Abstract
The objective of this study was to assess the relative contributions of genetic and environmental factors to variation in palatal parameters in twins with completed maxillary growth. The subjects of this study comprised digital dental casts of 50 monozygotic and 35 dizygotic twin pairs. The subjects’ average age was 17.95 ± 2.83 years. Zygosity determination was carried out using 15 specific DNA markers and an amel fragment of the amelogenin gene. The interdental distances were measured between selected dental landmarks at the occlusal and gingival planes. The palatal height, surface area and volume were measured between the gingival plane and the midpalate suture. High heritability estimates were observed for all transverse intra-arch measurements. The palate height (a2 = 0.8), dental arch width in the molar area (a2 = 0.86), palatal surface area (a2 = 0.61) and palate volume (a2 = 0.69) were under strong additive genetic control. Moderate genetic dominance was observed for dental arch widths at the gingival line in the canine (d2 = 0.5) and premolar regions (d2 = 0.78–0.81). Sexual dimorphism was shown, with males exhibiting a greater arch width, palate surface area and volume than females (p < 0.01). The majority of palate parameters variation in twins was controlled by genetic effects, and most were highly heritable.
Similar content being viewed by others
Introduction
The size, shape and transverse dimension of the upper jaw are among the most important factors determining orthodontic treatment options for malocclusions, such as crossbite, dental crowding, lower anterior dentition irregularities and distal lower jaw position1,2. The palate form and volume are closely related to the width of the maxillary dental arch and have an impact on the position of the tongue and breathing function. Understanding facial skeletal and functional pattern changes throughout life and their control mechanisms is crucial for orthodontic treatment planning and subsequent stability3,4. There is ongoing discussion about the importance of genetic and environmental factors on maxillary dental arch and palatal morphology5.
A recent systematic literature review and meta-analysis demonstrated that maxillary arch dimensions have high heritability estimates 6. For the maxillary arch length heritability estimates were above moderate ranging from 0.42 to 0.92 7,8,9. Heritability for the palatal depth was estimated at 0.56 (95% CI range 0.22–0.90)6. The heritability of maxillary transversal dimensions such as intercanine and intermolar widths also have high estimates. Eugushi et al. 7 found these estimates equal 0.86 and 0.82 respectively. Similar estimates reported by Hughes et al.9 (0.84 and 0.87), Lapter et al.10 (0.69 and 0.58).
In contrast, there are studies showing that environmental factors have a greater influence on the formation of dental arches than previously thought11,12. Moreover, there is no doubt that soft tissue imbalance, including mouth breathing, irregular tongue position, irregular posture and other parafunctions, has a major impact on the upper dental arch and palatal formation. Studies have shown that mouth breathers have significantly smaller intermolar widths and palatal volumes and greater palatal heights13. A narrower and longer palate forms because of the short lingual frenulum, and these individuals have narrower arches in transverse dimensions and triangular arch shapes because of frontal tooth proclination 14. Tongue posture is also related to palatal width. Fatima and Fida reported significant differences in maxillary intercanine and intermolar widths at the cusp and gingival levels in patients with different resting tongue postures15. The combination of direct pressure on the teeth and an alteration in the pattern of resting cheek and lip pressures can change the tooth position and dental arch shape16. Mouth breathing allows the tongue to rest on the lower part of the oral cavity. This changes the equilibrium of the forces between the cheeks and the tongue, leading to the development of a narrow maxilla and increased palatal height17.
The similarity of twins within pair sources from shared genes and shared family environment. MZ twins share genetic effects and family environment to the full extent. DZ twins share 50% of additive genetic effects, 25% of non-additive genetic effects and 100% of family environment. MZ twins differ because of person-specific environment, DZ twins—because of unique environment and genes. Due to the underlying genetic and environmental similarities in related individuals, twin studies play a crucial role in understanding the aetiology of malocclusion by enabling the separation of genetic and environmental influences on dental arches and occlusion18.
Structural equation modelling (SEM) is a set of methods which allows checking the hypothesis about the structure of relationship between observed and unobserved (latent variables). The model is usually represented by path diagram which include variables and linear relationship between them (Fig. 1). The combination of twin method and SEM allows precise estimation of the role of genes and environment on the phenotype trait. Even in the postgenomic era, estimation of heritability from twin and sibling studies is foundational for investigating the genes involved in complex traits19.
Path diagram for the univariate twin model. Squares are latent variables (A—additive genetic factors, D—non-additive genetic factors, C—common environmental factors and E—unique environmental factors) shown with their respective path coefficients (a, d, c, e) indicating the relative importance of each of the contributing influences. Circles are observed variables, single-headed arrows are one-way (causal) relationships, and double-headed arrows are two way relationships (covariance).
However, the basic problems with the majority of previous twin studies are the reliability of the twin zygosity determination, the statistical methods used to analyse the twin data and the growth stage of the study sample. Regarding the first problem, for many years, zygosity determination was based on assessments of anthropological similarity, including tooth anatomy20. Although a comparison of physical appearance can provide a reasonably reliable means of determining zygosity, errors can occur in up to 15–20% of cases with this methodology21. The use of blood group determination, as well as serum and enzyme polymorphism analysis, improved the ability to assign zygosities to twins22. More recently, the use of highly polymorphic regions of DNA derived from blood or buccal cells has been shown to accurately measure zygosity in up to 90–95% of cases23. A more precise determination requires an increased number of highly polymorphic regions of DNA24.
The second problem lies in the statistical methods used to analyse the twin data. In twin studies performed 20–30 years ago, the heritability coefficient was calculated using the classical correlation approach. The essential limitation of the heritability coefficient is that it does not estimate the influence of the shared environment, and consequently, the calculated heritability coefficient could be inflated25. The path analysis and Dahlberg quotient used in the 1980s are also not appropriate for today's studies, and model-fitting methods should be used to obtain more accurate data6.
The third problem with twin studies relevant to maxillary dental arch and palate morphology is the maturity of the study sample. Many studies have assessed the maxilla in the intensive growth process of growing children11,26,27. The results of such studies on the heritability of maxillary dental arch and palate final parameters should be interpreted with caution because complete genetic predisposition to maxillary morphology can be detected only if growth is complete.
The aim of this study was to determine the genetic and environmental impacts on the maxillary arch and palatal morphology of twins with completed maxillary growth using structural equation modelling (SEM) and precise zygosity determination.
Results
Descriptive statistics
The results of the descriptive statistics are shown in Table 1. Compared with females, males had slightly greater increases in all parameters of dental arch width at the occlusal plane. The most notable difference at the occlusal plane was registered for 1IPW (p < 0.01), while at the canine and molar regions males had wider dental arches, but with lower statistical significance (p < 0.05). Dental arch widths of males at the gingival line also demonstrated higher values, but the differences had no statistical significance. The palate height at the second premolar and molar region, the palatal surface area and the palate volume in males were significantly greater than those in females (p < 0.01).
Measurement reliability analysis
The results of the measurement error analysis revealed no significant differences between the first and second measurements on the models. The ICCs showed high intrarater reliability for all measurements (0.90–0.96, p < 0.01). Dahlberg’s formula showed a random error of less than 1.0 mm for all linear measurements, 15 mm2 for the palate surface area and 40 mm3 for the palate volume.
Genetic analysis
The AIC values for each model were calculated (Table 2). The most parsimonious model and the lowest values were chosen. The AE and DE models were found to be the most parsimonious for variables. Variables with the best-fitting model of the contribution of factors (a2, c2, d2, e2) were counted. The results of the model-fitting analysis are summarized in Table 3. Variables representing interdental distances between cusp tips of teeth 1IPW, 2IPW and IMW showed high genetic determination (AE model), with a2 = 0.76, 0.72 and 0.86, respectively, and ICW had a strong dominant determination (DE), with d2 = 0.59.
The variables representing interdental distances at the palatal gum lines ICWG, 1IPWG, and 2IPWG had strongly dominant values of d2 = 0.5, 0.78, and 0.81, respectively, while IMWGL had an additive genetic factor of a2 = 0.78.
Variables showing interdental height 2IPH and IMH were affected by additive genetics (a2 = 0.7 and 0.8, respectively), while 1IPH and ICH were determined by dominant genetic factors.
The parameters representing the maxillary depth, palatal area and palatal volume were affected by additive genetic factors.
A model with specific environmental factors (e2) and common environmental factors (c2) was rejected. The AE and DE models were adequate for all variables. Heritability estimates were high for all widths, maxillary depths, palatal surface areas and palatal volumes, ranging from 0.48 to 0.8.
Principal components
Principal component analysis revealed that three principal components explained 69.3% of the total variance. The first component consisted of 1IPW, 1IPWG, 2IPW, 2IPWG, ICWG, IMW, and IMWG and explained 46.2% of the total variance, and the best-fitting model was DE. The palatal surface area and volume (IMW) were determined for the PC2 group, which showed an additive genetic determination of a2 = 0.62 with the best-fitting model AE. PC2 explained 23.2% of the total variance. The third component showed a correlation between two variables, ICW and maxillary depth, and these components showed strong genetic dominance (Table 4, Figs. 2 and 3).
Correlation coefficient densities of palatal variables. MZ monozygotic twins, DZ dizygotic twins, ICW intercanine width, ICWG interfirst premolar width, 2IPW intersecond premolar width, IMW interfirst molar width, ICWG intercanine width at the gum line, 1IPWG interfirst premolar width at the gum line, 2IPWG intersecond premolar width at the gum line, IMWG interfirst molar distance at the gum line, ICH intercanine palate height, 1IPH interfirst premolar palate height, 2IPH intersecond premolar palate height, IMH interfirst molar height, MD maxillary depth, PSA palate surface area, PV palate volume.
Discussion
There are many twin studies assessing genetic and environmental contributions to the upper arch form and palate parameters. However, it is challenging to compare different twin studies due to differences in the sample size, population, zygosity, and statistical methods used. The use of a model-fitting analysis allows the most accurate differentiation of sources of variation affecting the dental arch and palate form and size. This statistical method was used in our study.
Our results showed sexual dimorphism in palatal parameters. Compared with females, males exhibited slightly greater dental arch widths, whereas the palatal surface area and palate volume in males were significantly greater (p < 0.01).
According to our findings, the AE and DE models best explained the variance in the palatal parameters. Interdental distances at the gingival plane are mostly affected by the DE model, except for the IMWG. This means that palatal variances for patients with complete maxillary growth were due to additive genetic factors and specific non shared environmental factors. Distances at the gingival planes are mainly defined by dominant genetic factors.
The correlations for all parameters in the MZ twin analysis were greater than those in the DZ, which is likely due to genetic influences. The variance in the dental arch width and palatal morphology (depth, height, and volume area) had a high genetic contribution. Recent research in twin studies of palatal parameters confirms this finding18,26. In contrast, a longitudinal study with identical and fraternal twins performed by Chaaban et al.11 showed that heritability had a weak influence on palatal transverse variables and was more strongly affected by environmental factors. However, in the Chaaban et al.11 study, heritability patterns were retrospectively obtained from the Pearson correlation coefficient and Falconer’s heritability test.
Lione et al.27 reported that the maxillary arch form is determined by tongue pressure. The dental arch usually has an impact on palate form, but we did not find a remarkable environmental influence on the palatal area or volume variability. We did not examine whether the twins in our study were mouth breathers, so it was difficult to evaluate the tongue position and possible impact on the dental arch palate. Our results showed that genetic contributions have a remarkable impact on the variability of palatal parameters.
For patients with complete maxillary growth, the suture of the maxilla is ossified after approximately 13 years28, and treatment with conventional RME possibly causes only buccal inclination of the teeth, which can lead to relapse. To expand the palate, surgery and miniscrew-assisted RME may be needed. Palatal suture ossification may occur even at eleven years of age, and chronological age is not reliable for determining suture development29. According to our findings, the palatal surface area (a2 = 0.61), palate volume (a2 = 0.69), and maxillary arch depth (a2 = 0.56) are mostly affected by additive genetic factors. These findings suggest that miniscrew-assisted RME should be considered a better choice than conventional RME for maxillary expansion in patients older than 11 years for expansion stability. Although it has been proven that even with MARPE treatment, long-term stability is not reliable, dental and skeletal relapse are still observed over time30. The heritability estimates in the area of the first molars (a2 = 0.86) were the highest of all the parameters. These are teeth where the RME appliance is bonded, and due to the strong genetic influence on the width of the dental arch between the first molars, relapse is more likely to occur. The high heritability estimates maxillary intermolar width was reported by Eguchi et al.7 (0.82) and Hughes et al.9 (0.87).
The genetic influence on the dental arch width in the canine region is lower (a2 = 0.59), and possibly, environmental factors are more responsible for this transverse dimension. The genetic influence on the dental arch and palatal variables in the canine region showed reduced genetic dominance (ICW d2 = 0.59, ICWG d2 = 0.5, ICH d2 = 0.48). This is in agreement with findings of King et al. 31 and Cassidy et al. 32 reporting estimates of ICW at 0.53 and 0.56 respectively. This can be explained by the stronger environmental influence in this area, such as the position of the tongue, swallowing parafunction and mouth breathing. These abnormal functions can lead to a flatter and narrower palate and maxillary anterior tooth protrusion33.
The results of our study may have some clinical relevance and may be useful in predicting the response of different parts of the dental arch to various orthodontic interventions, especially maxillary dental arch expansion. The occlusal variables that are more influenced by genetic factors may be less receptive to corrective procedures; in contrast, traits that are more influenced by environmental factors may be more prone to respond positively and demonstrate a greater level of stability after orthodontic treatment. The most important finding from this study regarding orthotreatment planning is that changes in the dimensions of the dental arch and palate should not surpass the biological limits. The balance between bone, dental, and muscular structures should be maintained, as deviations from the original shape of the dental arch may increase the likelihood of treatment relapse to a genetic norm.
The present study has several strengths, including DNA-based zygosity determination and the use of a model-fitting analysis that allowed for a more accurate partitioning of different sources of variation affecting the palate parameters. This study also has some limitations. The complete dental and medical records were not available for all twins, and a questionnaire was used to determine whether previous orthodontic treatment was undertaken, which may have led to bias. Additionally, this study involved a sample of Lithuanian twins, and thus the generalizability of the findings to other populations may be limited.
Conclusion
Palatal dimensions have high heritability. The majority of dental arch widths at the occlusal line, palate height, palatal surface area, palate volume and maxillary arch depth were found to be under strong-to-moderate additive genetic control. Maxillary dental arch inter-canine width and widths at gingival line in premolar regions demonstrated dominant genetic determination. Sexual dimorphism was shown, with males exhibiting greater arch width, palate surface area and volume than females.
Methods
Study sample
The present study sample consisted of dental casts of 50 monozygotic (19 males and 31 females) and 35 dizygotic (19 males and 16 females) twin pairs of the same sex. Twins were selected from the Twin Centre of the Lithuanian University of Health Sciences (LSMU). All twins were of European ancestry. Their mean age was 17.95 ± 2.83 years. The protocol of the study was approved by the Regional Ethical Committee No. BE-2-41, and informed consent was given by the twins and their parents of any participant younger than 18 years. The study was conducted in accordance with relevant guidelines and regulations.
The following inclusion criteria were applied: (1) full adult dentition not including the third molars and (2) maxillary growth largely completed (defined as age > 15). The twins were excluded on the basis of the following criteria: (1) existing restorations involving landmarks on the cusps and incisal regions, (2) previous orthodontic treatment, (3) poor quality or damaged models, (4) excessive wear of the teeth, or (5) dental anomalies such as supernumerary or missing teeth.
Dental arch and palate measurements
Alginate dental impressions were obtained from the study participants. A three-dimensional 3Shape scanner (3Shape e3, Copenhagen, Denmark) with a reported accuracy of 7/10 μm was used. (Scan time 18 s, Resolution 2 cameras 5.0 megapixels) was used to obtain 3D data from dental casts (format STL) of maxillary dentition and palate.
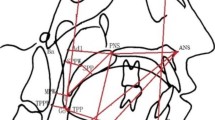
The definitions of the measurements used in the study are presented in Table 5. Linear measurements were calculated utilizing the selected dental landmarks as well as the maxillary occlusal plane as a plane of reference (Fig. 4). The maxillary occlusal plane was defined as the midpoint between a line connecting the central point of the incisal edges of the two maxillary central incisors and the mesiobuccal cusp tips of the maxillary first molars (Fig. 4a). The widths at the gingival level were measured connecting the appropriate points at the dentogingival junctions of the teeth on the palatal side (Fig. 4a). The gingival plane and distal plane were used as margins for the palate. The gingival plane was obtained by connecting the midpoints of the dentogingival junction of all permanent teeth on the palatal side (Fig. 4a). The distal plane was created through two points at the distal aspect of the first molars perpendicular to the gingival plane (Fig. 4b). Palate height was measured as the distance between the line connecting the centres of the dentogingival junctions of the canines, first and second premolars, and first molars on the palatal side and the highest point of the palatal vault on the midpalatal rafe (Fig. 4c). The measurements of the palate surface area and palate volume are presented in Fig. 4d and were performed according to the methods proposed by Kecik34 and Primožič et al.35.
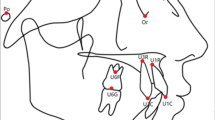
The upper dental arch parameters. (a) The upper dental arch widths were defined as the distances between the two reference points at the occlusal and dento-gingival junctions. The interdental distances were measured between the cusp tips of the canines, first premolars, second premolars, and first molars at the occlusal plane and between the centers of the dento-gingival junction of the canines, first premolars, second premolars and first molars at the palatal side. (b) The upper dental arch depth. Distance between a tangent from the incisal edge of the central incisors and a line connecting the contact point between the first molar mesiobucal cusps. (c) Palate height. Distance between the line connecting the centers of the dento-gingival junctions of second premolars on the palatal side and the highest point of the palatal vault on the midpalatal rafe. (d) Palate surface area—area below the gingival plane and limited by the distal plane; palate volume—volume below the gingival plane and limited by the palate surface and distal plane.
All linear landmark-based dimensions were calculated using the open-source universal 3D processing and animation software Blender 3.4.1 with the “3D Print Toolbox”. The digitization of the landmarks was conducted by a single investigator (VP). Prior to data collection, the investigator (VP) was calibrated in the use of the software.
Measurement error
Measurements were performed twice on the digital models by the same investigator, with a 1-month time interval on both members of 20 randomly selected twin pairs to determine measurement error. Intraobserver method error was assessed using the intraclass correlation coefficient (ICC) of reliability and the method suggested by Bland and Altman36. The estimated random error between the measurements was calculated using the Dahlberg formula:
Zygosity determination
Zygosity determination was carried out using a DNA test. The polymerase chain reaction set AmpFlSTR® Identifiler® (Applied Biosystems, USA) was used to amplify short tandem repeats, and 15 specific DNA markers (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TROX, D18S51, D5S818, FGA) and an amel fragment of the amelogenin gene were used for comparison of genetic profiles. Zygosity determination using this molecular genetic technique reached 99.9% accuracy21.
Heritability estimation
Heritability was estimated by structural equation modelling (SEM) with the OpenMx software package (http://openmx.psyc.virginia.edu) and R code examples provided at https://github.com/OpenMx/OpenMx. The variance of a trait was estimated by evaluating the contributions of three factors: the additive genetic factor (A), the shared environment (C), the nonadditive genetic factor (D), and the unique environment (E)37. As the C and D components cannot be estimated simultaneously in twins reared together, only the ACE (or ADE) models with two degrees of freedom were tested 38. Univariate ACE/ADE models were constructed with standardized path coefficients and expected variance and covariance matrices. The goodness of fit of the full and reduced ACE/ADE models were compared with a univariate saturated twin model imposing equal means and variance restriction across twins and zygosity to maximize information.
The Akaike information criterion (AIC) statistic and the difference in the chi-square (χ2) value relative to the chance in degrees of freedom provided an indication of the models’ goodness of fit. The most parsimonious model (lowest AIC value) to explain the observed variance was selected 39.
Principal component analysis
Principal component analysis (PCA) of the palatal measurements was performed using the “Psych package” (Procedures of Psychological, Psychometric and Personality Research) to reduce dimensionality and to assess correlations between variables. The principal components were rotated using varimax rotation. A variable was considered a component if the absolute value of the component loading was greater than 0.5.
Statistical analysis
Descriptive statistics included the mean and standard deviation. The normality of the data distribution was tested with the Shapiro–Wilk test. Parametric Student’s t tests were applied for comparisons of quantitative variables between two independent groups. Pearson’s correlation coefficient was calculated, and the difference between the two correlation coefficients was computed. Statistical analyses were performed in the statistical computing environment R (version 4.3.3). P values less than 0.05 were considered to indicate statistical significance.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
McNamara, J. A. Maxillary transverse deficiency. Am. J. Orthod. Dentofac. Orthop. 117, 567–570 (2000).
McNamara, J. A. The role of the transverse dimension in orthodontic diagnosis and treatment. in Growth Modification: What Works, What Doesn’t and Why? Monograph 36, Craniofacial Growth Series, Center for Human Growth and Development (ed. McNamara, J. A. J.) (The University of Michigan, 1999).
Bondevik, O., Espeland, L. & Stenvik, A. Dental arch changes from 22 to 43 years of age: Are they different in individuals with high versus low mandibular plane angle?. Eur. J. Orthod. 37, 367–372 (2015).
Cakan, D. G., Ulkur, F. & Taner, T. U. The genetic basis of facial skeletal characteristics and its relation with orthodontics. Eur. J. Dent. 6, 340–345 (2012).
Townsend, G. et al. Genetic and environmental influences on dentofacial structures and oral health: Studies of Australian twins and their families. Twin Res. Hum. Genet. 9, 727–732 (2006).
Giri, J. et al. Heritability of dental arches and occlusal characteristics: A systematic review and meta-analysis. Eur. J. Orthod. 45, 854–867 (2023).
Eguchi, S. et al. Genetic contribution to dental arch size variation in Australian twins. Arch Oral. Biol. 49, 1015–1024 (2004).
Corruccini, R. S. & Potter, R. H. Genetic analysis of occlusal variation in twins. Am. J. Orthod. 78, 140–154 (1980).
Hughes, T., Thomas, C., Richards, L. & Townsend, G. A study of occlusal variation in the primary dentition of Australian twins and singletons. Arch. Oral Biol. 46, 857–864 (2001).
Lapter, V., Slaj, M., Muretić, Z. & Weber, D. Orthodontic anomalies and differences in gnathometric variables in twins. Acta Stomatologica Croatica. 25, 25–31 (1991).
Chaaban, M. et al. Longitudinal changes in the dental arch width and symmetry in identical and fraternal twins. Am. J. Orthod. Dentofac. Orthop. 162, 704–713 (2022).
Negishi, S., Richards, L. C., Hughes, T., Kondo, S. & Kasai, K. Genetic contribution to palatal morphology variation using three-dimensional analysis in Australian twins. Arch. Oral Biol. 115, 104740 (2020).
Habumugisha, J. et al. Three-dimensional evaluation of pharyngeal airway and maxillary arch in mouth and nasal breathing children with skeletal Class I and II. BMC Oral Health 22, 320 (2022).
Fujita, Y., Ohno, Y., Ohno, K., Takeshima, T. & Maki, K. Differences in the factors associated with tongue pressure between children with class I and Class II malocclusions. BMC Pediatr. 21, 476 (2021).
Fatima, F. & Fida, M. The assessment of resting tongue posture in different sagittal skeletal patterns. Dent. Press J. Orthod. 24, 55–63 (2019).
Gultom, F. P., Muzdalifah, J. F., Hasriati, E. & Auerkari, E. I. Genetic, epigenetic, and environmental influences on dental arch variation. AIP Conf. Proc. 2344, 020010 (2021).
Vieira, B. B. et al. Influence of adenotonsillectomy on hard palate dimensions. Int. J. Pediatr. Otorhinolaryngol. 76, 1140–1144 (2012).
Lin, T.-H., Hughes, T. & Meade, M. J. The genetic and environmental contributions to variation in the permanent dental arch form: A twin study. Eur. J. Orthod. 45, 868–874 (2023).
Martin, N., Boomsma, D. & Machin, G. A twin-pronged attack on complex traits. Nat. Genet. 17, 387–392 (1997).
Lundstrom, A. & McWilliam, J. S. A comparison of vertical and horizontal cephalometric variables with regard to heritability. Eur. J. Orthod. 9, 104–108 (1987).
Vuollo, V. et al. Comparing facial 3D analysis with DNA testing to determine zygosities of twins. Twin Res. Hum. Genet. 18, 306–313 (2015).
Townsend, G., Hughes, T., Luciano, M., Bockmann, M. & Brook, A. Genetic and environmental influences on human dental variation: A critical evaluation of studies involving twins. Arch. Oral Biol. 54, S45–S51 (2009).
Nyholt, D. R. On the probability of dizygotic twins being concordant for two alleles at multiple polymorphic loci. Twin Res. Hum. Genet. 9, 194–197 (2006).
Šidlauskas, M. et al. Heritability of mandibular cephalometric variables in twins with completed craniofacial growth. Eur. J. Orthod. 38, 493–502 (2016).
Tenesa, A. & Haley, C. S. The heritability of human disease: Estimation, uses and abuses. Nat. Rev. Genet. 14, 139–149 (2013).
Birant, S., Koruyucu, M., Kasimoglu, Y., Veznikli, M. & Seymen, F. Assessment of dental arch parameters in Turkish twins. J. Clin. Pediatr. Dent. 46, 160–170 (2022).
Lione, R. et al. Palatal surface and volume in mouth-breathing subjects evaluated with three-dimensional analysis of digital dental casts—A controlled study. Eur. J. Orthod. 37, 101–104 (2015).
Thilander, B. Dentoalveolar development in subjects with normal occlusion. A longitudinal study between the ages of 5 and 31 years. Eur. J. Orthod. 31, 109–120 (2009).
Georgi, G. M. et al. Age-related changes in the midpalatal suture: Comparison between CBCT staging and bone micromorphology. Bone 179, 116984 (2024).
Zeng, W. et al. Long-term efficacy and stability of miniscrew-assisted rapid palatal expansion in mid to late adolescents and adults: A systematic review and meta-analysis. BMC Oral Health 23, 829 (2023).
King, L., Harris, E. F. & Tolley, E. A. Heritability of cephalometric and occlusal variables as assessed from siblings with overt malocclusions. Am. J. Orthod. Dentofac. Orthop. 104, 121–131 (1993).
Cassidy, K. M., Harris, E. F., Tolley, E. A. & Keim, R. G. Genetic influence on dental arch form in orthodontic patients. Angle Orthod. 68, 445–454 (1998).
Yu, M. et al. Orthodontic appliances for the treatment of pediatric obstructive sleep apnea: A systematic review and network meta-analysis. Sleep Med. Rev. 72, 101855 (2023).
Kecik, D. Three-dimensional analyses of palatal morphology and its relation to upper airway area in obstructive sleep apnea. Angle Orthod. 87, 300–306 (2017).
Primožič, J., Perinetti, G., Richmond, S. & Ovsenik, M. Three-dimensional longitudinal evaluation of palatal vault changes in growing subjects. Angle Orthod. 82, 632–636 (2012).
Martin Bland, J. & Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327, 307–310 (1986).
Neale, M. C. & Cardon, L. R. Methodology for Genetic Studies of Twins and Families (Springer Science & Business Media, 1992).
Grayson, D. A. Twins reared together: Minimizing shared environmental effects. Behav. Genet. 19, 593–604 (1989).
Akaike, H. Factor analysis and AIC. Psychometrika 52, 317–332 (1987).
Acknowledgements
We wish to express our sincere thanks to the twins and their families who agreed to participate in this study. This work was supported by the Lithuanian University of Health Sciences Research Foundation.
Author information
Authors and Affiliations
Contributions
M.Š.—conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing of the original draft, review and editing.V.P. – data curation, methodology. M.Š. – data collection, formal analysis. A.Š. – supervision, formal analysis, writing, review and editing, project administration, data collection, funding acquisition. S.J. – statistical analysis and formal analysis. K.L. – formal analysis, funding acquisition, supervision, writing, review and editing, project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Šidlauskienė, M., Papievis, V., Šidlauskas, A. et al. Genetic and environmental impact on variation in the palatal dimensions in permanent dentition: a twin study. Sci Rep 14, 19785 (2024). https://doi.org/10.1038/s41598-024-70985-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70985-4
- Springer Nature Limited