Abstract
To evaluate the impact of neoadjuvant chemotherapy on perioperative immune function in breast cancer patients, focusing on CD3+, CD4+, CD8+, and natural killer (NK) cells, as well as the CD4+/CD8+ ratio. We retrospectively reviewed medical records of breast cancer patients who underwent surgery with or without neoadjuvant chemotherapy at our medical center from January 2020 to December 2022. Patients were matched 1:1 based on propensity scores. Immune cell proportions and the CD4+/CD8+ ratio were compared on preoperative day one and postoperative days one and seven. Among matched patients, immune cell proportions and the CD4+/CD8+ ratio did not significantly differ between those who received neoadjuvant chemotherapy and those who did not at any of the three time points. Similar results were observed in chemotherapy-sensitive patients compared to the entire group of patients who did not receive neoadjuvant chemotherapy. However, chemotherapy-insensitive patients had significantly lower proportions of CD4+ and NK cells, as well as a lower CD4+/CD8+ ratio, at all three time points compared to patients who did not receive neoadjuvant chemotherapy. Neoadjuvant chemotherapy may impair immune function in chemotherapy-insensitive patients, but not in those who are sensitive to the treatment.
Similar content being viewed by others
Introduction
Breast cancer is one of the most common malignancies in women, and its incidence is increasing1. Although surgery is effective for many patients2, the prognosis may be poor for those with specific molecular signatures, larger tumors, or lymph node involvement3,4. Neoadjuvant chemotherapy, also known as preoperative or induction chemotherapy5,6,7,8, is often given to breast cancer patients to reduce tumor size, prevent spread, and improve the chances of a favorable prognosis after surgery6,9.
Neoadjuvant chemotherapy has several roles in breast cancer management10,11,12,13. Firstly, it aims to shrink the tumor, facilitating surgical resection and possibly enabling breast-conserving surgery in patients with initially unresectable tumors14. Secondly, it targets micrometastases, reducing the risk of distant metastasis and improving long-term survival15. Additionally, neoadjuvant chemotherapy can help assess tumor response to treatment, guiding further therapeutic decisions and prognosis16. The principles and mechanisms of neoadjuvant chemotherapy include tumor shrinkage, minimization of dissemination, and potential enhancement of surgical outcomes. Furthermore, neoadjuvant chemotherapy may affect immune cells, specifically CD4+ and CD8+ cells, potentially altering immune function and anti-tumor responses17. Understanding these effects is crucial for our study’s rationale and elucidating the implications of neoadjuvant chemotherapy on immune parameters.
However, not all breast cancer patients exhibit a favorable response to initial chemotherapy, underscoring the challenge of chemotherapy resistance18. Tumor heterogeneity, genetic variations, and immune responses are contributing factors to this resistance19,20. Understanding these mechanisms is vital for optimizing treatment strategies. Despite its benefits, neoadjuvant chemotherapy may harm normal tissue and immune cells in the body21. Specifically, it may decrease the counts of CD4+ and CD8+ T cells in peripheral blood, thereby weakening the patient's anti-tumor responses22,23 and their basic immune defenses against postoperative infection24.
Here, our aim was to investigate and validate the potential adverse effects of neoadjuvant chemotherapy on perioperative immune function in breast cancer patients treated at our medical center.
Materials and methods
Study population
A retrospective analysis was performed on the medical records of 622 breast cancer patients who underwent surgery at the Third Hospital of Nanchang, China, from January 2020 to December 2022. Patients were divided into two groups: those who received surgery following neoadjuvant chemotherapy (Group A) and those who underwent surgery without prior neoadjuvant chemotherapy (Group B). Due to the retrospective nature of the study, the Ethics Committee of The Third Hospital of Nanchang waived the need for obtaining informed consent. Nonetheless, this study, identified by the ethics review number K-lw2023002, received approval from the Ethics Committee of The Third Hospital of Nanchang, thus ensuring compliance with ethical guidelines and patient privacy.
Stringent eligibility and exclusion criteria were established to ensure the homogeneity and safety of the study population. Eligibility criteria included patients under 70 years old, confirmed with histopathological diagnosis of breast cancer from surgical samples. Disease staging (I-III) was determined through comprehensive evaluation, including breast and axillary ultrasonography with biopsy, mammography, or CT scans of cranial, thoracic, and abdominal regions. Positron emission tomography (PET) imaging was used in certain cases to aid staging. Moreover, patients needed to demonstrate sufficient hematological, renal, hepatic, and pulmonary functions to tolerate interventions effectively. Conversely, exclusion criteria included patients intolerant to general anesthesia, those with distant metastases, or concurrent autoimmune diseases or other malignancies. Patients with a history of chemotherapy, radiotherapy, or other antitumor therapies were also excluded to isolate the effects of neoadjuvant chemotherapy. Furthermore, pregnant or lactating individuals were excluded due to potential risks associated with chemotherapy exposure. These comprehensive criteria aimed to ensure the validity, safety, and homogeneity of the study cohort.
Staging definitions
In this study, tumor staging was assessed utilizing the TNM classification system, with "T" representing primary tumor size and extent, "N" indicating regional lymph node involvement, and "M" indicating distant metastases. Specifically, the "T" category includes T0 (no evidence of primary tumor), T1 (tumor size ≤ 2 cm), T2 (tumor size > 2 cm but ≤ 5 cm), T3 (tumor size > 5 cm), and T4 (tumor of any size with direct extension to the chest wall or skin). The "N" category is further divided into N0 (no regional lymph node metastasis), N1 (metastasis to movable ipsilateral level I, II axillary lymph nodes), N2 (metastasis to ipsilateral level III axillary lymph nodes or infraclavicular lymph nodes), and N3 (metastasis to ipsilateral internal mammary lymph nodes)25.
Treatment
Patients in Group A received surgery in conjunction with neoadjuvant chemotherapy. The primary chemotherapy regimens were as follows, with detailed dosages and cycles: Cyclophosphamide + Epirubicin + Fluorouracil (CEF): Cyclophosphamide was administered at a dose of 600 mg/m2, Epirubicin at 90 mg/m2, and Fluorouracil at 600 mg/m2. These were administered on day 1 of each 28-day cycle, with most patients receiving 2–4 cycles before surgery, which was performed 2-4 weeks after the last chemotherapy course. CEF-docetaxel (CEF-T): This regimen consisted of the CEF components as described above, with the addition of Docetaxel administered at a dose of 75 mg/m2 on day 1 of each cycle. The regimen was administered over 28-day cycles, with most patients receiving 2-4 cycles. Cyclophosphamide + Methotrexate + Fluorouracil + Docetaxel (CMF-T): Cyclophosphamide was administered at 600 mg/m2, Methotrexate at 40 mg/m2, Fluorouracil at 600 mg/m2, and Docetaxel at 75 mg/m2. These were administered on day 1 of each 28-day cycle, similar to the CEF-T regimen, with 2-4 cycles being most common.
Adjustment Protocols for Chemotherapy Regimens: In cases of significant toxicity (e.g., neutropenia, anemia, or liver function abnormalities), dosages were adjusted according to standardized guidelines, which included dose reduction or cycle extension as warranted26. Treatment could be paused for severe adverse reactions, with continuation or further adjustment based on a comprehensive evaluation by the medical team. Close monitoring, including hematologic, liver, and kidney function tests, as well as cardiac function assessments, was conducted throughout the chemotherapy period to ensure patient safety and treatment efficacy. Patients in Group B underwent surgery without any prior chemotherapy.
Outcomes
To adjust for preoperative clinicodemographic differences between patients, who received neoadjuvant chemotherapy or not, we took the two groups of patients and matched them 1:1 to each other using propensity scoring based on age, body mass index, clinical T and N stages, tumor pathology subtype, hormone status, Her2 status, and Ki-67 status.
The score-matched patients with or without neoadjuvant chemotherapy were compared based on percentages of CD3+, CD4+, CD8+, and the CD4+/CD8+ ratio on preoperative day 1 and postoperative days 1 and 7. Comparisons were also made between the score-matched groups regarding clinicodemographic profiles and postoperative complications.
Finally, we analyzed the diverse outcomes in subgroups of score-matched patients, who received neoadjuvant chemotherapy and, based on the tumor response, were classified as sensitive or insensitive. Patients were classified as sensitive if they showed partial or complete tumor response based on a previously described tumor response grading system27; otherwise, they were classified as insensitive.
Flow cytometry
Flow cytometry was utilized to quantify the proportions of CD4+ and CD8+ T lymphocytes in peripheral blood samples collected from patients on preoperative day 1 and postoperative days 1 and 7. Peripheral venous blood samples (10 mL) were drawn into EDTA-coated tubes and processed for peripheral blood mononuclear cell (PBMC) isolation using Ficoll-Paque PLUS (GE Healthcare). Blood samples were diluted 1:1 with phosphate-buffered saline (PBS) and layered over Ficoll-Paque, followed by centrifugation at 400 × g for 30 minutes at room temperature. The PBMC layer was carefully harvested, washed twice with PBS, and resuspended in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS). For flow cytometry staining, 1 × 106 PBMCs were incubated with Fc receptor blocking solution (Human TruStain FcX, BioLegend) for 10 minutes at 4°C to minimize nonspecific binding, and subsequently stained with APC-conjugated anti-CD3, FITC-conjugated anti-CD4, and PE-conjugated anti-CD8 monoclonal antibodies (BD Biosciences) for 30 minutes at 4 °C in the dark. Post-staining, cells were washed with PBS containing 2% FBS and resuspended in 500 μL of PBS for analysis. Data acquisition was performed using a BD FACSCanto II flow cytometer, with instrument settings optimized using unstained and single-stained controls for compensation, and fluorescence minus one (FMO) controls to set gating thresholds. A minimum of 10,000 events were recorded per sample. Data analysis was conducted using FlowJo software (version 10.7.1), where lymphocytes were gated based on forward and side scatter properties, followed by identification of CD3+ T cells and subsequent gating of CD4+ and CD8+ subsets within the CD3+ population. To ensure reproducibility and accuracy, all samples were processed and analyzed in triplicate, with inter-assay and intra-assay variations monitored by including a standard control sample in each run, maintaining a coefficient of variation (CV) below 10%. Detailed protocols for flow cytometry procedures can be referenced from previously reported studies28.
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD) if normally distributed, or median (range) if skewed. Categorical data were presented as counts (percentages). Differences between groups were assessed using appropriate statistical tests. Propensity score matching (PSM) was performed to minimize potential confounding factors, based on age, BMI, clinical T stage, and N stage. Post-PSM, these characteristics were comparable between the groups. One-way ANOVA was used to compare changes in T lymphocyte and natural killer cell subsets across different time points within the same group. Independent samples t-test was used to compare differences between groups at the same time point. Paired samples t-test was used to compare changes within the same group at different time points. Bonferroni correction was applied to control for type I errors in multiple comparisons. Statistical significance was defined as P ≤ 0.05. All analyses were performed using SPSS 17.0 (IBM, Chicago, IL, USA).
Ethics statement
The subjects included in this study were human participants, and the research was approved by the Ethics Committee of the Third Hospital of Nanchang City (Ethics review number: K-lw2023002).
Results
Patient demographics and clinical characteristics
A total of 439 patients were ultimately included in the analysis (Fig. 1). Among them, 119 (27.1%) received neoadjuvant chemotherapy, while the majority, 320 (72.9%), did not. The clinicodemographic characteristics of study participants before and after matching are presented in Table 1. Prior to propensity score matching, patients who received neoadjuvant chemotherapy were notably younger, had lower body mass index, and exhibited more advanced clinical T and N stages compared to those who did not. Following propensity score matching, there were no significant differences observed across any of the examined clinicopathologic characteristics between the two patient groups: those who underwent neoadjuvant chemotherapy (Group A, n=106) and those who did not (Group B, n=106).
Immune cell analysis
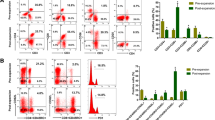
No significant differences in immune cell percentages (Table 2, Fig. 2) were observed between Group A and Group B at any time point examined. In both groups, the percentages of CD3+, CD4+, and the CD4+/CD8+ ratio decreased from preoperative day 1 to postoperative day 1, but reverted to preoperative levels by postoperative day 7. We hypothesize that the initial decrease in CD3+, CD4+, CD8+ levels, and the CD4+/CD8+ ratio following neoadjuvant chemotherapy may be attributed to the cytotoxic effects of the treatment on immune cells, particularly T lymphocytes. Subsequently, the observed increase could potentially be due to the recovery of immune function or compensatory mechanisms triggered by the body in response to the initial depletion.
The proportions of (A) CD3+ cells, (B) CD4+ cells, (C) CD8+ cells, and the ratio of (D) CD4+ to CD8+ cells were assessed at various time points in patients who received neoadjuvant chemotherapy (Group A, n = 106) and those who did not (Group B, n = 106). The timepoints "-1", "1", and "7" correspond, respectively, to preoperative day 1, postoperative day 1, and postoperative day 7. Values are mean ± SD. NCT, neoadjuvant chemotherapy; ns, not significant.
Subgroup analysis within group A
Patients in Group A were further divided into two categories based on their response to chemotherapy: Group A sensitive (n = 70) and Group A insensitive (n = 36). The sensitive subgroup in Group A showed no significant differences in immune cell percentages compared to the entirety of Group B across all three time points (Table 4, Fig. 3). However, they exhibited significantly lower T and N stages (Table 3).
The proportions of (A) CD3+ cells, (B) CD4+ cells, (C) CD8+ cells, ratio of (D) CD4+ to CD8+ cells were measured at different time points in all patients who did not receive NCT (Group B, n =106) and in patients who received NCT. Patients treated by NCT were divided into those sensitive (Group A sensitive, n = 70) or insensitive (Group A insensitive, n = 36) to NCT. The timepoints "-1", "1", and "7" correspond, respectively, to preoperative day 1, postoperative day 1, and postoperative day 7. Values are mean ± SD. NCT, neoadjuvant chemotherapy; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Conversely, the insensitive subgroup in Group A exhibited significantly lower percentages of CD4+ cells and a reduced CD4+/CD8+ ratio compared to Group B across all three time points (Table 4, Fig. 3B, D). However, the percentages of CD3+ and CD8+ cells were similar between these two groups (Fig. 3A, C), and there were no significant differences observed in clinical T or N stage (Table 3).
Postoperative complications
When comparing Group A with Group B, patients in Group A who were sensitive to chemotherapy exhibited a similar rate of postoperative complications. In contrast, patients in Group A who were insensitive to chemotherapy showed significantly higher rates of infection and fat necrosis compared to their sensitive counterparts (Table 3).
Discussion
Our single-center retrospective study suggests that neoadjuvant chemotherapy may induce immunosuppression in breast cancer patients who are insensitive to the treatment, while having minimal impact on those who are sensitive to it. Our study revealed that neoadjuvant chemotherapy decreased the levels of CD4+ and CD8+ cells, as well as the CD4+/CD8+ ratio in insensitive patients, suggesting an elevated risk of postoperative infection and a weakened anti-tumor response. Notably, insensitive patients in our study showed significantly higher rates of infection and fat necrosis than the entire group of patients who did not receive neoadjuvant chemotherapy.
The reliability of our study might exceed that of previous research because we matched patients who underwent neoadjuvant chemotherapy with those who did not based on propensity scoring. This scoring method took into account three key factors influencing the postoperative prognosis of breast cancer: age, body mass index, and clinical stage29,30,31. Before matching, these three variables differed significantly between patients in our study who received neoadjuvant chemotherapy or not, indicating that patients who receive such therapy tend to be younger and to have larger tumors and more aggressive disease. Our propensity score-matched patients who received neoadjuvant chemotherapy and those who did not did not differ significantly in postoperative complications, consistent with a previous report involving breast cancer patients32. However, our subgroup analysis suggests that such a conclusion may depend on whether patients are sensitive or insensitive to neoadjuvant chemotherapy. Indeed, our findings differ from those of a previous study that indicated that neoadjuvant chemotherapy could significantly reduce the percentages of CD3+ and CD4+ T cells while having no effect on the CD4+/CD8+ ratio33,34. The difference between these findings and ours may be partly attributed to the sensitivity or insensitivity of patients to neoadjuvant chemotherapy35,36,37,38. One possibility is that in patients sensitive to neoadjuvant chemotherapy, the treatment weakens the tumor, thus preserving immune cells, while in patients insensitive to chemotherapy, the tumor burden remains constant or may even increase, resulting in immune function suppression. Further complicating this hypothesis is the potential toxicity of neoadjuvant chemotherapy to immune cells, leading to perioperative immunosuppression39,40,41. The overall impact of chemotherapy on breast cancer patients may involve a multifaceted interplay of various factors affecting immune function42,43,44, including disease characteristics and preoperative interventions like immunotherapy, nutritional support, and radiotherapy45,46,47.
This study highlights the complex interplay between neoadjuvant chemotherapy and immune function, particularly in chemotherapy-insensitive patients. The potential of this research lies in its ability to inform more personalized treatment strategies. By identifying patients who are at higher risk of immune suppression, we can better tailor supportive interventions to improve outcomes. However, significant knowledge gaps remain, particularly regarding the mechanisms underlying the differential impact of chemotherapy on immune function. Addressing these gaps will require multi-center studies with standardized treatment protocols and comprehensive documentation of patient characteristics. Future research should also explore the role of genetic factors and adjunctive therapies, such as immunotherapy, to enhance the understanding of how neoadjuvant chemotherapy affects immune function. Over the next five years, we anticipate a more nuanced approach to treatment that integrates these findings, ultimately leading to improved patient care and outcomes in breast cancer treatment.
The interpretation of our study must consider several important limitations. Firstly, we acknowledge that we did not fully account for variations in patients’ baseline health or lifestyle factors, such as diet and exercise, which could introduce bias into our outcomes. Healthier individuals may inherently have better surgical outcomes, independent of the treatment type they receive. Additionally, the lack of detailed records regarding other concurrent treatments or interventions limits our ability to rule out their potential impact on our findings. This includes any additional medications or supportive therapies that were not documented in our analysis, which could confound the results. Moreover, the variation in chemotherapy regimens among participants presents another significant challenge. This diversity complicates direct comparisons between groups and may influence the observed outcomes, making it difficult to attribute differences solely to neoadjuvant chemotherapy. Given these limitations, our results should be interpreted with caution. Future research should aim for larger, more controlled studies that thoroughly document baseline health status, lifestyle factors, and all treatments received. Such studies would provide clearer insights into the effects of neoadjuvant chemotherapy on surgical outcomes and help to better understand the complex interactions between these variables.
Despite these limitations, our study provides evidence that neoadjuvant chemotherapy can significantly immunosuppress breast cancer patients who do not respond to such chemotherapy. Future studies should verify and extend our findings by analyzing a broader range of immune and inflammatory indicators as well as recurrence and other outcomes during follow-up. Such work should also take into account the potential influence of genetic polymorphism related to immune function. It may be possible to identify prognostic indicators that can differentiate patients more likely to benefit from such chemotherapy or from immediate surgery.
Conclusion
Neoadjuvant chemotherapy potentially impairs immune function in chemotherapy-insensitive patients, but not in those sensitive to it.
Data availability
The primary data can be acquired from the corresponding authors in compliance with privacy and ethical constraints.
References
Toohey, K. et al. systematic review of multimodal prehabilitation in breast cancer. Breast Cancer Res. Treat. https://doi.org/10.1007/s10549-022-06759-1 (2022).
Al-Masri, M., Aljalabneh, B., Al-Najjar, H. & Al-Shamaileh, T. Effect of time to breast cancer surgery after neoadjuvant chemotherapy on survival outcomes. Breast Cancer Res. Treat. 186, 7–13. https://doi.org/10.1007/s10549-020-06090-7 (2021).
Morgan, J. L. et al. Breast cancer surgery in older women: Outcomes of the Bridging Age Gap in Breast Cancer study. Br J Surg 107, 1468–1479. https://doi.org/10.1002/bjs.11617 (2020).
Rizzo, A., Cusmai, A., Acquafredda, S., Rinaldi, L. & Palmiotti, G. Ladiratuzumab vedotin for metastatic triple negative cancer: Preliminary results, key challenges, and clinical potential. Expert Opin. Investig. Drugs 31, 495–498. https://doi.org/10.1080/13543784.2022.2042252 (2022).
Krug, D. & Loibl, S. Neoadjuvant chemotherapy for early breast cancer. Lancet Oncol. 19, e129 (2018).
Tamirisa, N. & Hunt, K. K. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. Ann. Surg. Oncol. 29, 1489–1492 (2022).
Tian, H. et al. Platinum and taxane based adjuvant and neoadjuvant chemotherapy in early triple-negative breast cancer: A narrative review. Front. Pharmacol. 12, 770663 (2021).
Xu, H. D. & Zhang, Y. Q. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer using diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging. Neoplasma J. Exp. Clin. Oncol. 64, 430–436 (2017).
Inari, H., Teruya, N., Kishi, M., Horii, R. & Ohno, S. Survival in cytologically proven node-positive breast cancer patients with nodal pathological complete response after neoadjuvant chemotherapy. Cancers 12, 2633 (2020).
Caputo, R. et al. Sacituzumab govitecan for the treatment of advanced triple negative breast cancer patients: A multi-center real-world analysis. Front. Oncol. 14, 1362641. https://doi.org/10.3389/fonc.2024.1362641 (2024).
Rizzo, A. et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: Changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 18, 2301–2309. https://doi.org/10.2217/fon-2021-1647 (2022).
Rizzo, A. et al. Discontinuation rate and serious adverse events of chemoimmunotherapy as neoadjuvant treatment for triple-negative breast cancer: A systematic review and meta-analysis. ESMO Open 8, 102198. https://doi.org/10.1016/j.esmoop.2023.102198 (2023).
Sahin, T. K., Rizzo, A., Aksoy, S. & Guven, D. C. Prognostic significance of the royal marsden hospital (RMH) score in patients with cancer: A systematic review and meta-analysis. Cancers (Basel) https://doi.org/10.3390/cancers16101835 (2024).
Iwamoto, T., Kajiwara, Y., Zhu, Y. & Iha, S. Biomarkers of neoadjuvant/adjuvant chemotherapy for breast cancer. Chin. Clin. Oncol. 9, 27. https://doi.org/10.21037/cco.2020.01.06 (2020).
Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19, 27-39, https://doi.org/10.1016/s1470-2045(17)30777-5 (2018).
Kaufmann, M. et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann. Oncol. 18, 1927–1934. https://doi.org/10.1093/annonc/mdm201 (2007).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680. https://doi.org/10.1038/s41568-020-0285-7 (2020).
Ding, S., Chen, X. & Shen, K. Single-cell RNA sequencing in breast cancer: Understanding tumor heterogeneity and paving roads to individualized therapy. Cancer Commun. (Lond.) 40, 329–344. https://doi.org/10.1002/cac2.12078 (2020).
DeMichele, A., Yee, D. & Esserman, L. Mechanisms of resistance to neoadjuvant chemotherapy in breast cancer. N. Engl. J. Med. 377, 2287–2289. https://doi.org/10.1056/NEJMcibr1711545 (2017).
Chang, H. L., Schwettmann, B., McArthur, H. L. & Chan, I. S. Antibody-drug conjugates in breast cancer: overcoming resistance and boosting immune response. J. Clin. Invest. https://doi.org/10.1172/jci172156 (2023).
Ding, L., Wang, L., Yin, J., Fan, Z. & He, Z. Effects of neoadjuvant chemotherapy on respiratory function in patients with breast cancer. Chin. J. Cancer Res. 32, 36–42. https://doi.org/10.21147/j.issn.1000-9604.2020.01.05 (2020).
Garciamartinez, E. et al. Baseline CD4/CD8 tumor infiltrating lymphocytes (TIL) ratio predicts pathologic response to neoadjuvant chemotherapy (NC) in breast cancer. Cancer Res. 72(1024), P3-06–15 (2012).
Handgraaf, S. M. et al. Regulatory T cells play a crucial part in radio-immunotherapy resistance of brain tumors. Nat. Cancer 4, 590–591 (2023).
Itoi, N. et al. Infiltration of CD4, CD8, CD56, and Fox-P3-positive lymphocytes in breast carcinoma tissue after neoadjuvant chemotherapy with or without trastuzumab. Breast Dis. 38, 57–65 (2019).
Amin, M. B. et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99. https://doi.org/10.3322/caac.21388 (2017).
Schipilliti, F. M. et al. Datopotamab deruxtecan: A novel antibody drug conjugate for triple-negative breast cancer. Heliyon 10, e28385. https://doi.org/10.1016/j.heliyon.2024.e28385 (2024).
Meredith, K. L. et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann. Surg. Oncol. 17, 1159–1167 (2010).
Tan, J.-T. et al. Comparison of postoperative immune function in patients with thoracic esophageal cancer after video-assisted thoracoscopic surgery or conventional open esophagectomy. Int. J. Surg. 30, 155–160. https://doi.org/10.1016/j.ijsu.2016.04.052 (2016).
Gennari, A. et al. Body mass index and prognosis of metastatic breast cancer patients receiving first-line chemotherapy. Cancer Epidemiol. Biomark. Prevent. 22, 1862–1867 (2013).
Azim, H. A. & Partridge, A. H. Biology of breast cancer in young women. Breast Cancer Res. 16, 427 (2014).
Kimbung, S., Loman, N. & Hedenfalk, I. Clinical and molecular complexity of breast cancer metastases. Semin. Cancer Biol. 35, 85–95 (2015).
Lorentzen, T., Heidemann, L. N., Moller, S. & Bille, C. Impact of neoadjuvant chemotherapy on surgical complications in breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 48, 44–52. https://doi.org/10.1016/j.ejso.2021.09.007 (2022).
Wang, W. H., Xu, H. Y., Zhao, Z. M., Zhang, G. M. & Lin, F. W. Dynamic and significant changes of T-cell subgroups in breast cancer patients during surgery and chemotherapy. Int. Immunopharmacol. 65, 279–283. https://doi.org/10.1016/j.intimp.2018.09.039 (2018).
Costa, A. D., Vyrynen, S., Chawla, A., Zhang, J. & Nowak, J. A. Proc. Abstracts: AACR Virtual Special Conference on Pancreatic Cancer; September 29-30, (2020).
Rizzo, A. et al. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: The MOUSEION-02 study. Expert Opin. Drug Metab. Toxicol. 17, 1455–1466. https://doi.org/10.1080/17425255.2021.2029405 (2021).
Viscardi, G. et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: A systematic review and meta-analysis. Eur. J. Cancer 177, 175–185. https://doi.org/10.1016/j.ejca.2022.09.031 (2022).
Comes, M. C. et al. Explainable 3D CNN based on baseline breast DCE-MRI to give an early prediction of pathological complete response to neoadjuvant chemotherapy. Comput. Biol. Med. 172, 108132. https://doi.org/10.1016/j.compbiomed.2024.108132 (2024).
Fanizzi, A. et al. Prognostic power assessment of clinical parameters to predict neoadjuvant response therapy in HER2-positive breast cancer patients: A machine learning approach. Cancer Med. 12, 20663–20669. https://doi.org/10.1002/cam4.6512 (2023).
Lundy, J., Iii, E. J. L., Hamilton, S. & Dvm, P. C. Halothane, surgery, immunosuppression and artificial pulmonary metastases. Cancer 41, 827–830 (2015).
Napoletano, C. et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J. Cell. Mol. Med. 14, 2748–2759 (2010).
Benisovich, V. I., Silverman, L., Slifkin, R., Stone, N. & Cohen, E. Cisplatin-based chemotherapy in renal transplant recipients. A case report and a review of the literature. Cancer 77, 160–163 (2015).
Strasser, A., Jost, P. J. & Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 30, 180–192 (2009).
Asano, Y. et al. Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression. J. Transl. Med. 16, 87 (2018).
Zheng, Y., Chen, Z., Han, Y., Han, L. & Shen, L. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat. Commun. 11, 6268 (2020).
Parkin, J. & Cohen, B. An overview of the immune system: The lancet. Lancet 357, 629–653 (1997).
Venter, C. et al. Nutrition and the immune system: A complicated tango. Int. J. Appl. Mech. 12, 818 (2020).
Lundholm, K., Daneryd, P., Bosaeus, I., Krner, U. & Lindholm, E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer 100, 1967–1977 (2010).
Funding
This study was supported by the Major Project of Nanchang Science and Technology (Contract Grant Number: HKZ2020-133-1 to Z Li).
Author information
Authors and Affiliations
Contributions
J.T., Z.L. and P.D. conceived the study. Q.J. and H.H. collected the data. Q.J. and Z.X. analyzed the data. Q.J. wrote the manuscript. J.T., Z.L. and P.D. revised the manuscript. H.H. conducted the statistical analysis. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, QH., Hu, H., Xu, ZH. et al. Impact of neoadjuvant chemotherapy on perioperative immune function in breast cancer patients: a propensity score-matched retrospective study. Sci Rep 14, 18738 (2024). https://doi.org/10.1038/s41598-024-69546-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69546-6
- Springer Nature Limited