Abstract
Post-transplantation cancer is a significant cause of mortality among kidney transplant recipients (KTR). The incidence of post-transplantation cancer varies based on geographic region and ethnicity. However, data on KTR from South East Asia, where characteristics differ from other parts of Asia, is lacking. We conducted a retrospective cohort study at a transplant center in Thailand to investigate the incidence of post-transplantation cancer and mortality rates. Factors associated with post-transplantation cancer and patient outcomes were analyzed using competing-risks regression. The study included 1156 KTR with a post-transplant follow-up duration of 5.1 (2.7–9.4) years. The age- and sex-adjusted incidence rate of post-transplant cancer was highest for urothelial cancer (6.9 per 1000 person-years), which also resulted in the highest standardized incidence ratio (SIR) of 42.5 when compared to the general population. Kidney cancer had the second-highest SIR of 24.4. Increasing age was the factor associated with an increased risk of post-transplant cancer (SHR 1.03; 95% CI 1.01–1.05). Human leukocyte antigen (HLA) DR mismatch was associated with a decreased risk of post-transplant cancer (SHR 0.72; 95% CI 0.52–0.98). Post-transplantation cancer was significantly associated with patient mortality (HR 3.16; 95% CI 2.21–4.52). Cancer significantly contributes to KTR mortality, and the risk profile for cancer development in Thai KTRs differs from that of Western and most Asian counterparts. Further research is essential to explore appropriate screening protocols for countries with high rates of urothelial and kidney cancer, including Thailand.
Similar content being viewed by others
Introduction
Kidney transplantation offers substantial advantages for patients with end-stage renal disease (ESRD) in terms of both patient survival and quality of life when compared to those who remain on dialysis1,2. Thanks to advancements in the development of immunosuppressive medications, tissue typing, and organ allocation systems, the short-term survival of kidney transplant recipients (KTR) has significantly improved on a global scale, typically exceeding 95% at 1-year3,4,5. The rate of acute rejection in the first year post-transplant has been reduced to less than 10–15%4,6. Despite these remarkable achievements, the long-term survival of both patients and kidney allografts has not seen significant improvements over the past decade. Cardiovascular disease, infections, and cancer remain significant causes of death among KTR7,8,9,10.
The risk of post-transplant cancer is higher for transplant recipients than in the general population. Several factors contribute to this increased risk among solid-organ transplant recipients11,12,13,14. While immunosuppressive medications are necessary for suppressing the allorecognition process, they also compromise viral immunity, leading to a higher incidence of viral-associated malignancies, such as Kaposi sarcoma, post-transplant proliferative disease (PTLD), anogenital cancers, and hepatocellular cancer12,15,16. Tumor surveillance and containment are also hampered by the use of immunosuppressive medications, both in the induction and maintenance regimens17. Commonly used immunosuppressants, like calcineurin inhibitors (CNIs), promote angiogenesis and tumor growth through increased expression of transforming growth factor β1 (TGFβ1) and vascular endothelial growth factor (VEGF)11. Furthermore, kidney transplant recipients have specific risks for developing kidney and urothelial cancers, such as prolonged dialysis resulting in acquired cystic kidney disease, or a history of aristolochic acid nephropathy as a cause of ESRD12,14.
While skin cancer, lip cancer, and lymphoma are commonly recognized as post-transplantation cancers with the highest standardized incidence ratios (SIR) in cohorts from the US and European countries, data from East Asian countries, including Hong Kong, Taiwan, and South Korea, demonstrate that the post-transplant cancer incidences were different from those in Western countries11,18,19,20,21,22,23,24,25. Geographic region and ethnicity evidently influence the incidence of these post-transplantation cancers. To date, data regarding cancer after kidney transplantation in the Southeast Asian region, where the population differs from other parts of Asia, are lacking. Our study aims to elucidate the incidence, risk factors, and outcomes of post-kidney transplantation cancers in Thailand.
Methods
Study design and study population
We conducted a retrospective cohort study comprising KTR who underwent kidney transplantation at Praram 9 Hospital, Bangkok, Thailand, during the period from January 1, 1992, to December 31, 2022. Praram 9 Hospital is a specialized kidney transplantation center performing approximately 80 cases annually. KTR with a minimum of 30-day follow-up data were included in the cohort, while recipients with primary non-functioning kidney allografts were excluded from the analysis. Patient and kidney allograft statuses were recorded until the last follow-up date. The date of cancer diagnosis was documented upon presentation. Second primary cancers were identified if they occurred in organs distinct from the primary cancer with unrelated tissue histology or if they were of the same type as the primary cancer but with an interval exceeding 5 years. Demographic information at the time of transplantation was documented, encompassing the age and gender of recipients and donors, recipient's body mass index (BMI), medical comorbidities, dialysis duration, human leukocyte antigen (HLA) mismatch, panel reactive antibody (PRA) status, type of kidney transplantation (living donor vs. deceased donor), total ischemic time, induction regimen, and initial maintenance regimen at the time of hospital discharge.
Outcomes measurement
The primary outcome of this study was the incidence of post-kidney transplantation cancer. We conducted an analysis of the median time elapsed from kidney transplantation to the diagnosis of cancer, in addition to examining the mortality rates among KTR who developed specific types of cancer. The risk factors associated with post-transplantation cancer were evaluated. Allograft survival and patient survival were determined, with post-transplantation cancer included as one of the independent factors.
Statistical analysis
Continuous variables were presented as either mean ± standard variation (SD) for normally distributed data or median and interquartile range (IQR) for non-normally distributed data. Categorical data were expressed as the count and percentage. The incidence of post-transplantation cancer was calculated per 1000 person-years (PY) and accompanied by a presentation of the corresponding 95% confidence interval (95% CI). Age and sex were utilized to compute adjusted incidence rates per 1000 PY for each specific cancer. Poisson regression was used to analyze the SIR by comparing with the cancer incidence in the general Thai population as reported by the National Cancer Institute26. Mortality rates for post-transplantation cancer were computed among KTR who developed cancer and compared to KTR without cancer to ascertain the mortality rate ratio. However, due to the unavailability of records on cancer mortality in the general population of Thailand, we refrained from conducting a standardized mortality ratio (SMR) compared with the non-transplant population. In the context of factors associated with cancer development, univariable and multivariable competing-risks regression analyses were conducted, using death as a competing event, and reported the results as subhazard ratios (SHR). Additionally, competing-risks regression was utilized to visualize the cumulative incidence function of deaths attributed to cancer, considering non-cancer deaths as competing events. The median time from kidney transplantation to cancer death were compared with non-cancer deaths by the Wilcoxon rannk-sum test. Competing-risks regression was also conducted to identify factors associated with graft loss, considering death as a competing event. Univariable and multivariable Cox proportional hazard regression analyses were performed to assess factors associated with mortality. In order to mitigate the potential for immortal time bias before the cancer diagnosis, we conducted separate analyses for the time period before and after the cancer diagnosis. This approach involved treating these time segments as time-updated variables for each KTR, thus ensuring a precise allocation of time at risk subsequent to a cancer diagnosis. In all the models, variables with a p-value of less than 0.1 in the univariable models were incorporated into the multivariable models. A significance level of p < 0.05 was considered statistically significant. All statistical analyses were conducted using Stata 17.0 (StataCorp LLC, College Station, TX).
Results
Patient characteristics
A total of 1156 KTR with complete data were included in the cohort (Table 1). The mean age at transplantation was 52.2 ± 12.6 years. Living donor kidney transplantation accounted for 36.8% of cases, and 9.0% received preemptive transplantation. The majority of KTR received basiliximab as an induction therapy (76.2%), while antithymocyte globulin was utilized in 9.2% of cases. The median follow-up time after transplantation was 5.1 (2.7–9.4) years.
Post-transplantation cancer incidence and mortality
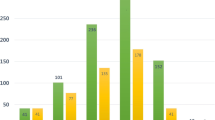
Table 2 presents the overall numbers and percentages of post-kidney transplantation cancer. Urothelial cancer was the most common primary cancer (31.9%), followed by hepatocellular cancer (14.3%). Urothelial cancer also ranked as the leading second primary cancer (25%). The incidence rate, age- and sex-adjusted incidence rate, and standardized incidence ratio (SIR) for each cancer are displayed in Table 3. Urothelial cancer exhibited the highest adjusted incidence rate (6.9 per 1000 person-years), followed by hepatocellular cancer (2.4 per 1000 person-years). Urothelial cancer and kidney cancer had the highest SIRs, with values of 42.5 and 24.4, respectively. Figure 1 illustrates the cumulative incidence of each post-transplant cancer and the median time from kidney transplantation to cancer diagnosis.
Using competing-risks regression, factors associated with the development of post-transplant cancer were identified as shown in Table 4. Increasing age at transplantation was linked to an increased risk of post-transplant cancer (SHR 1.03; 95% CI 1.01–1.05; p-value < 0.001) in the multivariable model. Interestingly, an increasing number of HLA DR mismatches was associated with a decreased SHR of post-transplant cancer (SHR 0.72; 95% CI 0.52–0.98; p-value = 0.038).
Secondary analyses were conducted, focusing on KTR who developed hepatocellular carcinoma. Using a multivariable model for competing-risks regression, it was found that age (SHR 1.05; 95% CI 1.01–1.09; p-value = 0.031), hepatitis B virus surface antigen (HBsAg) positivity (SHR 6.43; 95% CI 1.45–28.04; p-value = 0.013), and anti-hepatitis C virus (anti-HCV) positivity (SHR 20.69; 95% CI 4.11–104.26; p-value < 0.001) among KTR were strongly associated with the development of post-transplant hepatocellular carcinoma.
Table 5 presents the mortality rates of KTR with post-transplant cancer. Hepatocellular cancer displayed the highest mortality rate (145.1 per 1000 person-years), followed by lung cancer (97.8 per 1000 person-years) and gastrointestinal tract cancer (83.7 per 1000 person-years). Figure 2 illustrates the cumulative incidence of cancer-related deaths compared to infection and cardiovascular-related deaths. The median duration from kidney transplantation to cancer-related death was 7.9 (3.9–11.1) years, which was significantly longer than the time to infection-related deaths (4.4 (0.5–9.3) years; p-value = 0.004). However, it was comparable to the time to cardiovascular-related deaths (8.2 (3.9–13.9) years; p-value = 0.256).
Factors associated with KTR death and allograft loss
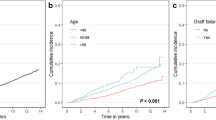
Table 6 presents the results of univariable and multivariable Cox proportional hazard regression for death. The multivariable model demonstrated that post-transplantation cancer was significantly associated with death (HR 3.16; 95% CI 2.21–4.52; p-value < 0.001). Other factors contributing to death included recipient age (HR 1.04; 95% CI 1.02–1.05; p-value < 0.001), preemptive transplantation (HR 0.39; 95% CI 0.21–0.70; p-value = 0.002), donor male sex (HR 1.66; 95% CI 1.16–2.39; p-value = 0.006), and donor age (HR 1.02; 95% CI 1.01–1.03; p-value = 0.002). Figure 3 illustrates the survivor function of KTR with and without post-transplantation cancer, adjusted for recipient age and sex, comorbidities, preemptive transplantation, dialysis vintage, type of transplantation, donor age and sex, total ischemic time, and delayed graft function.
Survivor function of kidney transplant recipients with and without post-transplantation cancer, adjusted for recipient age and sex, comorbidities, preemptive transplantation, dialysis vintage, type of transplantation, donor age and sex, total ischemic time, and delayed graft function. The hazard ratio of post-transplantation cancer for death was 3.16 (95% CI 2.21–4.52; p-value < 0.001).
Factors affecting graft failure are detailed in Table 7. From the multivariable analysis, recipient age (SHR 0.98; 95% CI 0.97–0.99; p-value = 0.005), donor age (SHR 1.02; 95% CI 1.01–1.03; p-value < 0.001), delayed graft function (SHR 2.20; 95% CI 1.45–3.33; p-value < 0.001), and tacrolimus use (SHR 0.45; 95% CI 0.30–0.67; p-value < 0.001) were associated with graft failure. Post-transplantation cancer was not found to be associated with graft loss.
Discussion
This study represents the largest cohort of post-kidney transplantation cancer cases within the South East Asia region. Our findings demonstrate that urothelial cancer has the highest incidence rate among post-transplantation cancers. The incidence rates of urothelial cancer and kidney cancer were 42.5 and 24.4 times higher, respectively, compared to the general non-transplant population. While post-transplantation cancer was significantly associated with patient mortality, its incidence was lower than that of deaths resulting from infection and cardiovascular causes. The occurrence of post-transplantation cancer did not affect graft failure. Notably, recipient age was identified as a factor increasing the risk of post-transplantation cancer, whereas an increased number of HLA-DR mismatches was associated with a decreased risk. Notably, it was unsurprising that the presence of HBsAg and anti-HCV positivity among KTR was associated with the occurrence of post-transplant hepatocellular carcinoma.
Numerous studies have reported varying incidence rates of post-transplantation cancer across different geographic regions and ethnicities. Studies conducted in the United States, European countries, and the Australia-New Zealand registry indicated that the most commonly occurring post-transplantation cancers, as determined by SIRs, were lip cancer, Kaposi sarcoma, and non-melanoma skin cancer21,24,25,27,28,29. In contrast, among Asian populations, the incidence of post-transplantation cancers differs by country. Research conducted in Hong Kong revealed that non-Hodgkin lymphoma and kidney cancer were the predominant post-transplantation cancers19. A Korean cohort reported comparable SIRs for Hodgkin and non-Hodgkin lymphoma, non-melanoma skin cancer, and Kaposi sarcoma22. Intriguingly, the risk of bladder and kidney cancer in a Taiwanese cohort was exceptionally high20, consistent with the SIRs observed in our study.
Several explanations have been proposed to account for the significantly increased risk of urothelial and kidney cancer in KTR. While the mechanisms underlying the elevated risk of post-transplantation cancer typically involve the effects of immunosuppression, which reduce tumor surveillance and increase oncogenic viral replication, specific biological changes in ESRD patients predispose them to urothelial and kidney cancer30,31,32,33,34. For instance, peroxiredoxin, an antioxidant enzyme, is upregulated and highly expressed in dialysis kidneys with acquired cystic kidney disease and renal cell carcinoma, in contrast to renal cell carcinoma in non-dialyzed kidneys35. This finding suggests that one of the pathogenetic mechanisms of renal cell carcinoma in dialysis patients may involve increased oxidative stress, as indicated by the heightened antioxidant signal observed, potentially resulting in cumulative DNA damage35. Factors like hepatocyte growth factor (HGF), hypoxia-inducible factor protein 2 (HIP-2), hypoxia-inducible factor 1-alpha (HIF-1-alpha), and phosphorylated nuclear factor-kappa B (NF-kB) have been found to be upregulated in acquired cysts in chronic kidney disease (CKD) patients associated with renal cell carcinoma36,37. Additionally, uremic toxins, such as p-cresyl sulfate, have been linked to epithelial-mesenchymal transition (EMT), stress fiber redistribution, and the migration of malignant urothelial cells, leading to multifocal urothelial carcinomas in ESRD patients38.
Notably, the heightened risk of urothelial and kidney cancer observed in our Thai cohort, as well as in the Taiwan cohort, may be influenced by additional factors unique to these regions. First, aristolochic acid, a known mutagenic carcinogen, is found in traditional medicine compounds in Taiwan and China39. Kidney and urothelial cancer have a higher prevalence in patients with aristolochic acid nephropathy as a cause of ESRD39. In Thailand, over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) and herbs play a significant but under-recognized role in CKD40. The use of the dried root of Aristolochia tagala, a plant containing aristolochic acid, has been reported in Thai traditional medicine41. It is plausible that KTR who developed urothelial and kidney cancer in our study might have a history of using herbs and traditional medicine. However, the retrospective nature of the study limits this information, as not every KTR underwent a native kidney biopsy to confirm the diagnosis before transplantation. This is especially pertinent among the group defined as having hypertensive nephropathy, as there may be other causes of ESRD within this population. Second, the risk of bladder cancer is associated with the cumulative dose of cyclophosphamide, which is one of the first-line treatments for lupus nephritis in systemic lupus erythematosus (SLE) patients42,43. Studies have shown that Asian SLE patients have a higher prevalence of renal involvement and disease severity compared to Caucasians44,45. It is plausible that the heightened risk of urothelial cancer is potentially linked to the increased utilization of cyclophosphamide and its higher cumulative dosage in Asian populations, which may contribute to the elevated incidence of urothelial cancer. Lastly, BK polyomavirus (BKV) has been established as a causative factor for urothelial cancer in KTR46,47. The prevalence of BKV reactivation after kidney transplantation varies and tends to be higher in Asian populations48,49,50,51. Additionally, recent research has shown that the risk of BKV-associated nephropathy is higher in Asians than in Caucasians52. These findings could contribute to the higher incidence of urothelial cancer in KTR of Asian ethnicity compared to those in Western countries. Furthermore, differences in BKV subtypes among geographic regions may also be linked to the varying incidence of post-transplantation urothelial carcinoma53,54.
The significantly increased risk for urothelial and kidney cancer in KTR has prompted questions regarding screening protocols. Candidates for kidney transplantation typically undergo pre-transplant evaluation, which often includes screening for urinary tract cancer55. However, recommendations for post-transplant screening have been limited. Wong et al. demonstrated that routine post-transplant kidney cancer screening (annually or biennially) may not be cost-effective56. However, their study was based on data from countries with average post-transplant kidney cancer incidence. Further research is needed, especially in the context of the higher incidence of post-transplantation kidney cancer observed in Thailand and Taiwan. The proposed screening protocol currently involves biennial ultrasonography for high-risk KTR (those over 60 years of age with a dialysis history of over 5 years or those with native Bosniak stage 1 or 2 kidney cysts)57. More frequent screening is suggested for KTR with congenital cystic kidney disease or cysts classified as Bosniak stage 2F or higher14,57. For urothelial and bladder cancer, there are no routine screening guidelines58. However, urine cytology and cystoscopy may be recommended for high risk KTR, such as those with a history of high-dose cyclophosphamide exposure, regular use of compound analgesics, or a smoking history of more than 30 pack-years14.
Surprisingly, an increased number of HLA DR mismatches were associated with a lower risk of post-transplantation cancer in this study. The underlying mechanism behind this finding remains unclear. Gao et al. demonstrated a similar protective effect of HLA mismatch in heart and lung transplant recipients against post-transplant skin cancer59. In this US national population-based cohort, HLA DR mismatch exhibited the strongest protective effect against skin cancer development. These results align with the findings from our study regarding the protective effect of HLA DR mismatch. It is postulated that a higher number of HLA mismatches enhance the immune response against tumor and oncogenic viral antigens by activating antigen-presenting cells. Additionally, allogenic T lymphocyte activation may cross-react with tumor antigens, leading to improved tumor surveillance and control59,60.
This study addresses a significant gap in the literature by presenting a large cohort study of post-kidney transplant malignancies in the South East Asian region, where comprehensive data on this topic has been lacking. We conducted thorough analyses to examine the risks and outcomes associated with post-kidney transplantation malignancies compared to other causes of death among KTR. This includes the presentation of mortality rates and mortality risk ratios, which have not been extensively reported in previous studies. Furthermore, we provided detailed insights into the median times to cancer occurrence. Notably, our findings shed light on the impact of HLA DR mismatch, highlighting a promising area for future research.
However, it is important to acknowledge the study's limitations. First, our cohort lacked details on allograft rejection episodes and immunosuppressive medication concentrations and doses. The overall level of immunosuppression or the use of mammalian target of rapamycin inhibitors (mTORi) could potentially affect the incidence of post-transplantation cancer61,62. The study did include data on immunosuppression at the time of first hospital discharge after transplantation, and the majority of patients had unchanged regimens throughout the post-transplantation course. However, since more than 95% of KTR were discharged from the hospital with CNI and mycophenolic acid (MPA), the information regarding the use of mTORi was limited in our cohort. Further studies with adequate power are required to evaluate the effect of mTORi on post-transplant malignancy. Additionally, anti-CD20 antibody is not routinely prescribed as an induction therapy in Thailand, resulting in limited data on this medication in our cohort. Second, the record of post-transplant cancer surveillance was not available. This includes information such as the development of new native kidney cysts after transplantation or de novo hepatitis virus infections, which may be associated with post-transplantation cancer63. Third, BKV reactivation surveillance was not consistently conducted in every case, particularly in the earlier era. This limitation prevented the inclusion of BKV as an independent factor for post-transplantation urothelial cancer. Finally, the calculation of SMR compared to the general population was not performed, as mentioned in the methods section. However, the study did analyze the mortality rate ratio compared to KTR without cancer to determine the impact of each post-transplant cancer.
In conclusion, this study reveals that the risk of developing cancer after kidney transplantation among Thai KTR is significantly increased, particularly for urothelial and kidney cancer. These findings diverge from those in Western countries and most of Asia. Increasing age was associated with an increased risk of post-transplantation cancer, while HLA DR mismatch was associated with a decreased risk. Future research exploring options for incidence-based post-transplantation cancer surveillance and conducting cost-effectiveness analyses are urgently needed to mitigate the burden of post-transplant cancer in Thailand.
References
Tonelli, M. et al. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 11(10), 2093–2109 (2011).
Kaballo, M. A. et al. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 11(3), 389–393 (2018).
Boenink, R. et al. The ERA Registry Annual Report 2019: Summary and age comparisons. Clin. Kidney J. 15(3), 452–472 (2022).
Lentine, K. L. et al. OPTN/SRTR 2021 annual data report: Kidney. Am. J. Transplant. 23(2 Suppl 1), S21-s120 (2023).
Udomkarnjananun, S. et al. The First Asian kidney transplantation prediction models for long-term patient and allograft survival. Transplantation 104(5), 1048–1057 (2020).
Lee, H. S., Kang, M., Kim, B. & Park, Y. Outcomes of kidney transplantation over a 16-year period in Korea: An analysis of the National Health Information Database. PLoS ONE 16(2), e0247449 (2021).
Noppakun, K. et al. A 25-year experience of kidney transplantation in Thailand: Report from the Thai Transplant Registry. Nephrology 20(3), 177–183 (2015).
Ying, T. et al. Death after kidney transplantation: An analysis by era and time post-transplant. J. Am. Soc. Nephrol. 31(12), 2887–2899 (2020).
Thai Society of Transplantation. 2021 Annual Report of Organ Transplantation in Thailand 2021. https://www.transplantthai.org/?page=annual-report-old.
Awan, A. A. et al. Trends in the causes of death among kidney transplant recipients in the United States (1996–2014). Am J Nephrol. 48(6), 472–481 (2018).
Au, E., Wong, G. & Chapman, J. R. Cancer in kidney transplant recipients. Nat. Rev. Nephrol. 14(8), 508–520 (2018).
Al-Adra, D., Al-Qaoud, T., Fowler, K. & Wong, G. De Novo malignancies after kidney transplantation. Clin. J. Am. Soc. Nephrol. 17(3), 434–443 (2022).
Schreiber, B., Abdelrahim, M., Abudayyeh, A. & Murakami, N. Emerging concepts in managing malignancy in kidney transplant patients. Semin. Nephrol. 42(1), 63–75 (2022).
Serkies, K., Dębska-Ślizień, A., Kowalczyk, A., Lizakowski, S. & Małyszko, J. Malignancies in adult kidney transplant candidates and recipients: Current status. Nephrol. Dial. Transplant. 38(7), 1591–1602 (2023).
Vanichanan, J., Udomkarnjananun, S., Avihingsanon, Y. & Jutivorakool, K. Common viral infections in kidney transplant recipients. Kidney Res. Clin. Pract. 37(4), 323–337 (2018).
Nimitpanya, P., Limpanavongsaen, P., Udomkarnjananun, S., Jutivorakool, K. & Vanichanan, J. Post-transplant lymphoproliferative disorder of naso-orbital region in adult renal transplant recipients: A case report and literature review. Transplant. Proc. 52(9), 2731–2735 (2020).
Engels, E. A. Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am. J. Transplant. 19(12), 3223–3232 (2019).
Engels, E. A. et al. Spectrum of cancer risk among US solid organ transplant recipients. Jama 306(17), 1891–1901 (2011).
Cheung, C. Y. et al. Malignancies after kidney transplantation: Hong Kong renal registry. Am. J. Transplant. 12(11), 3039–3046 (2012).
Li, W. H. et al. Malignancies after renal transplantation in Taiwan: A nationwide population-based study. Nephrol. Dial. Transplant. 27(2), 833–839 (2012).
Yanik, E. L., Clarke, C. A., Snyder, J. J., Pfeiffer, R. M. & Engels, E. A. Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J. Am. Soc. Nephrol. 27(5), 1495–1504 (2016).
Jeong, S. et al. Incidence of malignancy and related mortality after kidney transplantation: A nationwide, population-based cohort study in Korea. Sci. Rep. 10(1), 21398 (2020).
Kim, B. et al. De Novo cancer incidence after kidney transplantation in South Korea from 2002 to 2017. J. Clin. Med. 10(16), 3530 (2021).
Friman, T. K. et al. Cancer risk and mortality after solid organ transplantation: A population-based 30-year cohort study in Finland. Int. J. Cancer 150(11), 1779–1791 (2022).
Piselli, P. et al. Variation in post-transplant cancer incidence among Italian kidney transplant recipients over a 25-year period. Cancers (Basel) 15(4), 1347 (2023).
Ministry of Public Health Thailand. Cancer Registry in Thailand 2016–2018. https://www.nci.go.th/th/cancer_record/cancer_rec1.html. (2018).
Vajdic, C. M. et al. Cancer incidence before and after kidney transplantation. Jama 296(23), 2823–2831 (2006).
Benoni, H. et al. Relative and absolute cancer risks among Nordic kidney transplant recipients-a population-based study. Transpl. Int. 33(12), 1700–1710 (2020).
Blosser, C. D., Haber, G. & Engels, E. A. Changes in cancer incidence and outcomes among kidney transplant recipients in the United States over a thirty-year period. Kidney Int. 99(6), 1430–1438 (2021).
Han, Y. et al. Interplay between chronic kidney disease (CKD) and upper tract urothelial carcinomas (UUC): Foe or friend?. Oncotarget 7(33), 53951–53958 (2016).
El-Zaatari, Z. M. & Truong, L. D. Renal cell carcinoma in end-stage renal disease: A review and update. Biomedicines 10(3), 657 (2022).
Lees, J. S. et al. The “other” big complication: How chronic kidney disease impacts on cancer risks and outcomes. Nephrol. Dial. Transplant. 38(5), 1071–1079 (2023).
Xie, X. et al. Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis. Open Life Sci. 18(1), 20220553 (2023).
Yan, L., Chen, P., Chen, E. Z., Gu, A. & Jiang, Z. Y. Risk of bladder cancer in renal transplant recipients: A meta-analysis. Br. J. Cancer 110(7), 1871–1877 (2014).
Fushimi, F. et al. Peroxiredoxins, thioredoxin, and Y-box-binding protein-1 are involved in the pathogenesis and progression of dialysis-associated renal cell carcinoma. Virchows Arch. 463(4), 553–562 (2013).
Konda, R. et al. Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J. Urol. 171(6 Pt 1), 2166–2170 (2004).
Konda, R. et al. Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappaB is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J. Urol. 180(2), 481–485 (2008).
Peng, Y. S., Syu, J. P., Wang, S. D., Pan, P. C. & Kung, H. N. BSA-bounded p-cresyl sulfate potentiates the malignancy of bladder carcinoma by triggering cell migration and EMT through the ROS/Src/FAK signaling pathway. Cell Biol. Toxicol. 36(4), 287–300 (2020).
Das, S. et al. Aristolochic acid-associated cancers: A public health risk in need of global action. Nat. Rev. Cancer 22(10), 576–591 (2022).
Cha’on, U. et al. High prevalence of chronic kidney disease and its related risk factors in rural areas of Northeast Thailand. Sci. Rep. 12(1), 18188 (2022).
Tripatara, P. et al. The safety of Homnawakod herbal formula containing Aristolochia tagala Cham. in Wistar rats. BMC Complement. Altern. Med. 12, 170 (2012).
Chou, W. H. et al. Cyclophosphamide-associated bladder cancers and considerations for survivorship care: A systematic review. Urol. Oncol. 39(10), 678–685 (2021).
Anders, H. J. et al. The management of lupus nephritis as proposed by EULAR/ERA 2019 versus KDIGO 2021. Nephrol. Dial. Transplant. 38(3), 551–561 (2023).
Thumboo, J. & Wee, H.-L. Systemic lupus erythematosus in Asia: Is it more common and more severe?. APLAR J. Rheumatol. 9(4), 320–6 (2006).
Yap, D. Y. & Chan, T. M. Lupus nephritis in Asia: Clinical features and management. Kidney Dis. (Basel) 1(2), 100–109 (2015).
Wang, Y. et al. Viral integration in BK polyomavirus-associated urothelial carcinoma in renal transplant recipients: Multistage carcinogenesis revealed by next-generation virome capture sequencing. Oncogene 39(35), 5734–5742 (2020).
Zeng, Y., Sun, J., Bao, J. & Zhu, T. BK polyomavirus infection promotes growth and aggressiveness in bladder cancer. Virol. J. 17(1), 139 (2020).
Favi, E. et al. Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J. Clin. Cases 7(3), 270–290 (2019).
Ebrahimi, M., Mohebbi, A., Mostakhdem Hashemi, M. & Ashrafi, S. M. Prevalence of BK virus among iranian renal transplant recipients: A systematic review and meta-analysis. J. Clin. Basic Res. 4(4), 50–61 (2020).
Abeywardana, K. D. S. T., Rajamanthri, R. G. L. S., Wazil, A. W. M., Nanayakkara, N. & Muthugala, M. A. R. V. Longitudinal viral kinetic study of BK virus in renal transplant patients: A single-center study in Sri Lanka. J. Clin. Virol. Plus 2(4), 100125 (2022).
Udomkarnjananun, S. et al. A systematic review and meta-analysis of enzyme-linked immunosorbent spot (ELISPOT) assay for BK polyomavirus immune response monitoring after kidney transplantation. J. Clin. Virol. 140, 104848 (2021).
Gately, R. et al. Incidence, risk factors, and outcomes of kidney transplant recipients with BK polyomavirus-associated nephropathy. Kidney Int. Rep. 8(3), 531–543 (2023).
Zheng, H. Y. et al. Relationships between BK virus lineages and human populations. Microbes Infect. 9(2), 204–213 (2007).
Zhong, S. et al. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J. Gen. Virol. 90(Pt 1), 144–152 (2009).
Chadban, S. J. et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney Transplantation. Transplantation. 104(4S Suppl 1), S11–S103 (2020).
Wong, G., Howard, K., Webster, A. C., Chapman, J. R. & Craig, J. C. Screening for renal cancer in recipients of kidney transplants. Nephrol. Dial. Transplant. 26(5), 1729–1739 (2011).
Dharia, A., Boulet, J., Sridhar, V. S. & Kitchlu, A. Cancer screening in solid organ transplant recipients: A focus on screening liver, lung, and kidney recipients for cancers related to the transplanted organ. Transplantation. 106(1), e64–e65 (2022).
Hickman, L. A., Sawinski, D., Guzzo, T. & Locke, J. E. Urologic malignancies in kidney transplantation. Am. J. Transplant. 18(1), 13–22 (2018).
Gao, Y. et al. Association of HLA antigen mismatch with risk of developing skin cancer after solid-organ transplant. JAMA Dermatol. 155(3), 307–314 (2019).
Höhn, H. et al. Human papillomavirus type 33 E7 peptides presented by HLA-DR*0402 to tumor-infiltrating T cells in cervical cancer. J. Virol. 74(14), 6632–6636 (2000).
Hellemans, R., Pengel, L. H. M., Choquet, S. & Maggiore, U. Managing immunosuppressive therapy in potentially cured post-kidney transplant cancer (excluding non-melanoma skin cancer): An overview of the available evidence and guidance for shared decision-making. Transpl. Int. 34(10), 1789–1800 (2021).
Udomkarnjananun, S. et al. Therapeutic drug monitoring of immunosuppressive drugs in hepatology and gastroenterology. Best Pract. Res. Clin. Gastroenterol. 54–55, 101756 (2021).
Chancharoenthana, W. et al. Durability of antibody response against the hepatitis b virus in kidney transplant recipients: A proposed immunization guideline from a 3-year follow-up clinical study. Open Forum Infect. Dis. 6(1), ofy342 (2019).
Author information
Authors and Affiliations
Contributions
S.L. involved with study design, data collection, and writing the first draft of manuscript. N.N., K.B., U.P., N.A., P.L., W.L., R.K., and N.T. involved with data collection and manuscript review. V.M. involved with study design and manuscript review. S.U. involved with study design, statistical analysis, data presentation, writing the first draft of manuscript, manuscript review and edit.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study received approval from the Ethical Review Board of the Praram 9 Hospital, Bangkok, Thailand (RMD.R 002/2566) and the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 0856/66). It was conducted in compliance with the international guidelines for human research protection as described in the Declaration of Helsinki, The Belmont Report, CIOMS Guideline and International Conference on Harmonization in Good Clinical Practice (ICH-GCP). The patient data were de-identified to ensure complete anonymity and untraceability of individual patients. Informed consent for retrospective studies using anonymized existing data is exempted by the ethical committees.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Laowalert, S., Naitook, N., Boonnim, K. et al. Report on post-transplantation cancer in southeast Asia from the Thai kidney transplantation cohort. Sci Rep 14, 20154 (2024). https://doi.org/10.1038/s41598-024-71041-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71041-x
- Springer Nature Limited