Abstract
Subjects who have ischemia with non-obstructive coronary arteries (INOCA) experience angina pectoris with evidence of myocardial ischemia but without coronary stenosis. Few studies have investigated factors associated with its survival, especially insulin resistance. In this study, subjects with angina pectoris, without known diabetes mellites (DM), and with non-invasive tests showing myocardial ischemia were admitted for coronary angiography (CAG). Those whose CAG did not reveal stenosis and agreed to receive an oral glucose tolerance test (OGTT) 2 weeks after hospital discharge were enrolled for analysis. All-cause mortality was recorded, which served as the outcome of the study. A total of 587 subjects with INOCA, without known DM, and with OGTT data were analyzed. After OGTT and HbA1c tests, 86 subjects (14.7%) were newly diagnosed with DM and 59.8% had pre-DM. The median duration of follow-up was 7.03 years. Thirty-nine subjects died during the follow-up period. The incidence rate of mortality was 9.9 /1000 person-year. Those who died had a higher fasting glucose (101 ± 17 vs. 94 ± 13 mg/dl, p = 0.003) but a lower estimated glomerular filtration rate (eGFR) (54 ± 22 vs. 87 ± 30 ml/min, p < 0.001). In the Cox survival analysis, a higher fasting glucose (hazard ratio 1.053, p = 0.007) was associated with worse mortality for INOCA without DM (N = 501). On the contrary, a higher eGFR (hazard ratio 0.967, p = 0.012) was protective of better survival for non-diabetic INOCA (N = 501). In conclusion, for non-diabetic INOCA, higher fasting glucose was associated with worse mortality and higher eGFR was protective for better survival.
Similar content being viewed by others
Introduction
Subjects who have ischemia with non-obstructive coronary arteries (INOCA) experience angina-like symptoms, with ischemic evidence on stress electrocardiogram or isotope perfusion scan, but without coronary artery stenosis on coronary angiogram (CAG)1,2,3,4,5,6,7. Endothelium activation, inflammation, oxidative stress, and insulin resistance (IR) are proposed as possible mechanisms underlying INOCA1,4,7,8,9. Studies have revealed that chronic inflammation could mediate microvascular dysfunction and result in impaired coronary flow reserve in INOCA9,10.
Studies have reported that hyperinsulinemia during oral or intravenous glucose tolerance test was more prominent in the INOCA group than in controls, implying that IR could contribute to microvascular angina11,12. Using hyperinsulinemia and the euglycemia clamp test, other investigators found that subjects with INOCA had a higher degree of IR compared to controls13,14. A recent study showed that IR was associated with a decrease in coronary flow reserve during acute hyperglycemia challenge in the oral glucose tolerance test (OGTT), implying the detrimental impact of IR on coronary microvascular function15. Furthermore, subjects with INOCA are more commonly associated with metabolic syndrome, adiposity, and inflammatory derangements1,16,17,18. Obesity and associated inflammation could lead to global structural and functional abnormalities in the microvasculature17,18.
Previous studies in patients with INOCA showed that they have a favorable long-term prognosis19,20. However, data from a large national Danish patient registry showed that patients with stable angina without obstructive coronary disease have a higher risk of major adverse cardiovascular events and all-cause mortality compared to controls21. Some registry reports a two-fold increase in annual rates of major adverse cardiovascular events in subjects with INOCA22,23,24. Furthermore, patients with INOCA also have an adverse quality of life, functional status, and exercise capacity with relatively frequent visits to healthcare providers for persistent or recurring disability symptoms22,24.
Although the pathogenesis of INOCA may be related to IR, no studies have investigated its impact on long-term survival. The objectives of the present study are to investigate IR and glucose regulation in subjects with INOCA without known diabetes mellitus (DM) and to find associated factors related to survival.
Materials and methods
Study population
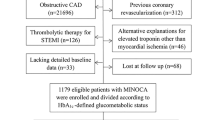
From April 5, 2009, to December 31, 2020, a total of 49,103 cardiac catheterization procedures were performed in our catheterization laboratories, including CAG, percutaneous coronary or peripheral vascular interventions, electrophysiological studies, catheter radiofrequency ablations, and pacemaker implantations. Among them, 25,560 catheterizations were for CAG or CAG plus percutaneous coronary intervention for angina pectoris or acute coronary syndromes. Among patients undergoing these procedures, 1538 patients with angina but without known DM, who were admitted for CAG with non-invasive tests showing evidence of myocardial ischemia, agreed to receive OGTT and blood tests for glycated hemoglobin (HbA1c) and circulating inflammatory markers 2 weeks after hospital discharge (Fig. 1)25. Subjects with significant coronary stenosis (SYNTAX score > 0) 26 or previous histories of surgical or percutaneous coronary revascularization before the index were excluded for analysis in this study. For INOCA (N = 587) (Fig. 1)25,27, we used the definition proposed by Lanza et al. 29 with some minor modifications that included 1. typical stable angina; 2. findings consistent with myocardial ischemia on non-invasive tests, such as diagnostic ST segment depression on exercise electrocardiogram or reversible perfusion defects on stress myocardial scintigraphy; 3. normal (or near normal) CAG (SYNTAX score = 0)26. All-cause mortality was recorded until 31 March 2021 and served as a follow-up result. Mortality data were matched from the National Death Registry and obtained from the Center for Collaboration on Health Information Application, Department of Health, Executive Yuan, Taiwan. The study protocol was approved by the Human Research Review Committee of Taichung Veterans General Hospital (Taichung, Taiwan).
Oral glucose tolerance test, glycated hemoglobin (HbA1c), and insulin resistance indices
Study subjects underwent blood tests and OGTT after 8 h of fasting to investigate abnormal glucose regulation. After collecting a fasting blood sample, a glucose load of 75 g was ingested for 5 min. Blood samples were taken 30 min and 120 min after test load. Blood glucose and insulin concentrations were measured in each sample. Serum insulin was determined using a commercially available assay kit (Roche Diagnostic, Mannheim, Germany). The detectable range was 0.2–1000 µIU/ml. The intra and inter-assay coefficients of variation for insulin were 1.9–2.0% and 2.5–2.8%, respectively. Fasting IR was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR), defined as fasting glucose mg/dl x fasting insulin μU/ml/40528,29. HbA1c was measured by boronate affinity high-performance liquid chromatography (CLC385TM, Primus Corporation, Kansas City, MO, USA). The intra- and inter-assay coefficients of variation for HbA1c [range 22 mmol (4.2%)–191 mmol/mol (19.6%)] were < 0.9% and < 2.9%, respectively.
Estimated glomerular filtration rate (eGFR)
Serum creatinine was obtained at index admission before cardiac catheterization and study recruitment. eGFR was calculated using the Cockcroft-Gault Eq. 30,31.
Statistical analysis
Continuous variables were expressed as median (interquartile range, 25th to 75th percentile) for non-normally distributed data or as mean ± standard deviation for normally distributed data. Categorical data were expressed as percentages. Differences in non-normally distributed continuous variables between INOCA that survived or died were compared using the Wilcoxon rank sum test, while normally distributed data were analyzed using Student’s t test. Categorical variables were compared using the Chi-square test or Fisher’s exact test as indicated. The incidence rate of mortality was calculated by the number of mortalities divided by the sum of the total observation person-years. Cox regression survival analyses were used to test significant variables associated with INOCA subjects who survived or died. Receiver operating characteristic curve (ROC) analyzes were performed using index admission fasting glucose or eGFR to differentiate survival vs. mortality during follow-up. A two-tailed p-value of < 0.05 was considered statistically significant. The SPSS 12.1 statistical software package (SPSS, Inc., Chicago, IL, USA) was used for all calculations.
Results
OGTT and HbA1c data for subjects with INOCA
A total of 587 subjects with INOCA without known DM were enrolled for investigation with OGTT and HbA1c. The mean fasting glucose was 95 ± 14 mg/dl, mean glucose at OGTT 2 h was 139 ± 45 mg/dl and the mean HbA1c was 5.8 ± 0.6 in the INOCA study cohort (Table 1). Subjects with INOCA who died during the follow-up period had significantly higher fasting glucose (101 ± 17 vs. 94 ± 13 mg/dl, p = 0.003) than those who survived (Table 1). On the contrary, subjects with INOCA who died during the follow-up period had significantly lower fasting insulin (8.4 ± 6.5 vs. 11.7 ± 10.0 µIU/ml, p = 0.049) than those who survived (Table 1). Other OGTT data and HbA1c were not significantly different between those who survived or died.
Revision of the diagnosis of abnormal glucose regulation after OGTT and HbA1c tests for subjects with INOCA (status without known DM)
After OGTT and HbA1c tests, only 25.6% of subjects with INOCA, a state without known DM, were in normal glucose regulation, while 86 subjects (14.7%) were newly diagnosed with DM and 351 (59.8%) had pre-DM (Table 2). The proportion of study subjects with normal glucose regulation, DM, and pre-DM was similar between those who survived or died during the follow-up period (Table 2).
Baseline demographic data from subjects with INOCA who had abnormal vs. normal glucose regulation after the investigation
For subjects with INOCA with abnormal glucose regulation, they were significantly older and had a higher BMI and systolic blood pressure (Table 3). Those with abnormal glucose regulation also had significantly lower eGFR (Table 3).
Baseline demographic data of subjects with INOCA who survived or died during the follow-up period
A total of 587 subjects with INOCA without known DM and with OGTT data were analyzed. After a median follow-up duration of 7.03 years, 39 subjects died. The incidence rate of all-cause mortality was 9.9 / 1,000 person-year. Among deaths, 12 were due to cardiovascular mortality and the incidence rate was 3.0/1000 person-year. Those who died were older and had a lower eGFR (54 ± 22 vs. 87 ± 30 ml/min, p < 0.001) and lower body mass index (Table 4).
Cox regression analysis of associated factors for all-cause death in INOCA without known DM
In the Cox survival regression analysis, a higher eGFR (hazard ratio 0.972, p = 0.006) was protective for better survival, while a higher fasting glucose (hazard ratio 1.024, p = 0.009) was related to worse all-cause mortality for INOCA without known DM. Other OGTT values, such as fasting insulin, or 2 h glucose or insulin were not associated with mortality (Table 5). Neither newly diagnosed DM nor pre-DM were associated with all-cause mortality.
Cox regression analysis of associated factors for all-cause death in non-diabetic INOCA (after excluding subjects with newly diagnosed DM)
After excluding subjects with newly diagnosed DM (N = 86), the remaining 501 non-diabetic INOCA were analyzed. Higher fasting glucose (hazard ratio 1.053, p = 0.007) was related to worse all-cause mortality, while higher eGFR was protective for less mortality (hazard ratio 0.967, p = 0.012). Furthermore, older age and higher diastolic blood pressure were associated with worse mortality (Table 6).
The receiver operating characteristic curve (ROC) analysis uses fasting glucose or eGFR data to differentiate between death and survival in non-diabetic INOCA
Using fasting glucose (mg/dl) ROC analysis to predict mortality in non-diabetic INOCA (N = 501), the area under the curve was 0.659 (95% confidence interval 0.565–0.753, p = 0.003) (Fig. 2A). Fasting glucose at 89.5 mg/dl showed a sensitivity of 86.7% and a specificity of 44.4% in predicting mortality during the follow-up period. Using eGFR ROC analysis (ml/min) to predict the survival of subjects with non-diabetic INOCA (N = 501), the area under the curve was 0.848 (95% confidence interval 0.795–0.901, p < 0.001) (Fig. 2B). A cut-off value of eGFR at 73.2 ml/min showed a sensitivity of 67.7% and a specificity of 93.3% to predict survival during the follow-up period.
(A) Analysis of the receiver operating characteristic (ROC) curve using fasting glucose (mg/dl) for predicting the mortality of non-diabetic INOCA (N = 501), the area under the curve was 0.659 (95% confidence interval 0.565–0.753, p = 0.003). (B) Analysis of the ROC curve using the estimated glomerular filtration rate (eGFR) (ml/min) to predict the survival of non-diabetic INOCA (N = 501), the area under the curve was 0.848 (95% confidence interval 0.795–0.901, p < 0.001).
Discussions
Although INOCA may be related to IR, no studies have investigated its impact on long-term survival. In this study, we found that the incidence rate of all-cause mortality in INOCA without known DM was 9.9 /1000 person-year. Furthermore, the incidence rate for cardiovascular mortality was 3.0/1000 person-year. For INOCA without known DM, after OGTT and HbA1c tests, 14.7% were newly diagnosed with DM and 59.8% had pre-DM (Table 2). After excluding subjects with newly diagnosed DM, we found that higher fasting glucose was significantly associated with worse mortality but higher eGFR was protective for better survival in non-diabetic INOCA.
In this study, the investigation of OGTT and HbA1c in subjects with INOCA without known DM revealed a high proportion of subjects with abnormal glucose regulation, including 14.7% with newly diagnosed DM and 59.8% with pre-DM (Table 2). Our previous studies showed that subjects with INOCA had a higher IR, more with hypertension, and a non-significantly higher body mass index compared to the control group2,3. Our group also demonstrated that subjects with CAD had significantly higher insulin at OGTT 2 h than those with INOCA25. We previously showed that newly diagnosed DM through OGTT was associated with an increased risk of composite adverse endpoints in patients with CAD32. Glucose at OGTT 2 h provided greater predictive power to predict major adverse endpoints in CAD than fasting glucose and HbA1c32. However, in this study, it is the higher fasting glucose that is helpful in predicting a worse mortality in non-diabetic INOCA. How fasting or post-challenge IR index translate into adverse events for CAD or INOCA requires further in vivo or in vitro studies to elucidate the underlying mechanism. However, it is worthwhile and productive to investigate abnormal glucose regulation in subjects with INOCA.
Regarding survival studies in INOCA, the WISE study reported the elevated risk of 10-year mortality among women with signs and symptoms of ischemia but without obstructive coronary artery disease (13%) compared to a nationally representative cohort of American women approximately the same age during the same period (2.8%)23. Data from a Danish national patient registry showed that INOCA patients have higher risks of all-cause mortality (hazard ratios of 1.29, p = 0.007) compared to controls21. In this study, the incidence rate of mortality for INOCA without known DM was 9.9 /1000 person-year. Few studies have investigated the associated factors for INOCA survival. This study was extended to show that higher fasting glucose was associated with all-cause mortality for non-diabetic INOCA. Other OGTT values, such as fasting insulin, glucose or insulin at OGTT 2 h, were not associated with mortality.
In this study, we did not work on the underlying mechanism of why higher fasting glucose was associated with worse mortality for non-diabetic INOCA. One study reported that INOCA subjects with normal glycemia had a lower percentage of coronary endothelial dysfunction compared to INOCA with pre-DM, as elucidated by intracoronary acetylcholine-induced coronary blood flow change33. At the 24th month of follow-up, major adverse events were higher in INOCA with pre-DM versus with normal glycemia33. Another report of takotsubo cardiomyopathy showed that patients with hyperglycemia at admission had higher norepinephrine and inflammatory makers and worse clinical outcomes34. Further work on glucose related endothelial dysfunction and inflammatory provocations is warranted to elucidate the underlying mechanism of higher fasting glucose-related worse mortality in non-diabetic INOCA.
There were limitations to this study. First, this INOCA study only used all-cause or cardiovascular mortality as a hard outcome, but did not record admissions for myocardial infarction, heart failure, or stroke. Second, this study conducted OGTT and HbA1c in subjects with INOCA and followed their outcome, but was an observational and association analysis without investigating the underlying mechanism. Therefore, we cannot elucidate the rationale why a higher fasting glucose, but not glucose at OGTT 2 h, was related to all-cause mortality. Third, our INOCA group had a heterogeneous degree of severity of ischemic evidence from radionuclide myocardium perfusion scan or treadmill exercise electrocardiogram, and we did not quantitatively compare their ischemic evidence6. Fourth, in this study, we did not investigate the coronary flow reserve data using non-invasive or invasive methods.
In conclusion, in this study, we found that the incidence rate for all-cause mortality was 9.9 /1000 person-year in INOCA without known DM. After investigation with OGTT and HbA1c tests, subjects with INOCA but without known DM revealed a high proportion (77.4%) of them with abnormal glucose regulation. Higher fasting glucose was significantly associated with worse mortality, while higher eGFR was protective for better survival in non-diabetic INOCA. Aggressive lifestyle modification and renal protection are warranted for these patients.
Data availability
The data sets generated and/or analyzed during the current study are not publicly available because the personal identification data were not anonymous or pseudonymized, but are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CAG:
-
Coronary angiogram
- DBP:
-
Diastolic blood pressure
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated rate of glomerular filtration rate
- HbA1c:
-
Glycated haemoglobin
- HOMA-IR:
-
Assessment of insulin resistance
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- INOCA:
-
Ischemia with non-obstructive coronary arteries
- IR:
-
Insulin resistance
- OGTT:
-
Oral glucose tolerance test
- ROC:
-
Receiver operating characteristic curve
- SBP:
-
Systolic blood pressure
References
Cannon, R. O. 3rd. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J. Am. Coll. Cardiol. 54, 877–885 (2009).
Liao, Y. C. et al. Leptin to adiponectin ratio as a useful predictor for cardiac syndrome X. Biomarkers 18, 44–50 (2013).
Liang, K. W. et al. Circulating adipokines and insulin resistance in subjects with combined cardiac and metabolic syndrome X. Diabetol. Metab. Syndr. 7, 83 (2015).
Spione, F., Arevalos, V., Gabani, R., Sabate, M. & Brugaletta, S. Coronary microvascular angina: A state-of-the-art review. Front. Cardiovasc. Med. 9, 800918. https://doi.org/10.3389/fcvm.2022.800918 (2022).
Taqueti, V. R. & Di Carli, M. F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J. Am. Coll. Cardiol. 72, 2625–2641. https://doi.org/10.1016/j.jacc.2018.09.042 (2018).
Reynolds, H. R. et al. Ischemia with nonobstructive coronary arteries: Insights from the ISCHEMIA trial. JACC Cardiovasc. Imaging 16, 63–74. https://doi.org/10.1016/j.jcmg.2022.06.015 (2023).
Mehta, P. K. et al. Ischemia and no obstructive coronary arteries (INOCA): A narrative review. Atherosclerosis 363, 8–21. https://doi.org/10.1016/j.atherosclerosis.2022.11.009 (2022).
Singh, M., Singh, S., Arora, R. & Khosla, S. Cardiac syndrome X: current concepts. Int. J. Cardiol. 142, 113–119 (2010).
Desideri, G. et al. Endothelial activation in patients with cardiac syndrome X. Circulation 102, 2359–2364 (2000).
Recio-Mayoral, A., Rimoldi, O. E., Camici, P. G. & Kaski, J. C. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc. Imaging 6, 660–667 (2013).
Arthur, H. M. et al. Women, cardiac syndrome X, and microvascular heart disease. Can. J. Cardiol. 28, 42–49 (2012).
Dean, J. D., Jones, C. J., Hutchison, S. J., Peters, J. R. & Henderson, A. H. Hyperinsulinaemia and microvascular angina (“syndrome X”). Lancet 337, 456–457 (1991).
Swan, J. W. et al. Insulin resistance syndrome as a feature of cardiological syndrome X in non-obese men. Br. Heart J. 71, 41–44 (1994).
Piatti, P. et al. Endothelial and metabolic characteristics of patients with angina and angiographically normal coronary arteries: Comparison with subjects with insulin resistance syndrome and normal controls. J. Am. Coll. Cardiol. 34, 1452–1460 (1999).
Botker, H. E. et al. Insulin resistance in microvascular angina (syndrome X). Lancet 342, 136–140 (1993).
Takei, Y., Tomiyama, H., Tanaka, N., Yamashina, A. & Chikamori, T. Association between insulin resistance, oxidative stress, sympathetic activity and coronary microvascular function in patients with early stage impaired glucose metabolism. Circ. J. 86, 866–873. https://doi.org/10.1253/circj.CJ-21-0549 (2022).
Jadhav, S. T. et al. Microvascular function, metabolic syndrome, and novel risk factor status in women with cardiac syndrome X. Am. J. Cardiol. 97, 1727–1731 (2006).
Bajaj, N. S. et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J. Am. Coll. Cardiol. 72, 707–717. https://doi.org/10.1016/j.jacc.2018.05.049 (2018).
Sorop, O. et al. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 113, 1035–1045. https://doi.org/10.1093/cvr/cvx093 (2017).
Lichtlen, P. R., Bargheer, K. & Wenzlaff, P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J. Am. Coll. Cardiol. 25, 1013–1018 (1995).
Lamendola, P. et al. Long-term prognosis of patients with cardiac syndrome X. Int. J. Cardiol. 140, 197–199. https://doi.org/10.1016/j.ijcard.2008.11.026 (2010).
Jespersen, L. et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 33, 734–744. https://doi.org/10.1093/eurheartj/ehr331 (2012).
Herscovici, R. et al. Ischemia and no obstructive coronary artery disease ( INOCA ): What is the risk?. J. Am. Heart Assoc. 7, e008868. https://doi.org/10.1161/JAHA.118.008868 (2018).
Kenkre, T. S. et al. Ten-Year Mortality in the WISE Study (Women's Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 10, https://doi.org/10.1161/CIRCOUTCOMES.116.003863 (2017).
Hansen, B. et al. Ischemia with no obstructive arteries (INOCA): A review of the prevalence, diagnosis and management. Curr. Probl. Cardiol. 48, 101420. https://doi.org/10.1016/j.cpcardiol.2022.101420 (2023).
Liang, K. W. et al. Post-challenge insulin concentration is useful for differentiating between coronary artery disease and cardiac syndrome X in subjects without known diabetes mellitus. Diabetol. Metab. Syndr. 9, 10 (2017).
Serruys, P. W. et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972 (2009).
Liang, K. W. et al. Differential expression of circulating vascular cell adhesion molecule-1 in subjects with coronary artery disease and cardiac syndrome X without known diabetes mellitus. Biomarkers 22, 798–804 (2017).
Lanza, G. A. Cardiac syndrome X: A critical overview and future perspectives. Heart 93, 159–166 (2007).
Reilly, M. P. et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation 110, 803–809 (2004).
Soyama, A. et al. Clinical usefulness of the thickness of preperitoneal and subcutaneous fat layer in the abdomen estimated by ultrasonography for diagnosing abdominal obesity in each type of impaired glucose tolerance in man. Endocr. J. 52, 229–236 (2005).
Wang, X. et al. Evaluation of the clinical value of 10 estimating glomerular filtration rate equations and construction of a prediction model for kidney damage in adults from central China. Front. Mol. Biosci. 11, 1408503. https://doi.org/10.3389/fmolb.2024.1408503 (2024).
Giron-Luque, F., Garcia-Lopez, A., Baez-Suarez, Y. & Patino-Jaramillo, N. Comparison of three glomerular filtration rate estimating equations with 24-hour urine creatinine clearance measurement in potential living kidney donors. Int. J. Nephrol. 2023, 2022641. https://doi.org/10.1155/2023/2022641 (2023).
Chen, W. L. et al. Newly diagnosed diabetes based on an oral glucose tolerance test predicts cardiovascular outcomes in patients with coronary artery disease: An observational study. Medicine (Baltimore) 101, e29557. https://doi.org/10.1097/MD.0000000000029557 (2022).
Sardu, C. et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: The CODYCE multicenter prospective study. Diabetes Care 42, 1946–1955. https://doi.org/10.2337/dc18-2356 (2019).
Paolisso, P. et al. Impact of admission hyperglycemia on heart failure events and mortality in patients with takotsubo syndrome at long-term follow-up: Data from HIGH-GLUCOTAKO investigators. Diabetes Care 44, 2158–2161. https://doi.org/10.2337/dc21-0433 (2021).
Funding
This study was partially supported by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-1123103C, 1123103D, 1113104C, 1113102D, 1103101C, 1103102D).
Author information
Authors and Affiliations
Contributions
K.W.L. designed the investigation and wrote the manuscript; W.H.S. designed the investigation; W.J.L. did the laboratory work; J.S.W. and W.L.L. reviewed the literature and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
We conducted this study in accordance with the Declaration of Helsinki. Our protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital (C08215; date of approval: February 2009 until February 2022). All study participants gave their informed consents.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, KW., Sheu, W.HH., Lee, WJ. et al. Higher fasting glucose is associated with poorer survival in non-diabetic subjects having ischemia with non-obstructive coronary arteries. Sci Rep 14, 20681 (2024). https://doi.org/10.1038/s41598-024-71796-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71796-3
- Springer Nature Limited