Abstract
Indian mustard (Brassica juncea; Brassicaceae) is an edible, oilseeds-yielding crop widely consumed as a food spice owing to its richness in nutrients with several health benefits. The current study aims to dissect the B. juncea metabolome heterogeneity among its different organs including leaf, stem, flower, and seed. Moreover, assessing the metabolome differences between two different varieties RH-725 and RH-761 grown at the same conditions. Gas chromatography-mass spectrometry (GC–MS) post-silylation was used to dissect the composition of nutrient metabolites coupled to multivariate data analysis. Variation in sulphur aglycones was measured using headspace-solid phase-microextraction HS-SPME coupled to GC–MS. A total of 101 nutrient metabolites were identified with the abundance of sugars represented by monosaccharides in all organs, except for seeds which were enriched in disaccharides (sucrose). α-Linolenic acid was detected as a marker fatty acid in leaf from RH-725 at 12.5 µg/mg. Malic acid was detected as a significant variant metabolite between the two varieties as detected in the leaf from the RH-725 variety at ca. 128.2 µg/mg compared to traces in RH-761. 7 Volatile sulphur compounds were detected at comparable levels in RH-725 and RH-761, with 3-butenyl isothiocyanate was the most abundant at 0.8–2 ng/mg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Brassicaceae also known as cruciferous crops are a common food spices widely consumed worldwide owing to its nutritional and medicinal value1. Rapeseed mustard is a major oil-producing seed in Brassica crops belonging to family Brassicaceae2. Indian mustard (Brassica juncea L. Czern & Coss.) is an important edible, oil-yielding crop covering about 90% of the cultivated area under brassica oilseeds in India3. Mustard is the third-largest source of vegetable oil in the world, after soybeans and palm oil3. Owing to its richness in micronutrient (vitamins, minerals) and bioactive metabolites (glucosinolates and polyphenolics) with potential health benefits, Indian mustard (Brassica juncea) is commonly consumed as a food spice in several diets2. Glucosinolates are important sulphur-containing phytochemicals enriched in brassica vegetables and to account for their unique sharp hot and pungent flavor and taste1. Owing to its richness in nutrients and phytochemicals i.e. glucosinolates, several biological activities were reported in mustard including anti-inflammatory, antioxidant, cholesterol lowering, anti-obesity, anticancer, and antibacterial2.
B. juncea has the potential for quicker seed germination, high productivity, and heat and drought tolerance, along with enhanced insect and disease resistance2,4. There is a need to expand on the production of food crops but, on the other hand, environmental stresses (biotic and abiotic) can negatively affect the overall yield of agricultural crops5. Drought stress is recognized as the main factor leading to decline in agricultural productivity, because water shortage is persistently related to other major abiotic stresses, such as high-temperature stress and salinity6. The Indian Mustard (B. juncea L.) varieties are being breed for specific purposes based on the environmental factors particularly for abiotic stresses7. Among abiotic stresses, water shortage is quite prevalent in the northern regions of India in which only source of water for irrigation lies on rainwater. The most popular and latest released mustard varieties include RH-725 and RH-761 grown in Northern India, and their detailed data were listed in Table S1. They are the national released varieties for ‘High seed and oil yield under rain fed condition with wider adaptability’ in year 2019 (Statutory Order (S.O.) 3220 (E) Dated 06.09.2019) and 2018 (S.O. 1379 (E) Dated 27.03.2018), respectively for timely sown and rain fed conditions in Haryana, Punjab, Delhi, Jammu and Northern Rajasthan by the Ministry of Agriculture and Farmers' Welfare, Government of India.
Recently, different metabolomics technologies were applied for metabolites profiling in foodstuff either in targeted or untargeted manner to ensure quality and assess variation among food sources or genotypes8. Gas chromatography coupled to mass spectrometry (GC–MS) is a well adopted analytical tool for profiling of nutrient metabolites post silylation9 that has extensively ben reported in plants metabolomics studies and more towards food applications10. Considering the complexity of metabolites datasets, chemometric analysis is typically employed for analyzing data set generated from MS-based analysis and assess variation between different samples using unsupervised principal component analysis (PCA) and supervised orthogonal projection to least squares discriminant analysis (OPLS-DA)11. As B. juncea is valued for its unique taste and aroma, analysis of aroma compounds across its accessions ought to be examined as typical in brassica vegetables12.
The current work aims to present the first comparative GC–MS based profiling approach of nutrients and sulphur compounds in Brassica juncea L. (Indian mustard) in context to its different organs including seed, leaf, flower, and stem represented by it two varieties RH 725 and RH 761. Such results aid in future valorization approaches of its unused parts based on such detailed chemical makeup. Moreover, quantification of metabolites among different organs from the two varieties is presented to aid future cultivation approach targeting certain component yield or for its standardization.
Results and discussion
The main goal of the current study was to assess metabolites heterogeneity in four organs derived from two different varieties in Indian mustard (Brassica juncea) in the context of their nutrient and sulfur aroma profiles using GC–MS-based metabolomics approach. Seed, leaf, flower, and stem of B. juncea obtained from the two varieties (RH-725 and RH-761) were included for comparative metabolites profiling analysed using chemometric tools.
Primary metabolites profiling in B. juncea organs via GC–MS analysis (post-silylation)
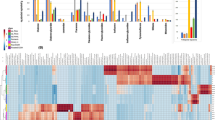
To assess variation in primary nutritive metabolites among B. juncea plant parts from two different varieties, GC–MS analysis was employed post-silylation. A total of 101 peaks (Table 1, Fig. 1) were annotated, including sugars (mono- and disaccharides), sugar alcohols, sugar acid, fatty acids/esters, organic acids, amino acids, nitrogenous compounds, sterols, phenolics and alcohols.
Sugars
Sugars were detected as the most dominant primary metabolites represented by 26 peaks identified in B. juncea different organs viz. leaf, stem, flower, and seed in both varieties. The highest levels of sugars represented by monosaccharides were detected in brassica stem RH-761 amounting for ca. 263.2 µg/mg, followed by leaf stem, and flower collected from RH-725 at 240.8, 197.65, and 147.7 µg/mg. In contrast, the highest levels of disaccharides were detected in seed from both varieties at 110–140 µg/mg, respectively. In contrast, the lowest sugar level was detected in the leaf RH-761 at ca. 4.3 µg/mg. Such variation in sugar levels indicate that B. juncea organs from the variety RH-725 are in general higher in sugar content, except only for the stem than RH-761. Glucose was the major sugar in leaf RH-725, stem and flower from both RH-761 and RH-725 detected at 26.7–126.4 µg/mg. Next to glucose, fructose and galactose were abundant in B. juncea leaf RH-725, stem and flower from both RH-761 and RH-725 at 30.3–62.03 and 10.5–97.5 µg/mg, respectively. Seeds from both varieties showed trace levels of glucose, fructose, and galactose, likely as storage organ to be richer in fatty acids. Interestingly, in seeds major sugar was represented by sucrose detected at much higher level in seeds with higher level in variety RH-725 at ca. 133.02 µg/mg compared to 98.6 µg/mg in RH-761. The abundance of sucrose in B. juncea seed pose it as the most palatable and edible part. Conclusively regarding sugar profile, organs from B. juncea variety RH-725 showed higher levels of monosaccharides than variety RH-761, except stem RH-761 that encompassed higher level of sugars among all organs. Among organs from both varieties, seeds contained higher levels of disaccharides. Free sugars such as stachyose, raffinose, melibiose, galactose, glucose and fructose were previously detected in B. juncea13.
Unlike free sugars, sugar alcohols were detected at much lower levels in B. juncea organs from different varieties being detected at comparable levels of 19.9 and 16.6 µg/mg in leaf and flower RH-725, respectively. Glycerol was detected as the most abundant sugar alcohol in leaf and flower for variety RH-725 at 18.3 and 15.9 µg/mg, respectively. Likewise, sugar acids were detected at high level in B. juncea flowers from the two varieties with higher abundance in variety RH-725 at ca. 33.2 compared to 14.9 µg/mg in RH-761. Gluconic acid, an important sugar acid used in food and pharmaceutical industry14 was detected as the most abundant sugar acid in brassica flower at ca. 30.3 and 13.4 µg/mg in RH-725 and RH-761, respectively. Retrieving sugars results and comparison among brassica organs from the two varieties revealed that variety RH-725 was the richest in free sugars and its derivatives represented by glucose, fructose, galactose, sucrose, glycerol, and gluconic acid.
Amino acids/nitrogenous compounds/phenolics
Amino acids play a pivotal role in human health owing to their nutritional and medicinal importance in the regulation of anti-oxidative, metabolic, and immune responses8. Amino acids were represented by 17 peaks detected at comparable higher levels in leaf and flower from both varieties compared to other organs and detected at higher level in leaf RH-725 and flower RH-725 at ca. 23.3 and 10.6 µg/mg, respectively. Such high amino acids level in leaf and flower from RH-725 pose it for improved nutritional makeup compared variety RH-761. Alanine, valine, and leucine were the most abundant amino acids detected among brassica organs, specially leaf and flower form RH-725 variety. Compared to the food sources of amino acids including meat, milk, cheese, and grains, B. juncea leaf and flower are considered as important source for nutritionally important amino acids. Amino acids were previously reported in Indian mustard seeds at high levels including phenylalanine, tyrosine, methionine, cystine, leucine, valine, and lysine15.
Nitrogenous compounds were detected at trace levels in all examined organs ranging from 0.2–2.5 µg/mg and 0.3–0.9 µg/mg in organs from RH-725 and RH-761, respectively, with only leaf RH-725 and flower RH-725 that showed relatively higher levels. Likewise, phenolics were detected at trace levels in organs from the two varieties except a comparable level was detected in seed from RH-725 and RH-761 at ca. 3–4µg/mg, though it should be noted that GC/MS is not suited for profiling of phenolics considering their polar nature and warranting for using LC/MS technique16. Several polyphenols were previously reported in B. juncea using high-performance liquid chromatography (HPLC)17. Sinapinic acidwas the major phenolic acid in B. juncea seed specially in variety RH-725 (3.03 µg/mg). Sinapinic acid is a chief phenolic acid previously identified in rapeseed with potential health benefits including anti-inflammatory, hepatoprotective, cardioprotective, anti-cancer, and anti-diabetic18.
Fatty acids/esters/sterols
Fatty acids/esters represented by 22 peaks were detected at relatively high levels in all organs of B. juncea with higher levels in leaf RH-725 at 56.6 µg/mg represented by saturated, monounsaturated and polyunsaturated fatty acids. α-Linolenic acid is a poly-unsaturated ω-3 fatty acid with positive effects on atherosclerosis incidence risk and hence reduce cardiovascular diseases risk19 found at high level in leaf RH-725 at 12.5 µg/mg. Palmitic acid was detected as the major saturated fatty acids in leaf RH-725 and seed RH-725 at 8.4 and 7.02 µg/mg, respectively. In a previous study, fatty acids were previously detected in B. juncea specially unsaturated fatty acids including oleic acid and palmitoleic acid20. Moreover, B. juncea oil was reported to contain a balanced levels of both saturated and unsaturated fatty acids specially both omega-6 and omega-3 polyunsaturated fatty acids13.
Regarding fatty acyl esters, 1-monopalmitin, an esterified product of palmitic acid previously identified in a variety of plant extracts and to exhibit antibacterial activities19, was detected at highest levels in leaf RH-725 of 10.2 µg/mg. Unlike fatty acids, sterols were detected at much lower levels in B. juncea represented mainly by two peaks identified as campesterol and β-sitosterol with relative high level in seed RH-725 at 2.4 µg/mg compared to other accessions. Such results indicated that B. juncea organs from variety RH-725 is the richest in fatty acids and sterols compared to that from RH-761.
Organic acids/alcohols/lactones
Organic acids were detected in all organs from the two varieties at comparable levels, with highest level found in leaf RH-725 at 176 µg/mg followed by flower RH-725 at 41.7 µg/mg. Organic acids are important in food products as natural preservative and can promote food digestion as well as aiding in protein utilization12. Malic acid was detected as the major form in B. juncea leaf RH-725 at ca. 128.2 µg/mg. Malic acid exhibits potential antioxidant capacity asides from its action as food preservative21. Such high level of malic acid in leaf from the variety RH-725 could be a marker for genetic variation between the two varieties RH-725 and RH-761 which has yet to be confirmed using QTL sequencing. Malic acid was previously detected in B. juncea flower in a study done by El Majdoub et al.22. Next to malic acid, hexanoic acid, a short-chain monocarboxylic acid was detected at high level in leaf RH-725 29.01 µg/mg. Compared to acids, alcohols were detected at trace levels in most samples represented mainly by phytol, a diterpene alcohol with potential health benefits23. Likewise, lactones were detected at trace levels in all organs, except in flower of RH-725 at 6.9 µg/mg versus 3.8 µg/mg in RH-761. Tetrahydroxypentanoic acid-1,4-lactone and gluconolactone were the two identified lactones found most abundant in flower RH-725.
HCA and PCA analysis of post-silylated primary metabolites from B. juncea organs from two different varieties
Unsupervised HCA and PCA analyses as multivariate data analysis tools were further employed to assess variations in primary metabolites among B. juncea organs (leaf, stem, flower, and seed) from two varieties (RH-725 and RH-761) (Table 1). HCA depicted a dendrogram of two distinct clusters (Fig. 2a), with seed from two varieties clustered separately in cluster 1, while other organs from the two varieties were divided in two subdivisions from cluster 2. Leaf RH-725 was clustered separately in sub cluster 2a. Moreover, stems from the two varieties were clustered against leaf-RH-761 and flower from the two varieties in subcluster 2b. However, clustering of organs from two different varieties together in cluster 2 indicated weak discrimination from HCA among varieties or even organs depending on silylated primary metabolites likely attributed to the weaker classification potential of primary compared to secondary metabolites24. A PCA model (Fig. 2b) prescribed by two orthogonal PCs accounted for 72% of the total variance, with segregation of leaf RH-725 alone at the positive left side of the score plot, whereas brassica seeds from two varieties were segregated together in the positive right side of PC1. Stems from both varieties were positioned towards the left side of PC1. Such unexpected clustering of leaf RH-725 specimens away from others is due to the large variations in metabolites content especially sugars, sugar alcohols, organic acids, fatty acid/esters, and amino acids. The corresponding loading plot (Fig. 2c) revealed for higher organic acids level in leaf RH-725 represented by malic acid and to account for its distant segregation. Moreover, the abundance of di-sugar i.e. sucrose (Peak 84) in seeds versus mono-sugar i.e. fructose, glucose, and galactose in other organs, suggestive that seeds are more rich in di sugars versus mono sugars in all other organs. PCA modelling results inferred that in particular leaf RH-725 and seeds (RH-725 and RH-761) were most distinguished from all other organs.
Unsupervised multivariate data analyses of Brassica juncea organs from different varieties derived from modeling of silylated primary metabolites dataset via GC–MS post silylation (n = 3). (a) HCA plot. (b) PCA score plot of PC1 vs. PC2 scores. (c) The respective loading plot for PC1 and PC2, providing mass peaks and their assignments. The metabolome clusters are placed in two-dimensional space at the distinct locations defined by two vectors of principal component PC1 = 51% and PC2 = 24%.
OPLS-DA Analysis of B. juncea organs from the two different varieties
Compared to the unsupervised PCA model, supervised orthogonal partial least squares discriminant analysis (OPLS-DA) was adopted to assess variations among B. juncea organs from the two different varieties (Fig. 3) by constructing two respective models of leaf RH-761 against leaf RH-725 (model 1), and seeds against all organs (model 2) from the two different varieties. Leaf model showed higher prediction power with Q2 = 0.98 and R2 = 0.99 (Fig. 3a) versus seed model at 0.95 and 0.99 (Fig. 3c) with p value < 0.157716 and 1.93251e-010, respectively. Additionally, loading S-plot (Fig. 3b) revealed the abundance of organic acids viz. malic acid, hexanoic acid and sugars viz. fructose, galactose and glucose in leaf RH-725 compared with RH-761. Likewise, loading S-plot (Fig. 3d) revealed that sucrose was abundant only in seeds from the two varieties compared with other organs being rich in monosaccharides viz glucose, fructose, and galactose. The distinct segregation of leaf RH-725 from leaf RH-761 indicated that primary metabolites provided a strong model for differentiation between the two different varieties (RH-725 and RH-761) warranting for studying gene sequencing for explaining differences among the two varieties for a proof of hypothesis.
GC–MS-based OPLS-DA score plot (a) derived from modeling silylated primary metabolites of Brassica juncea leaf-RH 761 versus leaf-RH 725 (n = 3). (c) Derived from modeling silylated primary metabolites of Indian brassica seed versus other 3 organs (n = 3). (b) and (d) The respective loading S-plots showing the covariance p [1] against the correlation p(cor) [1] of the variables of the discriminating component of the OPLS-DA model. Cut-off values of p < 0.157716 was used. Designated variables are highlighted, and identifications are discussed in the text.
Metabolites enrichment analysis
Metabolite enrichment analysis of the top 25 metabolites was employed in classification of B. juncea seed versus that in stem and leaf (Fig. 4). The top six mapped pathways with the greatest number of differentially expressed genes (DEGs) in the B. juncea seed versus stem and leaf group were as follows: galactose metabolism; starch and sucrose metabolism; pentose phosphate; steroids; fructose and mannose metabolism; and fatty acids biosynthesis. Based on the adjusted p-value, metabolites that were enriched in seed versus stem were identified, Fig. 5 revealed for “starch and sucrose metabolism” being significantly enriched in seed (p = 5.63e–14). Additionally, “fatty acid biosynthesis” was significantly enriched in seed represented by palmitic, oleic, and stearic acid. Likewise, steroids biosynthesis was significantly enriched in seed represented by campesterol and as expected considering its lipid rich nature as a storage organ.
Headspace-SPME–GC–MS volatiles analysis of B. juncea
Sulphur metabolites are highly characteristic for brassica crops especially seeds25 and contribute for their unique aroma and taste, and further health benefits so they are considered as potential markers to be exploited for B. juncea varieties classification. A total of 7 sulphur compounds were identified in two B. juncea varieties using headspace–solid-phase microextraction (HS-SPME) coupled to GC/MS targeting seeds aroma profile (Table 2, Fig. S1). These sulphur aroma metabolites included pungent thiosulfinates and sulfines are characteristic metabolites in brassica vegetables12. 3-Butenyl isothiocyanate was identified as the most abundant compound in B. juncea detected at 2.1 and 0.8 ng/mg in RH-725 and RH-761, respectively. In contrast, allyl isothiocyante a major sulphur compound in Brassica was detected at higher level in RH-761 than in RH-725 at fold ratio of 1.38. Compared to other previous studies, B. juncea is well known for its richness in sulphur compounds20. 3-Butenyl isothiocyanate and allyl isothiocyanate were previously identified in B. juncea seeds using GC–MS analysis20. Sulphur volatiles are abundant in Brassica vegetables being derived from the enzymatic hydrolysis of their intact glucosinolates26 and has to be further profiled in B. juncea using liquid chromatography mass spectrometry (LC/MS) more suited for profiling of the nonvolatile glucosinolates.
Conclusion
Metabolite heterogeneity targeting both nutrients and sulphur aroma compounds of B. juncea organs from two different varieties was assessed for the first time using a holistic untargeted GC/MS-based metabolomic approach. 101 Primary metabolites and 7 sulphur compounds were detected in B. juncea organs with distinct variations among organs and varieties. Glucose, fructose, and galactose were detected as the most abundant free sugars in B. juncea organs, except for seed in which sucrose predominated as the major form, especially in RH-725. Moreover, campesterol and β-sitosterol were detected at relatively high levels in seed RH-725 and suggestive that this seed presents a good source of phytosterols, while malic acid was abundant in the leaf from variety RH-725 and serves as a marker for distinguishing it from variety RH-761. Whether the enrichment of malic acid in RH-725 would add to its health benefits and shelf life as a natural preservative should be assessed using bioassays. Multivariate data analysis of nutrients revealed that organs belonging to RH-725 were the most rich in nutrients and posing it more for inclusion in food products or as food additive with stronger health potential. Our study provides evidence on the effect of the cultivation environment on metabolic variations among cultivars in Brassica genus. Our study provided the first complementary phytochemical evidence that supports the effect of cultivation environment on metabolic variations among cultivars highlights the nutritional determinants of B. juncea organs and highlights RH-725 with improved nutrient composition. Further future studies with an extended approach utilizing other analytical techniques targeting minerals, vitamins, and phytonutrients in Brassica to identify the best resources and elucidate the health outcomes of B. juncea is recommended.
Materials and methods
Plant material & cultivation
Brassica juncea L. Czern & Coss. organs including seed, leaf, flower, and stem were collected from two different varieties viz. RH 725 and RH 761 grown in Hisar, India during harvest season in 2022 (29.1492° N, 75.7217° E). The collected plant material was lyophilized and kept in the freezer at − 20 °C till analysis using GC–MS. All procedures were conducted in conformity with the pertinent rules and regulations.
GC–MS analysis of silylated primary metabolites
Freeze dried finely powdered plant part (100 mg) were extracted with 5 mL 100% methanol with sonication for 30 min and frequent vortex shaking. Three different samples from each B. juncea organ accession were analyzed under the same conditions to evaluate for biological replicates. Methanol extract (100 µL) was aliquoted in screw-cap vials and left to evaporate under a nitrogen gas stream until complete dryness. For derivatization, 150 µL of N-methyl- N-(trimethylsilyl)-trifluoroacetamide (MSTFA) previously diluted 1/1% with anhydrous pyridine was mixed with the dried methanol extract and incubated for 45 min at 60 °C prior to analysis using GC–MS. Separation of silylated derivatives was achieved on a Rtx-5MS (30-m length, 0.25-mm inner diameter and 0.25-m film). Quantitative analysis of primary metabolites followed the detailed protocol previously published in Ref.1. Soluble sugars, amino acids, organic acids and fatty acids were quantified using standard curves of glucose, glycine, citric and stearic acids and results were expressed as mg/g. Four serial dilutions were prepared from 10 to 600 ug/mL for establishing the standard curves. Calibration curves for glucose, glycine, citric acid and stearic acids displayed ca. 0.99 correlation coefficient.
SPME–GC–MS volatiles analysis
Freeze dried finely powdered seeds (100 mg) were placed in SPME screw-cap vials (1.5 mL) spiked with 10 µg (Z)-3-hexenyl acetate with fibers inserted manually above and placed in an oven kept at 50 °C for 30 min. HS-SPME analysis of the volatile compounds was performed as reported in Ref.8 with slight modifications. The fiber was subsequently withdrawn into the needle and then injected manually into the injection port of a gas chromatography–mass spectrometer (GC–MS). GC–MS analysis was adopted on an Agilent 5977B GC/MSD equipped with a DB-5 column (30 m × 0.25 mm i.d. × 0.25µm film thickness; Supelco) and coupled to a quadrupole mass spectrometer. The interface and the injector temperatures were both set at 220 ºC. Volatile elution was carried out using the following gradient temperature program: oven was set at 40 ºC for 3 min, then increased to 180 °C at a rate of 12 °C/min, kept at 180 °C for 5 min, finally increased at a rate of 40 °C/min to 240 °C and kept at this temperature for 5 min. Helium was utilized as a carrier gas with a total flow rate of 0.9 mL/min. For ensuring complete elution of volatiles, SPME fiber was prepared for the next analysis by placing it in the injection port at 220 °C for 2 min. For assessment of biological replicates, three different samples for each accession were analyzed under the same conditions. Blank runs were made during sample analyses. The mass spectrometer was adjusted to EI mode at 70 eV with a scan range set at m/z 40–500.
Metabolites identification and multivariate data analyses
Identification of both volatile and silylated components was performed by comparing their retention indices (RI) in relation to n-alkanes (C6-C30), mass matching to NIST, WILEY library database and with standards whenever available. Peaks were first deconvoluted using AMDIS software (www.amdis.net) before mass spectral matching. Peak abundance data were exported for multivariate data analysis by extraction using MS-Dial software under same conditions cited in Ref.27. Data were then subjected to principal component analysis (PCA), hierarchical clustering analysis (HCA) and partial least squares discriminant analysis (OPLS-DA) using SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). Markers were subsequently identified by analyzing S-plot, which was declared with covariance (p) and correlation (pcor). All variables were mean-centered and scaled to Pareto variance. Model validation was assessed by computing the diagnostic indices, viz. Q2 and R2 values, p value and permutation testing.
Plant ethics
The methods in plant collection and experimentation were carried out in accordance with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
The permissions of the plant collection were obtained in accordance with the guidelines prescribed by the American Society of Plant Taxonomists and adopted by the institutional research committee.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Baky, M. H., Shamma, S. N., Xiao, J. & Farag, M. A. J. F. C. Comparative aroma and nutrients profiling in six edible versus nonedible cruciferous vegetables using MS based metabolomics. Food Chem. 383, 132374 (2022).
Tian, Y. & Deng, F.J.C.-J.O.F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. CyTA J. Food 18, 704–718 (2020).
Jia, X. et al. Key odorant differences in fragrant Brassica napus and Brassica juncea oils revealed by gas chromatography–olfactometry, odor activity values, and aroma recombination. J. Agric. Food Chem. 68, 14950–14960 (2020).
Jamison, J., Khanal, S. K., Nguyen, N. H. & Deenik, J. L. J. A. Assessing the effects of digestates and combinations of digestates and fertilizer on yield and nutrient use of Brassica juncea (Kai Choy). Agronomy 11, 509 (2021).
Aneja, B., Yadav, N. R., Kumar, N. & Yadav, R. C. J. P. Hsp transcript induction is correlated with physiological changes under drought stress in Indian mustard. Physiol. Mol. Biol. Plants 21, 305–316 (2015).
Ahmad, P. & Prasad, M. N. V. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability (Springer Science & Business Media, 2011).
Gill, S. S. et al. Mustard: Approaches for Crop Improvement and Abiotic Stress Tolerance 1351–1368 (Wiley, 2012).
Baky, M. H., Shamma, S. N., Khalifa, M. R. & Farag, M. A. J. M. How does allium leafy parts metabolome differ in context to edible or inedible taxa? Case study in seven allium species as analyzed using MS-based metabolomics. Metabolites 13, 18 (2022).
Afifi, S. M., El-Mahis, A., Heiss, A. G. & Farag, M. A. Gas chromatography-mass spectrometry-based classification of 12 fennel (Foeniculum vulgare miller) varieties based on their aroma profiles and estragole levels as analyzed using chemometric tools. ACS Omega 6, 5775–5785 (2021).
Li, S. et al. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 61, 1448–1469 (2021).
Yun, J. et al. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 360, 130033 (2021).
Baky, M. H., Elshahed, M., Wessjohann, L. & Farag, M. A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 128, 577–591 (2022).
Rai, P. et al. The Brassica juncea Genome 1–13 (Springer, 2022).
Ramachandran, S., Fontanille, P., Pandey, A. & Larroche, C. J. F. T. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 44, 185–195 (2006).
Abul-Fadl, M., El-Badry, N. & Ammar, M. Nutritional and chemical evaluation for two different varieties of mustard seeds. World Appl. Sci. J. 15, 1225–1233 (2011).
Farag, M. A. et al. Comparative metabolite fingerprinting of four different cinnamon species analyzed via UPLC–MS and GC–MS and chemometric tools. Molecules 27, 2935 (2022).
Tian, Y. & Deng, F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. Cyta-J. Food 18, 704–718 (2020).
Quinn, L. et al. Sinapinic and protocatechuic acids found in rapeseed: Isolation, characterisation and potential benefits for human health as functional food ingredients. Irish J. Agric. Food Res. 56, 104–119 (2017).
Hu, Y. et al. Several natural phytochemicals from Chinese traditional fermented food-pickled Raphanus sativus L.: Purification and characterization. Food Chem. 15, 100390 (2022).
Bassan, P. et al. Extraction, profiling and bioactivity analysis of volatile glucosinolates present in oil extract of Brassica juncea var. raya. Physiol. Mol. Biol. Plants 24, 399–409 (2018).
Chen, H. et al. Quality chemistry, physiological functions, and health benefits of organic acids from tea (Camellia sinensis). Molecules 28, 2339 (2023).
Oulad El Majdoub, Y. et al. Chemical characterization of three accessions of Brassica juncea L. extracts from different plant tissues. Molecules 25, 5421 (2020).
Baky, M. H., Shawky, E. M., Elgindi, M. R. & Ibrahim, H. A. J. A. O. Comparative volatile profiling of Ludwigia stolonifera aerial parts and roots using VSE-GC-MS/MS and screening of antioxidant and metal chelation activities. ACS Omega 6, 24788–24794 (2021).
Farag, M. A., Weigend, M., Luebert, F., Brokamp, G. & Wessjohann, L. A. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC–Q-TOF-MS metabolomic profiles. Phytochemistry 96, 170–183 (2013).
Mondal, S. et al. Sulfur in seeds: An overview. Plants 11, 450 (2022).
Jang, M., Hong, E. & Kim, G. H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 75, M412–M416 (2010).
El-Hawary, E. A. et al. How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants 11, 131 (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.F. Conceptualization; Methodology, Supervision; Writing-original draft; Writing-review and editing, V. G., Methodology, Writing-original draft. M.H.B. Data curation, Methodology, Writing-original draft, Writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M.A., Goyal, V. & Baky, M.H. Comparative metabolome variation in Brassica juncea different organs from two varieties as analyzed using SPME and GCMS techniques coupled to chemometrics. Sci Rep 14, 19900 (2024). https://doi.org/10.1038/s41598-024-69865-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69865-8
- Springer Nature Limited