Abstract
The association between insulin resistance (IR) and the risk of all-cause mortality and cardiovascular mortality among osteoarthritis (OA) patients remains uncertain. This study aims to clarify the correlation between a novel marker of IR, the triglyceride glucose-body mass index (TyG-BMI), and the risk of all-cause mortality and cardiovascular mortality in OA patients. Data from the National Health and Nutrition Examination Survey (NHANES) spanning from 1999 to 2020 were analyzed. Multivariable Cox proportional hazards regression analysis and restricted cubic spline plots were employed to elucidate the association between the TyG-BMI index and the risk of all-cause mortality or cardiovascular mortality in OA patients. Additionally, subgroup analysis was conducted to explore potential interactions and identify populations at elevated risk of mortality. The study cohort comprised 4097 OA patients who were followed up for a period of 20 years, during which 1197 cases of all-cause mortality and 329 cases of mortality attributed to cardiovascular disease were recorded. Our findings revealed a U-shaped nonlinear relationship between the TyG-BMI index and the risk of all-cause mortality or cardiovascular mortality in OA patients, with the lowest mortality risk thresholds identified at 282 and 270, respectively. Moreover, surpassing these thresholds was associated with a 3% increase in the risk of all-cause mortality and a 5% increase in the risk of cardiovascular mortality for every 10-unit increment in TyG-BMI level. Among American OA patients, a U-shaped nonlinear relationship exists between the TyG-BMI index and the risk of all-cause mortality or cardiovascular mortality. These findings underscore the significant role of IR in the progression of OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) stands as the predominant form of chronic arthritis, inflicting pain, joint deformity, and escalating healthcare expenditures1, particularly among the elderly population, affecting an estimated 240 million individuals worldwide2,3. The etiology of OA is intricate, involving a nexus of genetic, biological, and biomechanical factors1. Recent decades have witnessed a surge in OA prevalence owing to demographic aging and mounting obesity rates4. Notably, mounting evidence underscores that OA patients face heightened susceptibility to cardiovascular fatalities and all-cause mortality relative to the general populace5,6,7. Consequently, the pursuit of prognostic biomarkers for OA to prognosticate and mitigate the long-term mortality risk represents a paramount endeavor, notably concerning cardiovascular-related mortality events.
Obesity is widely acknowledged as a cardinal risk factor precipitating the onset and progression of OA8. Historically, mechanical attrition resultant from excess weight, joint overburden, or malformations has been the predominant explanatory paradigm1,8. However, contemporary research posits OA as a multifaceted malady, implicating diverse factors such as adipose deposition, inflammatory mediators, and insulin resistance (IR) in the genesis and progression of obesity-associated OA8,9. Current scholarship delineates IR and type 2 diabetes mellitus (T2DM) as independent risk factors expediting OA progression10,11,12,13. IR denotes a state of diminished sensitivity and responsivity to insulin action14, intricately entwined with aberrations in glucose metabolism, lipid dysregulation, maladjusted inflammatory cascades, and oxidative stress13,15. Prolonged IR precipitates pathological alterations including the accumulation of inflammatory mediators within joints, cartilaginous degeneration, osteoporosis, and obesity, thereby fostering OA onset and advancement9. Moreover, the amalgamation of IR and microinflammation mechanisms has emerged as the predominant explanatory framework for OA9,13. Nevertheless, a lacuna persists in research concerning the potential of IR to prognosticate adverse outcomes such as all-cause mortality and cardiovascular mortality events among OA patients.
The triglyceride-glucose (TyG) index, serving as a surrogate marker for assessing IR, deviates from traditional methodologies (e.g., hyperinsulinemic-euglycemic clamp technique and IR steady-state model assessment) by virtue of its simplicity, cost-effectiveness, and widespread applicability16. Leveraging this index, a substantial body of literature posits that amalgamating the TyG index with obesity metrics such as waist-to-height ratio (WtHR), body mass index (BMI), and waist circumference (WC) markedly enhances the efficacy of IR assessment17. Not only does the construction of TyG-BMI bolster assessment precision, but its incorporation of obesity metrics also enhances the targeting of OA prediction, given obesity’s recognized status as an independent risk factor for OA. Consequently, employing the TyG-BMI index as a metric for evaluating IR to prognosticate the risk of all-cause or cardiovascular mortality in OA patients represents a pragmatic approach.
NHANES, a nationally representative cross-sectional survey in the United States, is meticulously crafted to gauge the health and nutritional status of the American populace. Leveraging the expansive dataset from NHANES, this study delves into the plausible correlation between the TyG-BMI index and mortality rates among OA patients. The primary objective is to furnish clinicians with a novel, convenient, and dependable tool, to discern the prognostic landscape of OA. Grasping the underpinnings of mortality risk factors in OA patients assumes pivotal significance for efficacious management and timely interventions aimed at enhancing patient survival rates.
Methods
Study design and data collection
This study employed a retrospective cohort study design, utilizing data extracted from the NHANES spanning from 1999 to 2020. NHANES, a comprehensive survey program representative of the U.S. population, provides detailed information accessible at http://www.cdc.gov/nchs/nhanes. Employing a complex, multi-stage, stratified design, NHANES conducts household interviews and examinations at Mobile Examination Centers (MECs). The database is meticulously managed and maintained by the National Center for Health Statistics (NCHS). Prior to participation, written informed consent was obtained from all individuals, with this study receiving approval from the NCHS Institutional Review Board (IRB). Additional details can be found at https://www.cdc.gov/nchs/nhanes/irba98.htm. Given NHANES’s provision of a publicly available database containing anonymous individual information, no further ethical approvals or informed consents were deemed necessary. Furthermore, the methodologies employed in this study strictly adhere to pertinent guidelines and regulations.
Study population
The study cohort comprised adult participants diagnosed with OA. Exclusion criteria encompassed: (1) individuals younger than 20 years old, (2) those lacking a diagnosis of arthritis or diagnosed with other arthritis types, (3) individuals without hematologic laboratory test or covariate data, (4) those with missing or undisclosed mortality data, and (5) individuals unable to obtain sample data weights.
Measurement of OA and TyG-BMI index
OA diagnosis was conducted via a questionnaire survey, utilizing questions such as “Has a doctor ever told you that you have arthritis?” (MCQ160a), with response options of “yes” or “no”, and “What type of arthritis is this?” (MCQ195; MCQ191; MCQ190), providing options, including rheumatoid arthritis, osteoarthritis, psoriatic arthritis, etc.
The hematologic laboratory indicators essential for this investigation were sourced from NHANES laboratory data. NHANES employs automated analyzers to enzymatically measure plasma triglyceride (TG) and fasting plasma glucose (FPG) levels from fasting blood samples collected from individuals who fasted for a minimum of 8 h but less than 24 h. The TyG-BMI index was calculated using the following formula: BMI = weight (kg)/height (m2); TyG index = Ln[1/2 fasting glucose (mg/dL) × fasting triglycerides (mg/dL)]; TyG-BMI = TyG index × BMI.
Ascertainment of mortality
Mortality rates within the study cohort were established by linking NHANES data with the National Death Index (NDI) mortality files, accessible through public links as of December 31, 2019. This linkage process was achieved using probabilistic matching algorithms. Additionally, causes of death were ascertained utilizing the International Classification of Diseases, Tenth Revision (ICD-10)18.
Covariates
This study comprehensively incorporated covariates based on independent risk factors associated with OA, as identified in prior research. These covariates encompass age, sex, race, income-to-poverty ratio, education level, BMI, diabetes, hypertension, smoking habits, alcohol consumption, and laboratory examinations, aimed at mitigating confounding bias. Trained interviewers utilized computer-assisted personal interviewing (CAPI) systems to conduct household and sampled population surveys, gathering demographic factors such as age, sex, race, income-to-poverty ratio, and education level. Physical measurements were obtained by health technicians at the Mobile Examination Center (MEC), with BMI calculated as weight (kg) divided by height (m) squared. The international BMI classification was employed, categorizing individuals as normal weight (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25–29.9 kg/m2), obese (BMI: 30–39.9 kg/m2), and morbidly obese (BMI ≥ 40 kg/m2).
Smoking status was categorized based on participants’ responses to survey questions (SMQ020: ever smoked 100 cigarettes, SMQ040: current smoking status), identifying never smokers, former smokers, and current smokers. Never smokers were defined as individuals who had never smoked 100 cigarettes in their lifetime and currently do not smoke, while current smokers were those who had smoked 100 or more cigarettes and currently smoke. Former smokers were individuals who had smoked 100 or more cigarettes but currently do not smoke. Alcohol consumption was delineated based on self-reported drinking frequency, encompassing heavy, moderate, light, and never drinkers. Heavy drinking was self-reported as consuming ≥ 4 or 5 drinks per day, moderate drinking as ≤ 3 drinks per day, light drinking as having consumed alcohol in the past but not in the last year or < 12 times, and never drinking as never having consumed alcohol.
Diabetes and hypertension status were identified through a combination of questionnaire responses and laboratory data to ensure accuracy. Questionnaires included queries such as “Has a doctor ever told you that you have diabetes?”, “Do you take insulin?”, “Do you take oral hypoglycemic drugs?”, or laboratory data such as fasting blood glucose concentration ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, oral glucose tolerance test (OGTT) blood glucose level ≥ 11.1 mmol/L, etc. Similarly, hypertension diagnosis relied on multiple blood pressure readings ≥ 130/80 mmHg or self-reported diagnoses by a doctor.
Statistical analysis
To ensure the national representativeness of our sample, we applied MEC weights as delineated in the NHANES weighting guidelines (https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx). Continuous variables are reported as weighted means ± standard errors, while categorical variables are presented as frequencies and weighted proportions. Differences between quartiles (Q1-Q4) of the TyG-BMI index were evaluated using the chi-square test (for categorical variables) or Kruskal-Wallis test (for continuous variables).
Multivariable Cox proportional hazards regression analysis was conducted to explore the association between the TyG-BMI index and mortality, adjusting for demographic characteristics (Model 2) and all covariates (Model 3). Hazard ratios (HR) and 95% confidence intervals (CI) were used to quantify these associations. Restricted cubic splines were employed to depict any potential nonlinear association between the TyG-BMI index and mortality, enabling visualization of survival risk discrepancies across various TyG-BMI index levels. Threshold effect analysis was performed to delineate the specific association between the TyG-BMI index and mortality in a segmented manner and to identify threshold inflection points.
Subgroup analyses were carried out to assess the potential influence of other variables on the relationship between the TyG-BMI index and mortality, thus validating the robustness of our findings and identifying populations with survival disadvantages. All p-values were two-sided, with statistical significance set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics 25.0 and R version 4.3.1.
Ethics approval
All participants provided written informed consent before undergoing the NHANES survey, and the survey received approval from the NCHS Institutional Review Board (IRB), as detailed at https://www.cdc.gov/nchs/nhanes/irba98.htm. As NHANES is a publicly available database with anonymized personal information, no additional ethical approval or informed consent was necessary.
Results
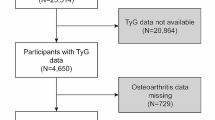
Between 1999 and 2020, NHANES conducted surveys encompassing a cohort of 126130 participants. After stringent exclusion criteria and data integrity checks, the final study cohort comprised 4097 patients diagnosed with OA (Fig. 1). The mean age of participants was 64.5 ± 14.5 years, with 63.8% being female and 36.2% male. Table 1 provides a comprehensive overview of baseline characteristics among patients, stratified by quartiles of the TyG-BMI index, revealing notable between-group disparities (p < 0.05) in age, gender, race, BMI, poverty-to-income ratio, smoking status, alcohol consumption, and diabetes prevalence. Over the ensuing 20-year follow-up period, encompassing individuals diagnosed with OA, a total of 1197 all-cause deaths and 329 cardiovascular-related deaths were recorded. Additionally, we assessed the differences in predictive abilities of TyG and TyG-BMI for mortality in OA patients using ROC curves. As illustrated in Fig. 2, TyG-BMI demonstrates superior performance compared to TyG in predicting both all-cause and cardiovascular mortality, aligning with findings from previous studies.
Cox regression analyses detailed in Table 2 delineate the nuanced associations between the TyG-BMI index and mortality, considering both continuous variables and quartile groupings. Upon evaluating the TyG-BMI index as a continuous variable through simple linear models, no consistent findings emerged across the three covariate-adjusted models. However, upon categorizing the TyG-BMI index into quartiles, individuals with OA in the second and third quartiles (Q2, Q3) exhibited a reduced risk of all-cause mortality compared to those in the lowest quartile (Q1), irrespective of adjustment models.
Illustrated in Fig. 3, restricted cubic spline plots elucidate a U-shaped nonlinear relationship between the TyG-BMI index in OA patients and both all-cause and cardiovascular mortality (nonlinear P < 0.05), adjusting for all covariates. Beyond a TyG-BMI index threshold of 282, heightened TyG-BMI levels were associated with elevated all-cause mortality risk in OA patients. Specifically, with every 10-unit increment in TyG-BMI level, there was a 3% rise in all-cause mortality risk (95% CI 2–5%, p < 0.001). Conversely, below this threshold, mortality risk displayed an inverse correlation with TyG-BMI level (HR = 0.995, 95% CI 0.994–0.997, p < 0.001, Table 3). Analogous U-shaped relationships were observed concerning TyG-BMI and cardiovascular mortality, with the lowest risk threshold identified at 270. Cardiovascular mortality risk exhibited a 4 and 5% alteration per 10-unit change in TyG-BMI level before and after the threshold, respectively. Additionally, from Fig. 3, it is visually discernible that upon reaching a TyG-BMI index level of 600, all-cause mortality risk in OA patients escalated up to fivefold, while cardiovascular mortality risk surged nearly tenfold compared to the threshold level.
Subgroup analyses scrutinized the impact of additional covariates on the TyG-BMI index’s association with mortality. Dichotomizing the TyG-BMI index based on the lowest risk threshold level, OA patients with higher TyG-BMI index values demonstrated a protective effect against all-cause mortality risk (HR = 0.77, 95% CI 0.68–0.87, Fig. 4) relative to those with lower TyG-BMI index levels (all-cause mortality < 282, cardiovascular mortality < 270). Furthermore, the presence of diabetes influenced this association (p for interaction = 0.021), with a more pronounced protective effect observed among non-diabetic patients. OA patients with diabetes symptoms exhibited a heightened survival disadvantage compared to those without diagnosed diabetes.
Discussion
The research conducted reveals a U-shaped nonlinear relationship between TyG-BMI levels and all-cause or cardiovascular mortality in patients with OA. The findings suggest that the lowest risk of death occurs at the inflection point of 282 and 270 TyG-BMI levels. When the TyG-BMI level exceeds the minimum risk threshold, there is a subsequent increase in the risk of all-cause mortality by 3% (95% CI 2–5%, p < 0.001) and cardiovascular mortality by 5% (2–7%, p < 0.001) for every 10-unit increase. Overall, maintaining TyG-BMI levels at the inflection point of the lowest risk could potentially lead to better long-term prognosis for OA patients.
OA is a complex disease that affects various joint tissues, including joint cartilage, subchondral bone, and synovium13. Previous studies have established a close relationship between T2DM and OA in terms of occurrence and development12,13,19. Epidemiological evidence indicates that the incidence of OA in T2DM patients is approximately 30%19. Additionally, research has identified 12 shared target genes between T2DM and OA20. Elena’s study demonstrates that while clinical manifestations differ, these two diseases are closely associated with cellular metabolic disorders and IR related to energy metabolism10.
IR plays a crucial role in the progression of OA, primarily through three main mechanisms: (1) direct interference with energy metabolism, (2) inflammatory activation, and (3) lipid metabolism disorder and obesity. Joint cartilage, which acts as a vital structure that absorbs mechanical stress between active bone surfaces, relies on insulin for maintenance10. Human chondrocytes express functional insulin receptors, and insulin can stimulate synthetic reactions, enhance the synthesis of type II collagen and proteoglycan, inhibit the expression of collagenase and matrix metalloproteinase (MMP)-1 and 13, and counteract the effects associated with interleukin (IL-1β)21. Insulin therapy in animal and human studies has shown improvements in cartilage degradation and the reversal of cartilage loss22,23. In OA patients, there is a decrease in the expression of insulin receptors, impaired ability of insulin-dependent glucose transporters (GLUTs) to stimulate glucose uptake, and subsequently, these factors impact cell metabolism, regeneration, and repair24.
Moreover, IR-induced inflammation poses significant challenges for OA patients, both locally within joints and systemically with low-level inflammation13. In individuals with OA, IR or sustained chronic hyperglycemia leads to excessive production of oxidative stress (free radicals), pro-inflammatory cytokines (IL-1β, TNF-α, prostaglandins), and advanced glycation end products (AGEs) in joint tissues, thereby contributing to a systemic inflammatory state13. This inflammation results in increased production of protein hydrolyzing enzymes that degrade the cartilage matrix (MMPs and aggrecanases)13. Furthermore, under conditions of IR, compensatory insulin secretion is required to meet energy metabolism requirements25. Studies have indicated that high insulin concentrations (10–500 nM) can contribute to cartilage degradation, macrophage infiltration, and chemokine production in OA26,27.
IR contributes to the redistribution of adipose tissue and disruptions in lipid metabolism28. Visceral obesity serves as a significant source of pro-inflammatory cytokines, fostering low-level chronic metabolic inflammation that can result in structural damage to joints29. Furthermore, leptin, a major adipokine secreted by adipose tissue, has been found to promote chondrocyte apoptosis and enhance the production of cytokines and MMPs by chondrocytes30. Additionally, IR and obesity are linked to elevated levels of free fatty acids (FFAs), which may similarly influence the progression of OA31.
IR is well-established as a contributor to cardiovascular disease32. However, the impact of lower IR on mortality remains underexplored. Our study found that low levels of TyG-BMI are linked to increased mortality in OA patients, suggesting that excessively low IR may adversely affect patient outcomes, consistent with previous research on TyG and mortality33,34,35. This phenomenon is not yet fully explained, and existing explanations are not entirely convincing. In an insulin-sensitive state, cellular synthesis and glucose metabolism are highly efficient, leading to reduced endogenous insulin production36. Insulin’s role as an endocrine hormone is not fully understood37,38. Many endocrine hormones have an optimal physiological concentration range, and deviations can be harmful39. Additionally, increased metabolic activity may accelerate aging and organ decline, potentially reducing life expectancy40. These explanations are based on current knowledge and warrant further experimental investigation to elucidate the underlying mechanisms.
Our study, based on a large sample cohort from the American NHANES database, sought to elucidate the correlation between the TyG-BMI index and all-cause mortality as well as cardiovascular mortality in patients with OA. The overarching goal was to identify novel prognostic indicators that could predict an increased risk of death in OA patients and provide clinicians with innovative methods for early identification of high-risk OA patients. These emerging biomarkers from peripheral blood offer accessibility, cost-effectiveness, and ease of dissemination.
Limitations
While our study has yielded significant findings, it is essential to acknowledge several limitations. Firstly, the reliance on cross-sectional laboratory test data may constrain our ability to accurately capture longitudinal changes or responses to intervention measures. To enhance our understanding of temporal fluctuations in IR status within the human body, dynamic monitoring of TyG-BMI index could offer more profound insights. Secondly, the data extracted from the NHANES database primarily stem from household interviews and questionnaire surveys, potentially introducing reporting inaccuracies or recall biases. Despite the rigorous validation procedures established by authoritative institutions like NCHS, inherent limitations and risks persist. Finally, although we carefully considered numerous covariates, unaccounted confounding factors may still impact the validity of our conclusions. Notably, due to limitations in the NHANES database, we were unable to include medication use as a covariate.
Conclusions
Our study unveils a U-shaped nonlinear relationship between TyG-BMI levels in OA patients and all-cause mortality or cardiovascular mortality, pinpointing a specific TyG-BMI index threshold level linked to the lowest mortality risk. This discovery equips clinicians with a fresh perspective and intervention target to promptly identify and intervene with OA patients at higher mortality risk. The research outcomes underscore the potential benefits of the novel TyG-BMI index in evaluating IR for predicting the prognosis of OA patients, as well as highlighting the potential therapeutic role of insulin in this context.
Data availability
The data supporting the conclusions of this article can be accessed at National Health and Nutrition Examination Survey (NHANES). (https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics) (https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory).
Abbreviations
- OA:
-
Osteoarthritis
- NHANES:
-
National Health and Nutrition Examination Survey
- TyG-BMI:
-
Triglyceride glucose-body mass index
- NCHS:
-
National Center for Health Statistics
References
Glyn-Jones, S. et al. Osteoarthritis. Lancet (London, England) 386, 376–387. https://doi.org/10.1016/s0140-6736(14)60802-3 (2015).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet (London, England) 393, 1745–1759. https://doi.org/10.1016/s0140-6736(19)30417-9 (2019).
Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 325, 568–578. https://doi.org/10.1001/jama.2020.22171 (2021).
Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: An update with relevance for clinical practice. Lancet (London, England) 377, 2115–2126. https://doi.org/10.1016/s0140-6736(11)60243-2 (2011).
Veronese, N. et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum. 46, 160–167. https://doi.org/10.1016/j.semarthrit.2016.04.002 (2016).
Mendy, A., Park, J. & Vieira, E. R. Osteoarthritis and risk of mortality in the USA: A population-based cohort study. Int. J. Epidemiol. 47, 1821–1829. https://doi.org/10.1093/ije/dyy187 (2018).
Constantino de Campos, G. et al. Osteoarthritis, mobility-related comorbidities and mortality: An overview of meta-analyses. Ther. Adv. Musculoskelet. Dis. https://doi.org/10.1177/1759720x20981219 (2020).
Nedunchezhiyan, U. et al. Obesity, inflammation, and immune system in osteoarthritis. Front. Immunol. 13, 907750. https://doi.org/10.3389/fimmu.2022.907750 (2022).
Duclos, M. Osteoarthritis, obesity and type 2 diabetes: The weight of waist circumference. Ann. Phys. Rehabil. Med. 59, 157–160. https://doi.org/10.1016/j.rehab.2016.04.002 (2016).
Tchetina, E. V., Markova, G. A. & Sharapova, E. P. Insulin resistance in osteoarthritis: Similar mechanisms to type 2 diabetes mellitus. J. Nutr. Metab. 2020, 4143802. https://doi.org/10.1155/2020/4143802 (2020).
Griffin, T. M. & Huffman, K. M. Editorial: Insulin resistance: Releasing the brakes on synovial inflammation and osteoarthritis?. Arthritis Rheumatol. (Hoboken, NJ) 68, 1330–1333. https://doi.org/10.1002/art.39586 (2016).
Courties, A. & Sellam, J. Osteoarthritis and type 2 diabetes mellitus: What are the links?. Diabetes Res. Clin. Pract. 122, 198–206. https://doi.org/10.1016/j.diabres.2016.10.021 (2016).
Veronese, N. et al. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 49, 9–19. https://doi.org/10.1016/j.semarthrit.2019.01.005 (2019).
Defronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795. https://doi.org/10.2337/db09-9028 (2009).
Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 22, 279. https://doi.org/10.1186/s12933-023-02030-z (2023).
Pacini, G. & Mari, A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract. Res. Clin. Endocrinol. Metab. 17, 305–322. https://doi.org/10.1016/s1521-690x(03)00042-3 (2003).
Dou, J. et al. Association between triglyceride glucose-body mass and one-year all-cause mortality of patients with heart failure: A retrospective study utilizing the MIMIC-IV database. Cardiovasc. Diabetol. 22, 309. https://doi.org/10.1186/s12933-023-02047-4 (2023).
Brämer, G. R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat. Q. Rapport Trimest. Stat. Sanit. Mond. 41, 32–36 (1988).
Louati, K., Vidal, C., Berenbaum, F. & Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 1, e000077. https://doi.org/10.1136/rmdopen-2015-000077 (2015).
Song, S. & Yu, J. Identification of the shared genes in type 2 diabetes mellitus and osteoarthritis and the role of quercetin. J. Cell. Mol. Med. 28, e18127. https://doi.org/10.1111/jcmm.18127 (2024).
Rosa, S. C. et al. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: Modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr. Cartil. 19, 719–727. https://doi.org/10.1016/j.joca.2011.02.004 (2011).
Kayal, R. A. et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 44, 357–363. https://doi.org/10.1016/j.bone.2008.10.042 (2009).
Tchetina, E. V., Antoniou, J., Tanzer, M., Zukor, D. J. & Poole, A. R. Transforming growth factor-beta2 suppresses collagen cleavage in cultured human osteoarthritic cartilage, reduces expression of genes associated with chondrocyte hypertrophy and degradation, and increases prostaglandin E(2) production. Am. J. Pathol. 168, 131–140. https://doi.org/10.2353/ajpath.2006.050369 (2006).
Qu, J. et al. PFKFB3 modulates glycolytic metabolism and alleviates endoplasmic reticulum stress in human osteoarthritis cartilage. Clin. Exp. Pharmacol. Physiol. 43, 312–318. https://doi.org/10.1111/1440-1681.12537 (2016).
Schenk, S., Saberi, M. & Olefsky, J. M. Insulin sensitivity: Modulation by nutrients and inflammation. J. Clin. Investing. 118, 2992–3002. https://doi.org/10.1172/jci34260 (2008).
Ribeiro, M., López de Figueroa, P., Blanco, F. J., Mendes, A. F. & Caramés, B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr. Cartil. 24, 731–739. https://doi.org/10.1016/j.joca.2015.10.017 (2016).
Qiao, L., Li, Y. & Sun, S. Insulin exacerbates inflammation in fibroblast-like synoviocytes. Inflammation 43, 916–936. https://doi.org/10.1007/s10753-020-01178-0 (2020).
Li, M. et al. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 7, 216. https://doi.org/10.1038/s41392-022-01073-0 (2022).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. https://doi.org/10.1146/annurev-immunol-031210-101322 (2011).
Courties, A., Gualillo, O., Berenbaum, F. & Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr. Cartil. 23, 1955–1965. https://doi.org/10.1016/j.joca.2015.05.016 (2015).
Wang, Y. et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthr. Cartil. 16, 579–583. https://doi.org/10.1016/j.joca.2007.09.007 (2008).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17, 122. https://doi.org/10.1186/s12933-018-0762-4 (2018).
Xiao, S., Zhang, Q., Yang, H. Y., Tong, J. Y. & Yang, R. Q. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: A retrospective study from NHANES database. Sci. Rep. 14, 13884. https://doi.org/10.1038/s41598-024-63886-z (2024).
Dang, K. et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc. Diabetol. 23, 8. https://doi.org/10.1186/s12933-023-02115-9 (2024).
Chen, J., Wu, K., Lin, Y., Huang, M. & Xie, S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc. Diabetol. 22, 320. https://doi.org/10.1186/s12933-023-02054-5 (2023).
Norton, L., Shannon, C., Gastaldelli, A. & DeFronzo, R. A. Insulin: The master regulator of glucose metabolism. Metab. Clin. Exp. 129, 155142. https://doi.org/10.1016/j.metabol.2022.155142 (2022).
Haeusler, R. A., McGraw, T. E. & Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19, 31–44. https://doi.org/10.1038/nrm.2017.89 (2018).
Myers, M. G. Jr. & White, M. F. Insulin signal transduction and the IRS proteins. Annu. Rev. Pharmacol. Toxicol. 36, 615–658. https://doi.org/10.1146/annurev.pa.36.040196.003151 (1996).
Kolb, H., Kempf, K., Röhling, M. & Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 18, 224. https://doi.org/10.1186/s12916-020-01688-6 (2020).
Poulose, N. & Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis. 5, 101–108. https://doi.org/10.14336/ad.2014.0500101 (2014).
Author information
Authors and Affiliations
Contributions
Study concept: DWB and QML. Study design: All authors. Acquisition, analysis, or interpretation of data: WW, FZ, YHL. Statistical analysis: WW, FA, YZL, and HRS. Drafting of the manuscript: WW and YHL. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, W., Zhou, F., Li, Y. et al. U-shaped association between triglyceride glucose-body mass index with all-cause and cardiovascular mortality in US adults with osteoarthritis: evidence from NHANES 1999–2020. Sci Rep 14, 19959 (2024). https://doi.org/10.1038/s41598-024-70443-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70443-1
- Springer Nature Limited