Abstract
Few studies have focused on the risk factors for necrotizing enterocolitis (NEC) in small for gestational age (SGA) infants. The aim of this study was to identify the risk factors for NEC in SGA newborns. This study included consecutive SGA neonates admitted to a tertiary hospital in Jiangxi Province, China from Jan 2008 to Dec 2022. Patients with NEC (Bell’s stage ≥ II) were assigned to the NEC group. Gestational age- and birth weight-matched non-NEC infants born during the same period at the same hospital were assigned to the control group. The risk factors associated with NEC were analyzed with univariate and logistic regression models. During the study period, 2,912 SGA infants were enrolled, 150 (5.15%) of whom developed NEC. In total, 143 patients and 143 controls were included in the NEC and control groups, respectively. Logistic regression analysis revealed that sepsis (OR 2.399, 95% CI 1.271–4.527, P = 0.007) and anemia (OR 2.214, 95% CI 1.166–4.204, P = 0.015) might increase the incidence of NEC in SGA infants and that prophylactic administration of probiotics (OR 0.492, 95% CI 0.303–0.799, P = 0.004) was a protective factor against NEC. Therefore, sepsis, anemia and a lack of probiotic use are independent risk factors for NEC in SGA infants.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is a common and devastating gastrointestinal emergency of preterm birth that occurs in 7–12% of very low birth weight infants1,2. The mortality rate associated with NEC ranges from 20 to 40%, and survivors are at increased risk for poor long-term growth and neurodevelopmental impairment despite improvements in medical technology and neonatal care over the past several years3,4. The exact etiology of NEC remains unclear, but multiple factors, such as formula feeding, prematurity, low birth weight, intestinal ischemia and abnormal microbial colonization, are considered risk factors5,6,7. Classification as small for gestational age (SGA) is assigned if a newborn has a birth weight < 10th percentile for their gestational age8, suggesting possible intrauterine growth retardation and growth insufficiency. The risk of developing NEC in SGA neonates is more than double that in appropriate for gestational age (AGA) neonates9. However, the risk factors associated with the development of NEC in SGA infants remain unclear. To our knowledge, few studies have focused on the risk factors for NEC in SGA infants. The aim of this study was to identify the potential risk factors for NEC in SGA infants.
Results
Clinical features
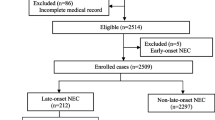
During the study period, 2,912 SGA infants were admitted to the Department of Neonatology, Jiangxi Hospital Affiliated to the Children's Hospital of Chongqing Medical University (CHCMU). Among these infants, 150 (5.15%) developed NEC (Bell’s stage ≥ II), 143 of whom were eligible for enrollment; the other 7 patients were excluded because they were discharged from the hospital during the first 24 h of hospitalization (n = 4) and had incomplete information (n = 3). Consequently, 143 matched SGA infants without NEC were included in the control group.
Table 1 shows the comparison of demographic characteristics between the two groups. No differences in neonatal baseline factors or maternal factors were found between the two groups (P > 0.05). Compared with non-NEC infants, infants with NEC required a longer duration of hospitalization (P = 0.046) and had higher overall mortality (P = 0.000). After adjustment, the mortality rate in the NEC group (Table 1).
was still significantly greater than that in the control group (P = 0.000).
Table 2 shows the risk factors associated with NEC identified with the univariate analysis. A higher incidence of anemia and sepsis and a lower incidence of prophylactic probiotic administration were found in neonates with NEC (P < 0.05). In infants with NEC, sepsis occurred at a mean of 2 (range 1–5) days before the onset of NEC; the time of sepsis onset after birth and the timing of NEC onset are presented in Fig. 1. No differences in the incidence of patent ductus arteriosus, respiratory failure, apnea, respiratory distress syndrome or polycythemia were found between the two groups (P > 0.05).
The most important risk factors for NEC in SGA infants in the less than P3 and P3–P10 subgroups are shown in Table 3. Specifically, sepsis was an important risk factor for NEC in SGA infants in the less than P3-P10 subgroup (P = 0.012), and anemia was an important risk factor for NEC in the P3 subgroup (P = 0.034). Additionally, prophylactic probiotics appeared to reduce the incidence of NEC in the P3 group (P = 0.005).
Table 4 shows the independent risk factors identified by the multivariate logistic regression model. Neonatal anemia (P = 0.015) and sepsis (P = 0.007) were considered independent risk factors for NEC in SGA infants, and SGA infants prophylactically administered probiotics were less likely to have NEC (P = 0.004).
To further clarify whether the presence of these risk factors affects the prognosis of NEC in infants, we compared subgroups of NEC survivors and nonsurvivors. Table 5 shows that the presence of sepsis significantly increased the mortality of NEC infants (P = 0.016), whereas the prophylactic administration of probiotics might reduce their mortality (P = 0.000). Here, anemia did not increase the mortality of NEC patients (P = 0.732).
Discussion
SGA infants are considered a high-risk population for NEC9,10. In this population, the incidence of NEC in SGA infants was 5.15% (150/2912). Other studies have reported that the incidence rate of NEC in SGA infants is between 3.2% and 6.02%9,11. Notably, a multicenter survey of the Chinese population revealed that the incidence rate of NEC in SGA infants may be as high as 20.41%12.
However, the exact risk factors for NEC in SGA infants remain unclear. We found that neonatal anemia and sepsis were risk factors for NEC, and prophylactic administration of probiotics might decrease the incidence of NEC in SGA infants. This study may provide scientific evidence for prevention and treatment strategies for NEC.
We found that SGA infants with sepsis were more vulnerable to NEC. The mechanism by which sepsis causes NEC is thought to be multifactorial. Bacteria from hematogenous and gut-derived infections can directly destroy intestinal epithelial cells, and bacterial products such as endotoxins can cause necrosis of the intestinal tract13,14. Various inflammatory mediators, such as platelet-activating factor, tumor necrosis factor-α, interleukin (IL)-1, IL-6 and IL-10, contribute to the onset and progression of NEC15,16. We also found that anemia was associated with the development of NEC in SGA infants. Anemia can impair splanchnic perfusion, including that in the intestine, resulting in increased anaerobic metabolism and the production of byproducts such as lactic acid17,18. Additionally, anemia can impair the normal maturation of vascular autoregulation in the intestine19. These effects can trigger a cascade of events leading to ischemic-hypoxemic mucosal gut injury, predisposing neonates to NEC17,18.
Finally, we found that prophylactic probiotics were associated with a lower incidence of NEC in SGA infants. Several clinical trials have demonstrated that probiotic administration after birth decreases the incidence of NEC in neonates20,21. Inappropriate bacterial colonization in the gastrointestinal tract plays an key role in the development of NEC. Probiotics may promote the colonization of beneficial microbiota, inhibit the growth of pathogens, improve the function of the gut mucosal barrier, and prevent the incidence of NEC5,22. Therefore, a lack of probiotic use may be associated with a higher incidence of NEC23,24.
In this study, the overall mortality rate of the NEC group was significantly greater than that of the control group, and after adjusting for mortality due to NEC, the mortality rate in the NEC group was still significantly greater than that of the control group. Sepsis, anemia, respiratory failure, and other factors might significantly increase the mortality rate of patients with NEC7,25,26; therefore, the higher mortality rate in the NEC group observed here might be closely related to the presence of multiple comorbidities in the SGA infants themselves.
There are several limitations in this study, including errors and bias inherent to the retrospective nature of the study. Moreover, this was only a single-center study and might not represent the characteristics of the entire Chinese SGA population. Therefore, prospective multicenter studies are needed to clarify the high-risk factors for NEC in the SGA population.
In conclusion, sepsis, anemia and a lack of probiotic use were independent risk factors for NEC in SGA infants in the present study. Thus, more attention should be given to SGA neonates with anemia and sepsis in future medical practices. Additionally, prophylactic probiotic use may reduce the incidence of NEC in SGA neonates.
Methods
Study population
This study was designed as a 1:1 matched case–control study. Consecutive SGA neonates who were admitted to the Department of Neonatology, Jiangxi Hospital Affiliated to Children’s Hospital of Chongqing Medical University (CHCMU) from Jan 2008 to Dec 2022, were included. This retrospective study was approved by the Ethics Committee of Jiangxi Hospital Affiliated to CHCMU (Approval No. 2016–19), and use of the database containing the evaluated data was permitted by the Ethics Committees of Jiangxi Hospital Affiliated to CHCMU. The requirement for informed consent was waived by the Ethics Committee of Jiangxi Hospital Affiliated to CHCMU. All study protocols were carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. SGA was defined as a birth weight < 10th percentile for each newborn’s gestational age according to the growth chart for Chinese neonates8,27. SGA neonates with diagnosed NEC (Bell’s stage ≥ II) were included in the NEC group28. The SGA neonates without NEC admitted during the same period to the same hospital (the admission time of the control group infants did not differ from that of the NEC group by more than 3 months) were screened as possible controls; those matched for gestational age (difference of < 3 days) and birth weight (difference of < 100 g) were selected. When there were multiple candidate infants , one infant was randomly selected for inclusion in the control group by a computer. Neonates whose medical information was incomplete or who were discharged from the hospital during the first 24 h of hospitalization were excluded from the study.
Data collection
The demographic characteristics, including maternal factors such as maternal age, antibiotic exposure during pregnancy, antenatal glucocorticoid exposure, gestational hypertension, intrauterine cholestasis during pregnancy, anemia during pregnancy, gestational diabetes mellitus, premature rupture of the membrane (> 18 h), fetal distress and meconium-stained amniotic fluid, were recorded. The neonatal factors included gender, gestational age, birth weight, mode of delivery, feeding type, and Apgar score at 1 and 5 min. Risk factors prior to the occurrence of NEC, such as neonatal anemia, sepsis, patent ductus arteriosus, respiratory failure, apnea, respiratory distress syndrome and polycythemia, were also recorded. Laboratory examinations and clinical outcomes were collected retrospectively from the hospital’s neonatal database. Neonatal anemia was defined as a hemoglobin or hematocrit concentration greater than 2 standard deviations below the mean for postnatal age29. Sepsis that developed prior to the onset of NEC was diagnosed on the basis of clinical manifestations and the growth of bacteria on blood culture and ancillary tests such as leukopenia (WBC < 5 × 109/L) or leukocytosis (WBC > 25 × 109/L for ≤ 3 days or WBC > 20 × 109/L for > 3 days), a platelet count < 100 × 109/L, an immature-to-total neutrophil ratio (I:T ratio) ≥ 0.16, and a C-reactive protein > 8 mg/L30,31,32. NEC was defined according to the modified Bell’s criteria as Bell Stage II or greater28,33. All infants were treated with suitable and necessary interventions according to their conditions, with the possible interventions including cessation of enteral feeding, nasogastric suction and parenteral nutrition, antibiotic therapy and surgical intervention. The data were collected, reviewed, deidentified, and anonymously analyzed by the authors, and the Ethics Committee of Jiangxi Hospital Affiliated to CHCMU waived the requirement for informed consent because of the anonymized nature of the data and the scientific purpose of the study.
Statistical analysis
All analyses were conducted using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov‒Smirnov test was used to assess the normality of continuous variables. Normally distributed variables were analyzed using Student’s t test, and skewed variables were analyzed with the Mann‒Whitney U test. The chi-square test and Fisher’s exact test were used to compare categorical variables between the two groups. All potential risk factors related to NEC incidence were included in the multivariate regression model to identify independent risk factors for NEC. P < 0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Neu, J. Necrotizing enterocolitis: The mystery goes on. Neonatology 106, 289–295. https://doi.org/10.1159/000365130 (2014).
Thänert, R., Keen, E. C., Dantas, G., Warner, B. B. & Tarr, P. I. Necrotizing enterocolitis and the microbiome: Current status and future directions. J. Infect. Dis. 223, S257-s263. https://doi.org/10.1093/infdis/jiaa604 (2021).
Duess, J. W. et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes 15, 2221470. https://doi.org/10.1080/19490976.2023.2221470 (2023).
Vaidya, R. et al. Long-term outcome of necrotizing enterocolitis and spontaneous intestinal perforation. Pediatrics 150, e2022056445. https://doi.org/10.1542/peds.2022-056445 (2022).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264. https://doi.org/10.1056/NEJMra1005408 (2011).
Niño, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600. https://doi.org/10.1038/nrgastro.2016.119 (2016).
Samuels, N., van de Graaf, R. A., de Jonge, R. C. J., Reiss, I. K. M. & Vermeulen, M. J. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr. 17, 105. https://doi.org/10.1186/s12887-017-0847-3 (2017).
Chen, Y., Wu, L., Zou, L., Li, G. & Zhang, W. Update on the birth weight standard and its diagnostic value in small for gestational age (SGA) infants in China. J. Matern. Fetal Neonatal Med. 30, 801–807. https://doi.org/10.1080/14767058.2016.1186636 (2017).
Ree, I. M. et al. Necrotizing enterocolitis in small-for-gestational-age neonates: A matched case-control study. Neonatology 105, 74–78. https://doi.org/10.1159/000356033 (2014).
Su, Y. et al. Risk factors for necrotizing enterocolitis in neonates: A meta-analysis. Front. Pediatr. 10, 1079894. https://doi.org/10.3389/fped.2022.1079894 (2022).
Liu, C. et al. Association of small-for-gestational-age status with mortality and morbidity in very preterm Chinese infants. J. Matern. Fetal Neonatal Med. 36, 2258257. https://doi.org/10.1080/14767058.2023.2258257 (2023).
Huang, X. R. et al. Real-world evidence regarding the growth of very premature infants with small for gestational age after birth: A multicenter survey in China. BMC Pediatr. 23, 437. https://doi.org/10.1186/s12887-023-04245-1 (2023).
Hackam, D. & Caplan, M. Necrotizing enterocolitis: Pathophysiology from a historical context. Semin Pediatr Surg. 27, 11–18. https://doi.org/10.1053/j.sempedsurg.2017.11.003 (2018).
Kumar, R. et al. Inflammatory biomarkers and physiomarkers of late-onset sepsis and necrotizing enterocolitis in premature infants. medRxiv https://doi.org/10.1101/2023.06.29.23292047 (2023).
Luo, J., Yu, S., Xu, J., Sun, X. & Wang, R. Deciphering the role of TNF-α-induced protein 8-like 2 in the pathogenesis of necrotizing enterocolitis in neonatal rats. Exp. Ther. Med. 26, 443. https://doi.org/10.3892/etm.2023.12142 (2023).
Yan, X. L. et al. Succinate aggravates intestinal injury in mice with necrotizing enterocolitis. Front. Cell Infect. Microbiol. 12, 1064462. https://doi.org/10.3389/fcimb.2022.1064462 (2022).
Maheshwari, A., Patel, R. M. & Christensen, R. D. Anemia, red blood cell transfusions, and necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 47–51. https://doi.org/10.1053/j.sempedsurg.2017.11.009 (2018).
Singh, R. et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J. Perinatol. 31, 176–182. https://doi.org/10.1038/jp.2010.145 (2011).
Jiang, Z., Ye, G., Zhang, S. & Zhang, L. Association of anemia and platelet activation with necrotizing enterocolitis with or without sepsis among low birth weight neonates: A case-control study. Front. Pediatr. 11, 1172042. https://doi.org/10.3389/fped.2023.1172042 (2023).
Healy, D. B. et al. Neonatal outcomes following introduction of routine probiotic supplementation to very preterm infants. Acta Paediatr. 112, 2093–2101. https://doi.org/10.1111/apa.16923 (2023).
Zhou, K. Z. et al. Probiotics to prevent necrotizing enterocolitis in very low birth weight infants: A network meta-analysis. Front. Pediatr. 11, 1095368. https://doi.org/10.3389/fped.2023.1095368 (2023).
Lin, H. C. et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter, randomized, controlled trial. Pediatrics 122, 693–700. https://doi.org/10.1542/peds.2007-3007 (2008).
Barbian, M. E. & Patel, R. M. Probiotics for prevention of necrotizing enterocolitis: Where do we stand?. Semin. Perinatol. 47, 151689. https://doi.org/10.1016/j.semperi.2022.151689 (2023).
Wang, H. et al. Probiotics to prevent necrotizing enterocolitis and reduce mortality in neonates: A meta-analysis. Medicine 102, e32932. https://doi.org/10.1097/md.0000000000032932 (2023).
Bracho-Blanchet, E. et al. Prognostic factors related to mortality in newborns with necrotising enterocolitis. Cir. Cir. 83, 286–291. https://doi.org/10.1016/j.circir.2015.02.002 (2015).
Zhang, Y. et al. Predictive scores for mortality in full-term infants with necrotizing enterocolitis: Experience of a tertiary hospital in Southwest China. World. J. Pediatr. 12, 202–208. https://doi.org/10.1007/s12519-015-0063-x (2016).
Zhu, L. et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi. 53, 97–103 (2015).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7. https://doi.org/10.1097/00000658-197801000-00001 (1978).
Aher, S., Malwatkar, K. & Kadam, S. Neonatal anemia. Semin. Fetal Neonatal Med. 13, 239–247. https://doi.org/10.1016/j.siny.2008.02.009 (2008).
Subspecialty Group of Neonatology Pediatric Society Chinese Medical Association, Editorial Board Chinese Journal of Pediatrics. Protocol for diagnosis and treatment of neonatal septicemia. Zhonghua Er Ke Za Zhi. 41, 897–899 (2003).
Camacho-Gonzalez, A., Spearman, P. W. & Stoll, B. J. Neonatal infectious diseases: Evaluation of neonatal sepsis. Pediatr. Clin. North Am. 60, 367–389. https://doi.org/10.1016/j.pcl.2012.12.003 (2013).
Shah, B. A. & Padbury, J. F. Neonatal sepsis: An old problem with new insights. Virulence 5, 170–178. https://doi.org/10.4161/viru.26906 (2014).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. North Am. 33, 179–201. https://doi.org/10.1016/s0031-3955(16)34975-6 (1986).
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the study. Xiang-Ping Ding collected the clinical data drafted the manuscript. Xiang-Wen Hu, Shi Chen helped to collect the clinical information. Lu Guo and Zheng-Li Wang analyzed the data, Lu-Quan Li contributed to the critical revision. Wen-Yan Tang supervised the project and contributed to the conception and design of the study. Xiang-Ping Ding, Xiang-Wen Hu, Shi Chen, Lu Guo, Zheng-Li Wang, Lu-Quan Li and Wen-Yan Tang provided the final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, XP., Hu, XW., Chen, S. et al. Risk factors for necrotizing enterocolitis in small-for-gestational-age infants: a matched case–control study. Sci Rep 14, 19098 (2024). https://doi.org/10.1038/s41598-024-70351-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70351-4
- Springer Nature Limited