Abstract
Sepsis and hypertension pose significant health risks, yet the optimal mean arterial pressure (MAP) target for resuscitation remains uncertain. This study investigates the association between average MAP (a-MAP) within the initial 24 h of intensive care unit admission and clinical outcomes in patients with sepsis and primary hypertension using the Medical Information Mart for Intensive Care (MIMIC) IV database. Multivariable Cox regression assessed the association between a-MAP and 30-day mortality. Kaplan–Meier and log-rank analyses constructed survival curves, while restricted cubic splines (RCS) illustrated the nonlinear relationship between a-MAP and 30-day mortality. Subgroup analyses ensured robustness. The study involved 8,810 patients. Adjusted hazard ratios for 30-day mortality in the T1 group (< 73 mmHg) and T3 group (≥ 80 mmHg) compared to the T2 group (73–80 mmHg) were 1.25 (95% CI 1.09–1.43, P = 0.001) and 1.44 (95% CI 1.25–1.66, P < 0.001), respectively. RCS revealed a U-shaped relationship (non-linearity: P < 0.001). Kaplan–Meier curves demonstrated significant differences (P < 0.0001). Subgroup analysis showed no significant interactions. Maintaining an a-MAP of 73 to 80 mmHg may be associated with a reduction in 30-day mortality. Further validation through prospective randomized controlled trials is warranted.
Similar content being viewed by others
Introduction
Sepsis arises from dysregulated host immune responses to infection, culminating in life-threatening organ dysfunction, posing a significant public health concern1. Epidemiological investigations indicate a persistent rise in the incidence of sepsis, coupled with in-hospital mortality rates remaining high2. Early identification and appropriate management within the initial hours following sepsis onset can ameliorate outcomes3.
Hemodynamic management is integral to the clinical treatment of patients with sepsis4. Its objective is to enhance systemic or regional/microcirculatory blood flow5, facilitating the restoration of tissue oxygenation4. Hence, maintaining an appropriate mean arterial pressure (MAP) is paramount. MAP is a key determinant of mean systemic filling pressure (Pmsf)3. Pmsf represents the pressure generated by the elastic recoil in the systemic circulation during a no-flow state6. Guyton’s model describes Pmsf as one of the determinants of venous return. According to Guyton’s theory, cardiac output is primarily governed by venous inflow7. Therefore, elevating MAP typically results in augmented tissue perfusion and increased supply-side perfusion of tissues. An appropriate MAP is essential to ensure adequate perfusion of vital organs. The 2021 International Guidelines for Management of Sepsis and Septic Shock establish the initial MAP target at 65 mmHg, rather than a higher value3. The European Society of Intensive Care Medicine, on the other hand, suggests a higher MAP in septic patients with a history of hypertension and in patients demonstrating clinical improvement with higher blood pressure8. The SEPSISPAM trial found no significant difference in 28-day or 90-day mortality between patients with septic shock receiving resuscitation targeting a high-target MAP (80—85 mmHg) compared to a low-target MAP (65—70 mmHg). However, among patients with chronic hypertension, those in the high-target group required less renal-replacement therapy than did those in the low-target group9. Concomitant research suggests that the optimal MAP should be individualized, as in specific patients such as those with arteriosclerosis or a history of systemic hypertension, a higher MAP may be warranted10. Therefore, controversy persists regarding the optimal MAP for patients with sepsis and primary hypertension. The purpose of this study is to utilize the Medical Information Mart for Intensive Care (MIMIC) database to explore the relationship between average MAP (a-MAP) within the initial 24 h of intensive care unit (ICU) admission and clinical outcomes in critically ill patients with sepsis and primary hypertension, aiming to offer insights for clinical decision-making.
Methods
Study population
The data for this retrospective cohort study were sourced from MIMIC IV (Version 2.2). MIMIC is an openly accessible database encompassing over 50,000 ICU inpatients at Beth Israel Deaconess Medical Center in Boston, Massachusetts, from the year 2008 to 2019. This comprehensive repository includes diverse patient information, comprising baseline characteristics upon admission, vital signs, laboratory examinations, diagnoses, and treatments. To safeguard patient privacy, the database underwent anonymization, obviating the necessity for further informed consent and ethical review prior to data utilization11. One of our co-authors (Xinhua Jia, certification number: 12022947) obtained access to this database through examination and qualification review, extracting the requisite data for our study.
The inclusion criteria for this study were: (1) patients diagnosed with sepsis; (2) patients with primary hypertension. According to the Sepsis-3 definition1, sepsis was defined as patients with suspected or confirmed infection, accompanied by an acute increase in total Sequential Organ Failure Assessment (SOFA) scores by ≥ 2. Among patients with positive culture results (1082/8810, or 12.28%), the top five positive culture specimens were: blood culture (35.1%), urine (33.0%), MRSA screen (8.9%), sputum (6.7%), and Staph aureus swab (6.1%) (Supplementary Table S1). The extraction of primary hypertension diagnosis was based on the International Classification of Diseases (ICD-9 codes 4010, 4011, 4019, and ICD-10 code I10) (Supplementary Table S2). Exclusion criteria encompassed individuals with missing MAP data, those under the age of 18, and patients with ICU stays lasting less than 24 h. For patients admitted to the ICU more than once, only the first ICU stay was considered12,13.
Variable extraction
In this study, data extraction was performed using PostgresSQL software (Version 13) and Navicat Premium software (Version 15) by executing Structured Query Language. The extracted variables included demographic data, such as age and sex; vital signs, including heart rate, respiratory rate, temperature, and a-MAP; and laboratory test results, including blood white cell count (WBC), platelets, hemoglobin, glucose, blood urea nitrogen (BUN), serum creatinine (sCr), blood sodium, blood potassium, and blood lactate levels. Comorbidities such as congestive heart failure, chronic pulmonary disease, diabetes, and renal disease, as well as disease severity scores, including the Charlson Comorbidity Index (CCI), Simplified Acute Physiology Score (SAPS-II), Oxford Acute Severity of Illness Score (OASIS), and SOFA score (Supplementary Table S3), were also documented. Additionally, we recorded the usage of vasopressor therapy, defined as the administration of one or more of the following: norepinephrine, epinephrine, dopamine, or vasopressin, and the occurrence of acute kidney injury (AKI)14 on the second and seventh day of ICU admission. Septic shock was defined as the use of vasopressor combined with a blood lactate level > 2 mmol/L1,15. All variables were extracted within the initial 24 h of ICU admission, with a-MAP referring to the average value monitored during the first 24 h post-ICU admission.
Primary outcome and secondary outcomes
The primary outcome was 30-day mortality, and the secondary outcomes included in-hospital mortality, length of hospital stay (Los hospital), length of ICU stay (Los ICU), and the incidence of AKI on Day 2 and on Day 7.
Statistical analysis
The study population was stratified into three groups based on the tertiles of a-MAP. Data are presented as mean ± standard deviation (SD) or median (interquartile range) for continuous variables, and as frequency or percentage for categorical variables. Baseline characteristics analysis involved assessing the statistical differences among the a-MAP groups using t-tests or one-way ANOVA for continuous variables and chi-square or Fisher's test for categorical variables. To investigate the association between a-MAP and 30-day mortality, we employed multivariable Cox regression analysis. In addition to an unadjusted model, three additional models were incorporated. Model 1 adjusted for age, sex, vital signs (including heart rate, respiratory rate, temperature, and urinary output on the first day), and admission years. Model 2, in addition to Model 1, included laboratory findings (such as WBC, BUN, sCr, blood sodium, blood potassium, and blood lactate levels). Model 3, the fully adjusted model, encompassed the variables adjusted in the aforementioned models, along with comorbidities such as congestive heart failure and diabetes, disease severity scores including CCI, SAPS-II, OASIS, SOFA score, and vasopressor therapy. After full model adjustment, we explored the nonlinear relationship between a-MAP and 30-day mortality using restricted cubic splines (RCS). To further investigate the relationship between a-MAP and 30-day mortality, we utilized Kaplan–Meier and log-rank analysis to plot survival curves. Subgroup analyses included four subgroups based on age (18–65 years vs. ≥ 65 years), sex (male vs. female), septic shock (no vs. yes), and admission years (2008–2010 vs. 2011–2013 vs. 2014–2016 vs. 2017–2019).
Data analyses were performed utilizing the statistical software packages R 4.2.2 (http://www.R-project.org, The R Foundation) and the Free Statistics Analysis Platform in Beijing, China. The main R packages used include: tableone, rms, ggplot2, survminer, forestplot. A two-tailed test was conducted, with statistical significance defined at a threshold of P < 0.05. Blood lactate level had the highest incidence of missing values (28.4%), followed by body temperature (5.7%). The remaining variables each had missing data rates below 1% (Supplementary Table S4). Before conducting data analysis, multiple imputation techniques were utilized to manage missing values.
Results
Study population
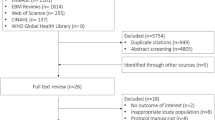
The data for this study were sourced from MIMIC-IV (Version 2.2), comprising 431,231 hospital admissions involving 299,712 patients. Among these admissions, there were 73,181 instances of ICU stays. After excluding patients with repeated ICU admissions (n = 22,261), the final cohort consisted of 50,920 patients undergoing their initial ICU admission. Among them, 22,633 met the diagnostic criteria for sepsis according to Sepsis-3. After further excluding patients without primary hypertension (n = 12,943), a total of 9,690 patients were identified as having sepsis and primary hypertension. Following exclusions for missing MAP data (n = 10), individuals under the age of 18 (n = 0), and those with ICU stays less than 24 h (n = 870), the final analysis included 8,810 patients. For a detailed overview of the inclusion and exclusion processes, refer to Fig. 1.
Baseline characteristics
In our study, a total of 8,810 patients with sepsis and primary hypertension during their initial ICU admission were included. The average age of these patients was 68 years, with females accounting for 42.6%. We grouped the patients based on tertiles of a-MAP and compared the baseline characteristics among the groups (Table 1). In the group with higher a-MAP, patients tended to be younger, demonstrated increased urine output on the first day, and exhibited higher platelet count, hemoglobin, blood glucose, and blood sodium levels. Conversely, they displayed lower WBC, BUN, blood potassium, and blood lactate levels. Regarding comorbidities, the group with higher a-MAP had fewer patients with congestive heart failure, chronic pulmonary disease, diabetes, and septic shock. In terms of scores, the SAPS-II and OASIS were lower in the group with higher a-MAP. Additionally, in this higher a-MAP group, the proportion of patients receiving vasopressor therapy was comparatively smaller.
Primary and secondary outcomes
We stratified a-MAP into tertiles and compared the incidence of primary and secondary outcomes among the three groups. As depicted in Table 2, the T2 group (73–80 mmHg) demonstrated the lowest rates of 30-day mortality and in-hospital mortality compared to the other two groups. Additionally, both Los hospital and Los ICU were the shortest in the T2 group (P < 0.001). However, with the increase in a-MAP, there was a gradual decrease in the incidence of AKI (P < 0.001).
a-MAP and 30-day mortality
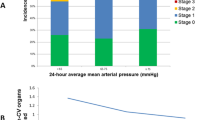
To explore the relationship between a-MAP and 30-day mortality, multivariable Cox regression analysis was conducted. The results (Table 3) revealed that using the T2 group (73–80 mmHg) as the reference, the unadjusted hazard ratios (HR) for T1 (< 73 mmHg) and T3 (≥ 80 mmHg) were 1.57 (95% CI: 1.37–1.78, P < 0.001) and 1.27 (95% CI: 1.11–1.46, P < 0.001), respectively. After adjustment, the HR values were 1.25 (95% CI: 1.09–1.43, P = 0.001) for T1 and 1.44 (95% CI: 1.25–1.66, P < 0.001) for T3. Following full model adjustment, the RCS (Fig. 2) illustrated a U-shaped relationship between a-MAP and 30-day mortality (nonlinear P < 0.001). Kaplan–Meier curves (Fig. 3) indicated that the T2 group (73—80 mmHg) had the highest survival rate (P < 0.0001).
Association between a-MAP and 30-day mortality in patients with sepsis and primary hypertension as indicated by hazard ratio. Solid and dashed lines represent the predicted value and 95% confidence intervals, respectively. The analyses are adjusted for age, sex, heart rate, respiratory rate, temperature, first day's urine output, admission years, WBC, platelet, BUN, sCr, sodium, potassium, lactate level, congestive heart failure, diabetes, CCI, SAPS-II, OASIS, SOFA score, and vasopressor therapy. Only 99% of the data are shown.
Subgroup analysis
As a form of sensitivity analysis, the study population was stratified into several subgroups (Fig. 4), including age, sex, septic shock, and admission years. Within each subgroup, the relationship between a-MAP and 30-day mortality was compared, and the results were consistent with the aforementioned findings.
Association between a-MAP and 30-day mortality according to baseline characteristics. Each stratification adjusted for all the factors (age, sex, heart rate, respiratory rate, temperature, first day's urine output, admission years, WBC, platelet, BUN, sCr, sodium, potassium, lactate level, congestive heart failure, diabetes, CCI, SAPS-II, OASIS, SOFA score, and vasopressor therapy) except the stratification factor itself. T1 (< 73 mmHg), T2 (73–80 mmHg), T3 ((≥ 80 mmHg).
Discussion
This retrospective cohort study identified a U-shaped correlation between a-MAP and 30-day mortality in critically ill patients with sepsis and primary hypertension. An a-MAP between 73—80mmHg may be associated with reduced 30-day mortality, in-hospital mortality, as well as shorter Los hospital and Los ICU in patients with sepsis and hypertension. Subgroup analysis results demonstrated the robustness of these findings. However, within the higher a-MAP group, incidences of AKI on both Day 2 and Day 7 were observed to be the lowest.
Sepsis presents as a life-threatening condition characterized by organ dysfunction and high mortality rates, constituting an increasingly severe global burden1,3. It is closely associated with microcirculatory alterations and tissue hypoxia16. Microvascular dysfunction has been found to correlate with organ dysfunction and mortality in septic patients17. Early improvement in microcirculatory perfusion during resuscitation has been linked to reduced organ failure18. Maintaining adequate perfusion pressure may serve as a crucial pathway toward improving microcirculatory perfusion16. MAP functions as a hemodynamic indicator ensuring appropriate perfusion pressure19. Adequate MAP levels can help mitigate inadequate perfusion and organ failure, representing a widely accepted target for resuscitation and vasopressor titration20. Prolonged low MAP during early sepsis has been associated with increased mortality, underscoring the importance of promptly restoring blood pressure21. Since the first definition of sepsis in 1991, discussions regarding the optimal MAP target have persisted. The Surviving Sepsis Campaign guidelines recommend an initial target MAP of 65 mmHg to maintain critical organ perfusion3. Capillary refill time (CRT), serving as a marker of skin perfusion, has been validated as an indicator of reperfusion. However, studies have demonstrated a poor correlation between MAP and CRT in septic shock patients, suggesting that a MAP > 65 mmHg does not necessarily guarantee normal CRT restoration22.
A retrospective cohort study revealed significantly diminished risks of 30-day, 60-day, and 100-day mortality in the high MAP group (> 65 mmHg) compared to the permissive low MAP group (60—65 mmHg), alongside longer ICU admission time and increased urine output in the high MAP group23. Conversely, continuous episodes of MAP < 73 mmHg were linked to the progression of AKI in critically ill patients with severe sepsis24, implying that setting initial MAP targets too low may jeopardize adequate organ perfusion.
Another prospective study involving 13 patients with septic shock25 observed that raising MAP above 65 mmHg using norepinephrine led to an elevation in cardiac output, enhancement of microvascular function, and a correlation with decreased blood lactate concentration. However, researchers highlighted significant interpatient variability in microvascular responses, indicating the necessity of personalized blood pressure targets.
Hypertension represents a severe and highly prevalent condition marked by functional and structural alterations in resistance arteries, resulting in heightened peripheral vascular resistance26. Research indicates that elevating MAP from 65 mmHg to normative levels in hypertensive patients with septic shock is associated with enhanced microcirculation. This enhancement is characterized by augmented perfused vessel density, a greater proportion of small perfused vessels, and increased microcirculatory flow index 27.
A randomized controlled trial9 comparing high MAP target (80—85 mmHg) and low MAP target (65—70 mmHg) for resuscitating septic shock patients revealed no difference in mortality rates. However, individuals in the high-target group with chronic hypertension necessitated less RRT, suggesting that septic shock patients with concomitant chronic hypertension may require a higher MAP to uphold microcirculation. Consequently, the optimal MAP setting remains unclear when patients with primary hypertension develop sepsis. Our study findings indicate a U-shaped relationship between a-MAP and 30-day mortality in patients with sepsis and primary hypertension. The lowest 30-day mortality was observed within the appropriate a-MAP range (73—80 mmHg), while the incidence of AKI was lowest in the higher a-MAP group (≥ 80 mmHg).
The human body possesses autoregulatory organs such as the heart, brain, and kidneys. Within these organs with autoregulatory function, blood flow remains relatively constant irrespective of changes in perfusion pressure, maintaining within a specific range of MAP values. The self-regulatory threshold of the kidneys is higher than that of the heart or brain. In the event that MAP falls below the self-regulatory threshold of the kidneys, renal blood flow might dip below "physiological" levels, potentially impacting renal function10. Long-standing hypertension can compromise the self-regulatory capacity of the kidneys28, leading to an elevated self-regulatory threshold. Consequently, during sepsis, hypertensive patients may necessitate higher MAP resuscitation targets.
Under normal conditions, arterial stiffness progressively and markedly increases from the elastic aorta to the muscular peripheral arteries, following a distinct gradient that attenuates the transmission of pulsatile pressure to the periphery, thus protecting the microcirculatory network. The heterogeneity of stiffness between elastic and muscular arteries causes the gradient to disappear29. In hypertension, the thickening and hypertrophy of larger conductive arterial walls ultimately lead to increased vessel stiffness and decreased arterial compliance30. Central arterial stiffening can result in a greater proportion of pulsatile power in the arterial pressure and blood flow waveforms perfusing high-flow organs, penetrating deeper into the microcirculation, where it may cause microvascular damage31. Consequently, in sepsis with hypertension, as MAP rises, microcirculatory perfusion increases accordingly. However, when MAP becomes significantly elevated, the pulsatile pressure exerted on the microcirculation may be significantly intensified due to arterial stiffness, exacerbating microcirculatory injury.
The mechanisms underlying the relationship between varying levels of a-MAP and 30-day mortality in critically ill patients with sepsis and primary hypertension currently lack clarity. Further research is warranted to validate these findings. Nonetheless, these results may provide clinicians with valuable insights for clinical decision-making. In the resuscitation of patients with sepsis, a fixed MAP target may not be universally applicable. Tailoring resuscitation targets to individual patients' medical histories could potentially improve prognostic outcomes for these individuals.
Our study has several limitations. Firstly, the inherent constraints of retrospective studies prevent the establishment of a causal relationship between a-MAP and 30-day mortality in patients with sepsis and primary hypertension. Additionally, as this is an observational study, target values for MAP cannot be artificially set. Secondly, Since MIMIC-IV has not yet released textual information, it is currently impossible to obtain patients' pre-admission medication information, nor can we obtain information related to premedication. Therefore, the extraction of primary hypertension in this study was based on admission diagnosis ICD codes, which indeed cannot exclude the possibility that some patients were missed due to incomplete diagnostic information. Thirdly, our data originate from the MIMIC database, where disparities in the site or method of blood pressure measurement may impact the accuracy of MAP measurements. Fourthly, despite adjusting for significant variables that may influence the relationship between a-MAP and 30-day mortality in patients with sepsis and primary hypertension in our model analysis, we cannot entirely eliminate the potential influence of residual confounding factors.
Conclusions
A U-shaped relationship between a-MAP and 30-day mortality is evident in critically ill patients with sepsis and primary hypertension. An a-MAP between 73 and 80 mmHg may be associated with a reduction in 30-day mortality. Further research is necessary to validate these findings.
Data availability
The data for our study is sourced from MIMIC-IV (version 2.2), accessible to all researchers under certain conditions via the following link: "https://physionet.org/content/mimiciv/2.2/".
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 (2016).
Fleischmann, C. et al. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 (2016).
Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
García-de-Acilu, M., Mesquida, J., Gruartmoner, G. & Ferrer, R. Hemodynamic support in septic shock. Curr. Opin. Anaesthesiol. 34, 99–106 (2021).
Scheeren, T. W. L. et al. Current use of vasopressors in septic shock. Ann. Intensive Care 9, 20 (2019).
Repessé, X. et al. Value and determinants of the mean systemic filling pressure in critically ill patients. Am. J. Physiol. Heart Circ. Physiol. 309, H1003-1007 (2015).
Persichini, R. et al. Venous return and mean systemic filling pressure: Physiology and clinical applications. Crit. Care 26, 150 (2022).
Cecconi, M. et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 40, 1795–1815 (2014).
Asfar, P. et al. High versus low blood-pressure target in patients with septic shock. N. Engl. J. Med. 370, 1583–1593 (2014).
Beloncle, F., Radermacher, P., Guerin, C. & Asfar, P. Mean arterial pressure target in patients with septic shock. Minerva Anestesiol. 82, 777–784 (2016).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1 (2023).
Zheng, J. et al. Association between wait time for transthoracic echocardiography and 28-day mortality in patients with septic shock: A cohort study. J. Clin. Med. 11, 215 (2022).
Yang, Q. et al. Association between the β-blocker use and patients with sepsis: A cohort study. Front. Med. (Lausanne) 10, 1272871 (2023).
Kellum, J. A. & Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Hu, W. et al. Identification of indications for albumin administration in septic patients with liver cirrhosis. Crit. Care 27, 300 (2023).
De Backer, D. et al. Microcirculatory alterations: Potential mechanisms and implications for therapy. Ann. Intensive Care 1, 27 (2011).
Hernandez, G. et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J. Crit. Care 28(538), e9-14 (2013).
Trzeciak, S. et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 34, 2210–2217 (2008).
Augusto, J.-F., Teboul, J.-L., Radermacher, P. & Asfar, P. Interpretation of blood pressure signal: physiological bases, clinical relevance, and objectives during shock states. Intensive Care Med. 37, 411–419 (2011).
Munroe, E. S. et al. Evolving management practices for early sepsis-induced hypoperfusion: A narrative review. Am. J. Respir. Crit. Care Med. 207, 1283–1299 (2023).
Vincent, J.-L. et al. Mean arterial pressure and mortality in patients with distributive shock: A retrospective analysis of the MIMIC-III database. Ann. Intensive Care 8, 107 (2018).
Putowski, Z. et al. Correlation between mean arterial pressure and capillary refill time in patients with septic shock: A systematic review and meta-analysis. J. Intensive Care Med. 38, 838–846 (2023).
Zhong, X. et al. Association between different MAP levels and 30-day mortality in sepsis patients: A propensity-score-matched, retrospective cohort study. BMC Anesthesiol. 23, 116 (2023).
Poukkanen, M. et al. Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: Data from the prospective observational FINNAKI study. Crit. Care 17, R295 (2013).
Thooft, A. et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit. Care 15, R222 (2011).
Savoia, C. & Schiffrin, E. L. Vascular inflammation in hypertension and diabetes: Molecular mechanisms and therapeutic interventions. Clin. Sci. (Lond) 112, 375–384 (2007).
Xu, J.-Y. et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: A prospective open label study. Crit. Care 19, 130 (2015).
Guan, Z., Makled, M. N. & Inscho, E. W. Purinoceptors, renal microvascular function and hypertension. Physiol. Res. 69, 353–369 (2020).
Zhang, Y., Lacolley, P., Protogerou, A. D. & Safar, M. E. Arterial stiffness in hypertension and function of large arteries. Am. J. Hypertens 33, 291–296 (2020).
Brown, I. A. M. et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 38, 1969–1985 (2018).
Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 128, 864–886 (2021).
Acknowledgements
We extend our sincere gratitude to the Clinical Scientists' team and the Free Statistics Analysis Platform for their invaluable support.
Author information
Authors and Affiliations
Contributions
X.J., A.Z., and K.M. contributed to conceptualization, methodology. X.J. and H.Z. were responsible for data curation and original draft preparation. W.S. handled visualization. X.J., A.Z., and K.M. participated in reviewing and editing the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics declarations
The Institutional Review Boards of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts, and the Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, have granted approval for the establishment of the database. Informed consent has been obtained for initial data collection, with all participants fully aware and consenting. All research procedures involving human participants adhere to institutional and/or national ethical standards and are in accordance with the 1964 Helsinki Declaration and its subsequent revisions or equivalent ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, X., Zhang, H., Sui, W. et al. Association between average mean arterial pressure and 30-day mortality in critically ill patients with sepsis and primary hypertension: a retrospective analysis. Sci Rep 14, 20640 (2024). https://doi.org/10.1038/s41598-024-71146-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71146-3
- Springer Nature Limited