. The SST dynamics on the foraging grounds of little auks in the Western Zone are generally in agreement with multi-year patterns of air and SST anomalies recorded in Nuuk in West Greenland (latitude 64.4° N) 59. This area was characterized by a period of warmer SSTs prior to 1890, a colder-than-normal period that ended between 1910 and 1920, and a warm period thereafter, that ended in the 1970s; then from the 1996–2000 period onwards, climatic conditions changed again to warmer-than-normal conditions 59. A remarkably strong warming of coastal summer air temperature has been recorded in every coastal region of Greenland between 1952 and 2017 67 . Due to a lack of water temperature data before 1870 it is hard to assess whether the observed SST dynamic is concordant with postulated climate warming after the “Little Ice Age”2,66 (after ~ 185068). Observed SST fluctuations are driven by various factors such as climatic variations within the North Atlantic Subpolar Gyre, coupled processes associated with the Atlantic Multidecadal Oscillation, and overlying atmospheric pressure patterns59,69,70.
Ice data in the present study, including the whole of the second half of the nineteenth century, suggest slight (Northern and Western Zone) to moderate (Southern Zone) decreases in SIC in the 1850-1880s. Observed general decrease of sea ice concentration in last decades (but see North SIC Zone) is in concordance with other studies showing decreasing trend between 1979 and 2018 in March and September in various regions of the Arctic including the study area71. Increase in SIC in North SIC Zone between ~ 1960 and ~ 1995 is in agreement with increasing trend recorded in March between 1953 and 2001 in the waters between Greenland and Baffin Island between 65 and 73oN72. Patterns of sea ice changes are driven mainly by the external forcing from natural processes (local melting within the Arctic Ocean, and export of sea ice through sea ice drift) and anthropogenic sources (the loss of Arctic sea ice is directly correlated with anthropogenic CO2 emissions)71,73. In the studied area two main types of sea ice show great interannual variability governed by factors like the outflow of sea ice from the Arctic Ocean, and wind conditions in neighbouring seas45. The presence of ice close to little auk breeding sites may be also affected by local processes such as the calving of glaciers. Such coastal sea ice may serve as local foraging microhabitats for little auks51. However, this type of ice is not included in the large scale ice concentration models because of its relatively low spatial resolution and systematic errors occurring in algorithms for coastal regions74.
In general, we found that periods with SST increase and SIC reduction, especially in the last few decades (since the 1990s), coincided with the main little auk population decline. This pattern was particularly notable at the largest colony in the studied area – on Horse Head (APPE) (Fig. 7). Analysis of a relationship between SIC and SST in years with presence of little auks at breeding sites revealed that birds were present when SST values in foraging areas were low (within the range of Calanus glacialis presence and functioning) independently on sea ice concentration ranging from 0 to 76%. However, presence of little auks was also possible with mean SST values above the thermal threshold of Calanus glacialis (> ~ 6 ℃). Apparently, birds in years with high SST might have still found areas with lower SST in some parts of foraging areas. These areas (sometimes located far from the colony) were characterized by moderate or high SIC enabling regular foraging on energy-rich prey associated with marginal ice zone (MIZ) (Calanus glacialis and the symphagic amphipod Apherusa glacialis)75,76. A similar foraging strategy has been reported at the Northwest Spitsbergen colony in Magdalenefjorden where little auks foraged regularly in the MIZ, located 100–150 km from the colony, when foraging areas close to the colony were characterized by relatively high SST33,34,35. Thanks to such a flexible foraging strategy some breeding sites may exist for longer, even with suboptimal environmental conditions close to the colony. Moreover, even in areas with relatively high SST values, in the Western SST Zone, 53% of multi-year water temperatures within potential foraging areas of little auks (mean 5.7 ℃) were close to or exceeded potential thermal thresholds of occurrence of cold water copepods C. glacialis and C. hyperboreus (~ 6 ℃). At least in 25% of the years studied, conditions were still optimal for cold water copepods (0–25% range for SST: 0.7–5.0 ºC). It may have provided the chance for little auks to still breed in this area, even without MIZ presence in the range of foraging trips, given the possibility of feeding in various microhabitats (e.g. at glacier melt-water fronts, thermal fronts, eddies) as reported from East Greenland, Svalbard and Franz Josef Land(25,33,34,51,77). However, in a longer perspective, higher parental efforts to deliver optimal food may affect their reproductive performance and survival, in turn resulting in negative population dynamics and viability34. Thus, presence/absence of breeding little auks at particular sites (especially those with at least periodically high SST) may by driven by multiple episodes of local extirpation/emigration and recolonization.
Mean sea surface temperature (SST) dynamics in July–August (1871–2008) (A) and maximal sea ice concentration (SIC) dynamics in July–August (1850–2017) (B) (GAM fit) in potential foraging areas of little auks breeding at Appalersalik / Horse Head (APPE). Black squares represent colony size estimates according to 21,36,101,102 and C. Egevang (personal inf.). A grey horizontal dashed line indicates a physiological upper thermal threshold for Calanus glacialis presence and functioning in Svalbard39,64.
There are only scarce data on zooplankton community composition in the study area. It has been reported that in the Disko Bay (69°N; at the edge of the Northern and Western SST Zones, and southern part of the Northern SIC Zone) in the 1990s, Atlantic Calanus finmarchicus and the two larger Arctic species C. hyperboreus and C. glacialis contributed equally to the copepod biomass5,78. Only in the most recent decades (2005–2018) with the reduction in sea ice cover, have the Arctic copepods declined, and, currently, C. finmarchicus dominates the planktonic biomass in this area5. Given the above-mentioned data and observed dynamics of mean SST with periodic occurrence of colder periods (Fig. 3) and larger amounts of sea ice (Fig. 4 and ref45), one can assume that copepods were available for little auks at least on some foraging grounds, securing their breeding in the area. It cannot be excluded that some sites were periodically abandoned (or present in very small numbers) and then recolonized given alternating patterns of change in environmental conditions. Periodic breeding may not have been detected due to lack of systematic and extensive surveys in the whole study area. Moreover, it cannot be excluded that in some sites, especially those with only singular birds recorded, observed little auks were prospecting subadults individuals or / non-breeding adults.
Observed patterns of SIC change in all zones, especially an abrupt decline after 2000, is in concordance with multiyear autumn ice cover variability in Northern Baffin Bay79. We found that in Southern and Western SIC Zones (with lower SIC values than in the Northern Zone), no breeding sites were active at the beginning of the twenty-first century (Fig. 6). This coincidence may be interpreted in terms of a relationship between zooplankton communities and sea ice. A zooplankton study from Disko Bay (south of the Northern SIC Zone) revealed that spring biomasses of preferred prey of little auks, copepods C. hyperboreus and C. glacialis, were significantly positively correlated with sea ice cover while C. finmarchicus biomass was positively correlated with fraction of warm Atlantic water masses5. The authors of that paper concluded that it is unlikely that Calanus populations were directly impacted by the sea ice, rather that sea ice could serve as a proxy for changes in the ocean and atmospheric circulation patterns that influence the advection of the three Calanus species into Disko Bay.
The process of altering the Arctic ecosystems towards a more temperate state, so-called atlantification or borealization, has already been observed at different trophic levels, and in several regions of the Arctic including Barents Sea, Chukchi Sea, Bering Sea and Greenland (e.g.5,80,81,82,83,84). It is associated with reduction of ice cover and increase in water temperature resulting in changes in the terrestrial and marine ecosystems5,85,86. The studies from Svalbard have revealed that borealization affects top- or meso- predators like seabirds through an effect on their foraging and their reproductive performances (e.g.24,27,85,87). In the consequence, colonies of Arctic species in Svalbard (Brünnich’s guillemot Uria lomvia, ivory gull Pagophila eburnea, glaucous gull Larus hyperboreus, and little auk) have generally declined in size in 2009–2018 while colonies of boreal species (Black-legged kittiwake Rissa tridactyla, northern fulmar Fulmarus glacialis, common guillemot Uria aalge, northern gannet Morus bassanus, and great skua Stercorarius skua) have been increasing27.
In West Greenland borealization process has been documented in Disko Bay (69°N) and included a decline of Arctic copepods and domination of smaller Calanus finmarchicus with lower energy content5. Given little auks’ high energy requirements88 and preference for cold water copepods during the chick-rearing period16,54,89, a borealization of zooplankton communities in West Greenland may soon lead to extirpation of Greenland little auk populations from the Low Arctic zone. This scenario is supported by the low number of breeding sites functioning in the twenty-first century and rapidly retreating sea ice raising prospects of ice-free Arctic seas by 205071,73,90 precluding little auk foraging in MIZ.
Existing estimates of little auk population size summarized in this study indicate a relatively small population, especially compared with Northwest Greenland (north of 75 o N; 33 million pairs91). It cannot be excluded that it was larger and had been reduced by earlier climate change episodes. On the other hand, despite dramatic changes in the West Greenland marine ecosystem in the nineteenth and twentieth centuries, the possible main little auk prey items, cold water copepods, were still available5,92. Thus, it is also possible that the West Greenland population south of 74o N, breeding at the edge of the species range in the Low Arctic zone, was never numerous. Such a small local population is prone to stochastic processes93 so even in temporary optimal foraging conditions they may decrease considerably or even become extirpated because of some local catastrophes, such as disease, predation, overhunting etc.
The little auk is not the only declining seabird species in West Greenland south of 74° N. The numbers of Brünnich’s guillemots breeding there have decreased significantly over the past several decades. The decline has been most severe between Disko Bay and southern Upernavik District. Unsustainable hunting for birds at colonies during the breeding season is the most obvious reason for the observed decline of Brünnich’s guillemots; however, in contrast to a heavy harvest of little auks from the large breeding aggregations in Northwest Greenland14, the harvest in the study area of this species has been very limited (DB unpublished data). On the other hand, other factors such as fisheries bycatch in the 1960s and 1970s is likely to have contributed to population decreases over that period66,94,95,96,97,98. Whilst hunting may not be an important factor affecting the decline of the little auk population in West Greenland south of 74° N, declines in the little auk population may be linked not only directly to environmental changes in the sea but also to more complex changes in the marine ecosystem affecting the species through changes in plankton communities.
Our study provides valuable findings on the little auk population breeding in West Greenland. However, it has some obvious limitations associated with quality of historical data. First, due to a limited number of historical sources concerning the population size of breeding little auks, and a lack of detailed description of survey methods used in many sources, we cannot provide reliable quantitative assessments of the number of breeding pairs in the study area, especially before the 1930s. The majority of studies and surveys where we found information about little auk presence were not focused on searching for little auks. Only studies on Horse Head were dedicated to studying little auks and from this largest colony in the study area reliable colony estimates are available21. Second, available SST and SIC data for long time series are only partially remote sensed (from the 1970th). Data from the 19th and first part of the twentieth century base on modelling and/or historical observations. These combined data have some obvious limitations and a relatively small spatial resolution. SST data from coastal regions considered here are often characterized by lower data certainty compared to the open sea99,100. Also sea ice concentration algorithms suffer from systematic errors in coastal regions74. Nevertheless, patterns of environmental change in the past described in our study are concordant with these reported in other studies (e.g.ref1,59,67) and fill the gap of knowledge about the response of zooplanktivorous mesopredators to variable climatic conditions in West Greenland.
Conclusions
Currently, the breeding population of the little auk in Low Arctic West Greenland is very small (several dozen pairs) and limited to a few breeding sites. Even at the beginning of the twentieth century, the species was not very numerous as a breeder (few thousand pairs). Increases in SST and decreases in SIC, especially in the last few decades, were apparently associated with abandonment of breeding sites previously occupied by little auks. Population declines may be also attributed to more complex changes in the marine ecosystem at finer spatial and temporal scales and/or cannot be easily detected. It cannot be excluded that the local populations in this region were always small and, as such, prone to stochastic processes. In any case, currently observed increases in SST, decreases in SIC and associated borealization of zooplankton communities (leading to dominance of suboptimal food in the foraging areas), may lead to the extirpation of West Greenland little auk populations south of 74o N. This study emphasizes the need for multi-disciplinary studies to better understand the processes driving population dynamics in the present era of climate change.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Drinkwater, K. F. The regime shift of the 1920s and 1930s in the North Atlantic. Prog. Oceanogr. 68, 134–151 (2006).
Evans, P. G. H. & Nettleship, D. N. Conservation of the Atlantic Alcidae. In the Atlantic Alcidae (eds Nettleship, D. N. & Birkhead, T. R.) 428–488 (Academic Press, 1985).
Bode, A. Synchronized multidecadal trends and regime shifts in North Atlantic plankton populations. ICES J. Mar. Sci. 81, 575–586 (2024).
Chust, G. et al. Are Calanus spp. shifting poleward in the North Atlantic? A habitat modelling approach. ICES J. Mar. Sci. 71, 241–253 (2014).
Møller, E. F. & Nielsen, T. G. Borealization of Arctic zooplankton—smaller and less fat zooplankton species in Disko Bay. Western Greenl. Limnol. Oceanogr. 65, 1175–1188 (2020).
Rantanen, M. et al. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 31(3), 1–10 (2022).
Descamps, S. et al. Climate change impacts on wildlife in a High Arctic archipelago—Svalbard. Norway. Glob. Chang. Biol. 23, 490–502 (2017).
Wassmann, P., Duarte, C. M., Agustí, S. & Sejr, M. K. Footprints of climate change in the Arctic marine ecosystem. Glob. Chang. Biol. 17, 1235–1249 (2011).
Wojczulanis-Jakubas, K., Jakubas, D. & Stempniewicz, L. The Little Auk Alle alle: An ecological indicator of a changing Arctic and a model organism. Polar Biol. 45, 163–176 (2022).
Keslinka, L. K., Wojczulanis-Jakubas, K., Jakubas, D. & Neubauer, G. Determinants of the little auk (Alle alle) breeding colony location and size in W and NW coast of Spitsbergen. PLoS ONE 14, e0212668 (2019).
Stempniewicz, L. BWP update. Little Auk (Alle alle). J. Birds West. Palearct. 3, 175–201 (2001).
González-Bergonzoni, I. et al. Small birds, big effects: The little auk (Alle alle) transforms high arctic ecosystems. Proc. R. Soc. B Biol. Sci. 284, 20162572 (2017).
Skrzypek, G. et al. Diversification of nitrogen sources in various tundra vegetation types in the High Arctic. PLoS ONE 10, e0136536 (2015).
Mosbech, A. et al. On the crucial importance of a small bird: The ecosystem services of the little auk (Alle alle) population in Northwest Greenland in a long-term perspective. Ambio 47, 226–243 (2018).
Frandsen, M. S., Fort, J., Rigét, F. F., Galatius, A. & Mosbech, A. Composition of chick meals from one of the main little auk (Alle alle) breeding colonies in Northwest Greenland. Polar Biol. 37, 1055–1060 (2014).
Jakubas, D. et al. Foraging effort does not influence body condition and stress level in little auks. Mar. Ecol. Prog. Ser. 432, 277–290 (2011).
Kwasniewski, S. et al. The impact of different hydrographic conditions and zooplankton communities on provisioning Little Auks along the West coast of Spitsbergen. Prog. Oceanogr. 87, 72–82 (2010).
Karnovsky, N. et al. Foraging distributions of little auks Alle alle across the Greenland Sea: Implications of present and future Arctic climate change. Mar. Ecol. Prog. Ser. 415, 283–293 (2010).
Bradstreet, M. S. W. & Brown, R. G. B. Feeding ecology of the Atlantic Alcidae. In the Atlantic Alcidae (eds Nettleship, D. N. & Birkhead, T. R.) 263–318 (Academic Press, 1985).
Boehnke, R. et al. Supplementary diet components of little auk chicks in two contrasting regions on the West Spitsbergen coast. Polar Biol. 38, 261–267 (2015).
Evans, P. G. H. Ecology and behaviour of the Little Auk Alle alle in West Greenland. Ibis (Lond.1859) 123, 1–18 (1981).
Boehnke, R., Balazy, K., Jakubas, D., Wojczulanis-Jakubas, K. & Błachowiak-Samołyk, K. Meso-scale variations in diet composition of little auk chicks in north-west spitsbergen. Polar Res. 36, 1409585 (2017).
Hovinen, J. E. H. et al. Climate warming decreases the survival of the little auk (Alle alle), a high Arctic avian predator. Ecol. Evol. 4, 3127–3138 (2014).
Descamps, S. et al. Consequences of Atlantification on a Zooplanktivorous Arctic Seabird. Front. Mar. Sci. 9, 878746 (2022).
Jakubas, D. et al. Flexibility of little auks foraging in various oceanographic features in a changing Arctic. Sci. Rep. 10, 8238 (2020).
Hovinen, J. E. H. et al. Fledging success of little auks in the high Arctic: Do provisioning rates and the quality of foraging grounds matter?. Polar Biol. 37, 665–674 (2014).
Descamps, S. & Strøm, H. As the Arctic becomes boreal: ongoing shifts in a high-Arctic seabird community. Ecology 102, e03485 (2021).
Jakubas, D. et al. Gone with the wind—Wind speed affects prey accessibility for a High Arctic zooplanktivorous seabird, the little auk Alle alle. Sci. Total Environ. 852, 158533 (2022).
Jakubas, D., Wojczulanis-Jakubas, K. & Walkusz, W. Response of dovekie to changes in food availability. Waterbirds 30, 421–428 (2007).
Wojczulanis-Jakubas, K., Jakubas, D., Karnovsky, N. J. & Walkusz, W. Foraging strategy of little auks under divergent conditions on feeding grounds. Polar Res. 29, 22–29 (2010).
Amélineau, F., Grémillet, D., Bonnet, D., Bot, T. L. & Fort, J. Where to forage in the absence of sea ice? Bathymetry as a key factor for an arctic seabird. PLoS ONE 11, e0157764 (2016).
Jakubas, D. et al. Intra-seasonal variation in zooplankton availability, chick diet and breeding performance of a high Arctic planktivorous seabird. Polar Biol. 39, 1547–1561 (2016).
Jakubas, D. et al. Foraging closer to the colony leads to faster growth in little auks. Mar. Ecol. Prog. Ser. 489, 263–278 (2013).
Jakubas, D., Wojczulanis-Jakubas, K., Iliszko, L. M., Strøm, H. & Stempniewicz, L. Habitat foraging niche of a High Arctic zooplanktivorous seabird in a changing environment. Sci. Rep. 7, 16203 (2017).
Jakubas, D., Iliszko, L., Wojczulanis-Jakubas, K. & Stempniewicz, L. Foraging by little auks in the distant marginal sea ice zone during the chick-rearing period. Polar Biol. 35, 73–81 (2012).
Salomonsen, F. Grønlands Fugle. The birds in Greenland (Munksgaard København, 1950).
Węsławski, J. M., Stempniewicz, L., Mehlum, F. & Kwaśniewski, S. Summer feeding strategy of the little auk (Alle alle) from Bjørnøya. Barents Sea. Polar Biol. 21, 129–134 (1999).
Jakubas, D., Wojczulanis-Jakubas, K. & Petersen, A. A quiet extirpation of the breeding little auk Alle alle population in Iceland in the shadow of the famous cousin extermination. Sci. Total Environ. 808, 152167 (2022).
Carstensen, J., Weydmann, A., Olszewska, A. & Kwaśniewski, S. Effects of environmental conditions on the biomass of Calanus spp. in the Nordic Seas. J. Plankton Res. 34, 951–966 (2012).
CAVM Team. Circumpolar Arctic Vegetation Map. Conservation of Arctic Flora and Fauna (CAFF) Map No. 1.U.S. Fish and Wildlife Service, Anchorage, Alaska. (2003).
Moe, B. et al. Climate change and phenological responses of two seabird species breeding in the high-Arctic. Mar. Ecol. Prog. Ser. 393, 235–246 (2009).
Jakubas, D. & Wojczulanis-Jakubas, K. Subcolony variation in phenology and breeding parameters in little auk Alle alle. Polar Biol. 34, 31–39 (2011).
Kelsey, K. C., Højlund Pedersen, S., Leffler, A. J., Sexton, J. O. & Welker, J. M. Snow and vegetation seasonality influence seasonal trends of leaf nitrogen and biomass in Arctic tundra. Ecosphere 14, e4515 (2023).
Zheng, J., Jia, G. & Xu, X. Earlier snowmelt predominates advanced spring vegetation greenup in Alaska. Agric. For. Meteorol. 315, 108828 (2022).
Buch, E., Pedersen, S. A. & Ribergaard, M. H. Ecosystem variability in West Greenland waters. J. Northwest Atl. Fish. Sci. 34, 13–28 (2004).
Choquet, M. et al. Genetics redraws pelagic biogeography of Calanus. Biol. Lett. 13, 20170588 (2017).
Falk-Petersen, S., Pavlov, V., Timofeev, S. & Sargent, J. R. Climate variability and possible effects on arctic food chains: The role of calanus. In Arctic Alpine Ecosystems and People in a Changing Environment (eds Børre Ørbæk, J. et al.) (Springer, 2007).
Karnovsky, N. J. et al. Inter-colony comparison of diving behavior of an arctic top predator: Implications for warming in the Greenland Sea. Mar. Ecol. Prog. Ser. 440, 229–240 (2011).
Karnovsky, N. J., Kwaśniewski, S., Wȩsławski, J. M., Walkusz, W. & Beszczyńska-Möller, A. Foraging behavior of little auks in a heterogeneous environment. Mar. Ecol. Prog. Ser. 253, 289–303 (2003).
Hovinen, J. E. H. et al. At-sea distribution of foraging little auks relative to physical factors and food supply. Mar. Ecol. Prog. Ser. 503, 263–277 (2014).
Grémillet, D. et al. Arctic warming: Nonlinear impacts of sea-ice and glacier melt on seabird foraging. Glob. Chang. Biol. 21, 1116–1123 (2015).
Kwasniewski, S. et al. Interannual changes in zooplankton on the West Spitsbergen Shelf in relation to hydrography and their consequences for the diet of planktivorous seabirds. ICES J. Mar. Sci. 69, 890–901 (2012).
Scott, C. L., Kwasniewski, S., Falk-Petersen, S. & Sargent, J. R. Lipids and life strategies of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in late autumn, Kongsfjorden. Svalbard. Polar Biol. 23, 510–516 (2000).
Balazy, K. et al. Molecular tools prove little auks from Svalbard are extremely selective for Calanus glacialis even when exposed to Atlantification. Sci. Rep. 13, 13647 (2023).
Carton, J. A. & Giese, B. S. A reanalysis of ocean climate using simple ocean data assimilation (SODA). Mon. Weather Rev. 136, 2999–3017 (2008).
Jakubas, D., Wojczulanis-Jakubas, K. & Iliszko, L. M. Sex differences in foraging ecology of a zooplanktivorous little auk Alle alle during the pre-laying period: Insights from remote sensing and animal-tracking. Front. Zool. 21, 1–20 (2024).
Walsh, J. E., Chapman, W. L., Fetterer, F. & Stewart, J. S. Gridded Monthly Sea Ice Extent and Concentration, 1850 Onward, Version 2 [Data Set]. Boulder, Colorado USA. National Snow and Ice Data Center. (2019).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. (2022).
Stein, M. Warming periods off Greenland during 1800–2005: Their potential influence on the abundance of cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) in Greenlandic waters. J. Northwest Atl. Fish. Sci. 39, 1–20 (2007).
Fokkema, M. gamtree: GAM-based Recursive Partitioning. (2022).
Zeileis, A. & Hothorn, T. Parties, models, mobsters: A new implementation of model-based recursive partitioning in R. Packag. Vignette (2022).
Zeileis, A., Hornik, K. & Wien, W. Model-based Recursive Partitioning. J. Comput. Graph. Stat. 17, 492–514 (2008).
Borkovec, M. & Madin, N. ggparty: ggplot Visualizations for the partykit Package. (2019).
Alcaraz, M., Felipe, J., Grote, U., Arashkevich, E. & Nikishina, A. Life in a warming ocean: Thermal thresholds and metabolic balance of arctic zooplankton. J. Plankton Res. 36, 3–10 (2014).
Wood, S. N. Generalized Additive Models An Introduction with R (Chapman and Hall/CRC, 2017).
Nettleship, D. N. & Evans, P. G. H. Distribution and Status of the Atlantic Alcidae. In the Atlantic Alcidae (eds Nettleship, D. N. & Birkhead, T. R.) 53–154 (Academic Press, 1985).
Jiang, S., Ye, A. & Xiao, C. The temperature increase in Greenland has accelerated in the past five years. Glob. Planet. Change 194, 103297 (2020).
Matthews, J. A. & Briffa, K. R. The ‘Little Ice Age’: Re-evaluation of an evolving concept. Geografiska Annaler, Series A: Phys. Geogr. 87, 17–36 (2005).
Hanna, E. et al. The influence of North Atlantic atmospheric and oceanic forcing effects on 1900–2010 Greenland summer climate and ice melt/runoff. Int. J. Climatol. 33, 862–880 (2013).
Lapointe, F. et al. Annually resolved Atlantic sea surface temperature variability over the past 2900 y. Proc. Natl. Acad. Sci. U. S. A. 117, 27171–27178 (2020).
Stroeve, J. & Notz, D. Changing state of Arctic sea ice across all seasons. Environ. Res. Lett. https://doi.org/10.1088/1748-9326/aade56 (2018).
Stern, H. L. & Heide-Jørgensen, M. P. Trends and variability of sea ice in Baffin Bay and Davis Strait, 1953–2001. Polar Res. 22, 11–18 (2003).
Notz, D. & Stroeve, J. Observed Arctic sea-ice loss directly follows anthropogenic CO2 emission. Science (80- ) 354, 747–750 (2016).
Maaß, N. & Kaleschke, L. Improving passive microwave sea ice concentration algorithms for coastal areas: Applications to the Baltic Sea. Tellus Ser. A Dyn. Meteorol. Oceanogr. 62, 393–410 (2010).
Lønne, O. J. & Gulliksen, B. On the distribution of sympagic macro-fauna in the seasonally ice covered Barents Sea. Polar Biol. 11, 457–469 (1991).
Arndt, C. E., Gulliksen, B. & Lønne, O. J. 13 Sea ice fauna Ecosystem Barents Sea (Tapir Academic Press, 2009).
Amélineau, F. et al. Arctic climate change and pollution impact little auk foraging and fitness across a decade. Sci. Rep. 9, 1014 (2019).
Madsen, S. D., Nielsen, T. G. & Hansen, B. W. Annual population development and production by Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland. Mar. Biol. 139, 75–93 (2001).
Ballinger, T. J. et al. Abrupt northern baffin bay autumn warming and sea-ice loss since the turn of the twenty-first century. Geophys. Res. Lett. https://doi.org/10.1029/2022GL101472 (2022).
Ingvaldsen, R. B. et al. Physical manifestations and ecological implications of Arctic Atlantification. Nat. Rev. Earth Environ. 2, 874–889 (2021).
Gall, A. E., Morgan, T. C., Day, R. H. & Kuletz, K. J. Ecological shift from piscivorous to planktivorous seabirds in the Chukchi Sea, 1975–2012. Polar Biol. 40, 61–78 (2017).
Polyakov, I. V. et al. Borealization of the Arctic Ocean in response to anomalous advection from Sub-Arctic Seas. Front. Mar. Sci. 7, 516272 (2020).
Divoky, G. J., Lukacs, P. M. & Druckenmiller, M. L. Effects of recent decreases in arctic sea ice on an ice-associated marine bird. Prog. Oceanogr. 136, 151–161 (2015).
Will, A. et al. The breeding seabird community reveals that recent sea ice loss in the Pacific Arctic does not benefit piscivores and is detrimental to planktivores. Deep. Res. Part II Top. Stud. Oceanogr. 181, 104902 (2020).
Vihtakari, M. et al. Black-legged kittiwakes as messengers of Atlantification in the Arctic. Sci. Rep. 8, 1–11 (2018).
Csapó, H. K., Grabowski, M. & Węsławski, J. M. Coming home—Boreal ecosystem claims Atlantic sector of the Arctic. Sci. Total Environ. 771, 144817 (2021).
Ramírez, F. et al. Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. Sci. Rep. 7, 1–9 (2017).
Konarzewski, M., Taylor, J. & Gabrielsen, G. Chick energy requirements and adult energy expenditures of dovekies (Alle alle). Auk 110, 343–353 (1993).
Vogedes, D., Eiane, K., Båtnes, A. S. & Berge, J. Variability in Calanus spp. abundance on fine- to mesoscales in an Arctic fjord: Implications for little auk feeding. Mar. Biol. Res. 10, 437–448 (2014).
Jahn, A., Holland, M. M. & Kay, J. E. Projections of an ice-free Arctic Ocean. Nat. Rev. Earth Environ. 5, 164–176 (2024).
Egevang, C., Boertmann, D., Mosbech, A. & Tamstorf, M. P. Estimating colony area and population size of little auks Alle alle at Northumberland Island using aerial images. Polar Biol. 26, 8–13 (2003).
Pedersen, S. A. & Smidt, E. L. B. Zooplankton distribution and abundance in west Greenland waters, 1950–1984. J. Northwest Atl. Fish. Sci. 26, 45–102 (2000).
Lacy, R. C. Considering threats to the viability of small populations using individual-based models. Ecol. Bull. 48, 39–51 (2000).
Evans, P. G. H. & Waterston, G. The decline of the Thick-billed murre in Greenland. Polar Rec. (Gr. Brit) 18, 283–287 (1976).
Evans, P. G. H. & Kampp, K. Recent changes in Thick-billed Murre populations in West Greenland. In Studies of high-Iatitude seabirds.2. Conservation biology of Thick-billed Murres in the Northwest Atlantic. Occasional Paper Number 69 Canadian WildIife Service (eds Gaston, A. J. & Elliot, R. D.) (Authority of the Minister of Environment Canadian Wildlife Service, 1991).
Merkel, F. et al. Why is the last Thick-billed Murre Uria lomvia colony in central West Greenland heading for extinction?. Bird Conserv. Int. 26, 177–191 (2016).
Evans, P. G. H. The Seabirds of Greenland: Their Status and Conservation. In Status and Conservation of the World’s Seabirds (eds Croxall, J. P. et al.) 49–84 (ICBP, 1984).
Christensen, O. & Lear, W. H. Bycatches in salmon driftnets at West Greenland in 1972. Meddelelser om Grønland, Biosci. 205, 1–38 (1977).
Smit, A. J. et al. A coastal seawater temperature dataset for biogeographical studies: Large biases between in situ and remotely-sensed data sets around the coast of South Africa. PLoS ONE 8, e81944 (2013).
Xie, J., Zhu, J. & Li, Y. Assessment and inter-comparison of five high-resolution sea surface temperature products in the shelf and coastal seas around China. Cont. Shelf Res. 28, 1286–1293 (2008).
Boertmann, D., Mosbech, A., Falk, K. & Kampp, K. Seabird colonies in western Greenland (60˚ – 79˚30ʹ N. lat.). NERI Technical Report No. 170. (1996).
Joensen, A. H. & Preuss, N. O. Report on the ornithological expedition to Northwest Greenland 1965. Meddelelser om Grønland, Biosci. 191, 1–58 (1972).
Kampp, K. & Falk, K. The birds of Ydre Kitsissut Avalliit, Southwest Greenland. Meddelelser om Grønland, Biosci. 42, 1–25 (1994).
Boertmann, D. A annotated checklist to the birds of Greenland. Meddelelser om Grønland, Biosci. 38, 1–63 (1994).
Boertmann, D. & Rosing-Asvid, A. Seabirds and seals in Southeast Greenland. Results from a survey in July 2014. Scientific Report From DCE – Danish Centre for Environment and Energy. Scientific Report From DCE – Danish Centre for Environment and Energy vol. 117 (2014).
Boertmann, D. Seabird colonies and moulting harlequin ducks in South Greenland. National Environmental Research Institute, Denmark. Results of a survey in July 2003. Research Notes from NERI No. 191. (2004).
Vihtakari, M. ggOceanMaps: Plot Data on Oceanographic Maps using ‘ggplot2’. R package version 2.2.0, <https://mikkovihtakari.github.io/ggOceanMaps/>. (2024).
Acknowledgements
We thank five anonymous reviewers for their constructive and helpful comments.
Author information
Authors and Affiliations
Contributions
D.J. drafted the manuscript and performed statistical analyses. D.J., P.E., D.B. contributed to acquisition of data. All authors contributed to conception and design of the study, interpretation of data, revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jakubas, D., Wojczulanis-Jakubas, K., Petersen, A. et al. Decline in the West Greenland population of a zooplanktivorous seabird, the little auk Alle alle. Sci Rep 14, 20686 (2024). https://doi.org/10.1038/s41598-024-71823-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71823-3
- Springer Nature Limited