Abstract
Metabolic syndrome (MetS) is prevalent and significantly impacts global public health, with obesity being a major risk factor for cardiovascular diseases (CVD) and mortality. Traditional metrics like body mass index (BMI) have limitations in assessing obesity-related risks. The weight-adjusted waist circumference index (WWI) has emerged as a novel obesity metric, this study aimed to evaluate the association of WWI with CVD and mortality in MetS patients. This study used data from 12,641 participants with MetS, derived from the National Health and Nutrition Examination Survey (NHANES) conducted from 1999 to 2020. The WWI was calculated, and its association with CVD and mortality was assessed using multivariate logistic and Cox regression models. The study controlled for potential confounders and performed subgroup and sensitivity analyses to validate the robustness of the findings. The predictive performance of WWI was evaluated using the area under the receiver operating characteristic curve (ROC). Kaplan–Meier (KM) curves further were used to evaluate the associations between WWI and mortality of the MetS population. As WWI values escalated, there was a proportional rise in the risk of CVD and mortality in MetS. The fully adjusted continuous model revealed a 32.0% elevated likelihood of CVD development, a 69.5% increased probability of heart failure (HF), a 51.1% heightened risk for CVD mortality, and a 22.8% augmented risk for all-cause mortality with each one-unit increment in WWI. Comparing the highest to the lowest quartile of WWI, the top quartile exhibited a significantly increased risk of CVD (odds ratio [OR] = 1.883; 95% confidence interval [CI]: 1.276–2.633, p-value = 0.001), HF (OR = 2.909; 95% CI: 1.490–5.677, p-value = 0.002), CVD mortality (hazard ratio [HR] = 2.088; 95% CI: 1.279–3.409, p-value = 0.003), and all-cause mortality (HR = 1.394; 95% CI: 1.070–1.816, p-value = 0.014) among individuals with MetS. Sensitivity and subgroup analyses substantiated the consistency and stability of these associations across various demographic groups. The ROC analysis demonstrated that WWI outperforms BMI in predicting adverse outcomes in MetS. The KM curves validated that higher WWI values was correlated with diminished survival rates in MetS population. The WWI served as a significant indicator for assessing the risk of CVD and mortality in the MetS population. This study recommended the regular assessment of WWI in MetS individuals for evaluating their risk of CVD and mortality, potentially enhancing preventive and treatment strategies for this patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Metabolic syndrome (MetS), characterized by a group of metabolic abnormalities, poses a significant challenge to global public health due to its rising prevalence across the world1,2,3. In the United States, it is estimated that more than 30% of individuals, particularly among the elderly, suffer from MetS4,5. Recent epidemiological data from the U.S. indicated that the prevalence of MetS has risen from 27.6 to 32.3% over the past several decades6. There is a growing body of evidence that the constellation of symptoms associated with MetS, including insulin resistance, hypertension, and obesity, not only heightens the risk for various chronic metabolic disorders but also contributes to an increased likelihood of premature mortality in later years6,7,8,9. Despite these findings, pinpointing predictive biomarkers and formulating personalized guidance for individuals with MetS continues to be a complex endeavor10.
Obesity, a growing global epidemic, is increasingly being recognized for its widespread impact on health11. There is a well-established link between obesity and the increased risk of cardiovascular diseases (CVD) and mortality12,13. Although the body mass index (BMI) is commonly used to gauge obesity, it has limitations as it does not provide a clear picture of fat distribution across the body14,15. Waist circumference (WC) serves as a more direct and reliable measure of abdominal obesity and can sometimes be used to infer overall body fat content16. However, WC has its own limitations as it does not distinguish between subcutaneous fat and visceral fat17. Research indicates that visceral fat is more likely to release free fatty acids, which can significantly raise the risk of CVD compared to subcutaneous fat18,19,20. In clinical practice, imaging techniques to measure visceral fat and muscle mass are often impractical. To address this issue, Park Y et al. proposed a novel obesity metric termed the “weight-adjusted waist circumference index (WWI)”21. This index is calculated by dividing WC by the square root of an individual’s body weight. By doing so, the WWI reduced its correlation with BMI and leverages the advantages of WC to focus on central obesity22. As a result, the WWI could potentially offer a more precise indicator for assessing the risks associated with obesity. However, there has been a lack of research exploring the prognostic significance of the WWI within the MetS demographic. The possibility that the WWI could act as an indirect measure of health status within the MetS community remains an open question.
Consequently, our objective was to investigate the correlation of WWI with CVD and mortality within the MetS population. This study performed a comprehensive, prospective, population-based cohort study in the United States. It represented a first epidemiological examination dedicated to unraveling the prognostic connections of WWI in MetS.
Methods
Study population
This observational research drew on data collected over 11 cycles of the National Health and Nutrition Examination Survey (NHANES) conducted across the United States from 1999 to 2020. Annually, NHANES selected approximately 5000 individuals from 15 distinct geographical regions for inclusion in its study. For comprehensive insights into the survey’s methodology, one should refer to the NHANES Plan and Operations manual. The survey was approved by the National Center for Health Statistics Research Ethics Review Board, adhering to established protocols. Since this investigation was a secondary analysis of anonymized NHANES data, no additional ethical approval was warranted.
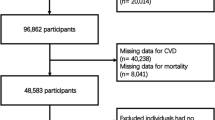
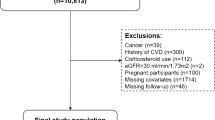
The research sample was derived from NHANES participants spanning the years 1999 to 2020. Initially, there were 116,876 potential candidates. The study initially excluded individuals under 18 years of age and those not diagnosed with MetS, totaling 101,725 participants. Subsequently, 2,101 individuals were excluded due to the absence of data on CVD and mortality. Finally, 409 individuals were excluded for lacking the physical data required to calculate the WWI and BMI. Consequently, the study ultimately included 12,641 participants, as illustrated in Fig. 1.
Measurement of WWI
The WWI is determined by dividing the WC, measured in centimeters, by the square root of the body weight, measured in kilograms. To guarantee the precision of these measurements, they were meticulously conducted by skilled medical staff and dedicated record-keepers. Body weight was precisely determined using a calibrated electronic scale, with participants dressed in their examination attire and standing barefoot, arms relaxed at their sides and facing forward, in accordance with the standardized procedure. For the measurement of WC, a tape measure was carefully placed at the intersection of the horizontal line above the superior border of the right patella with the midaxillary line, following a standardized technique23. This rigorous methodology ensures that the WWI is derived from accurate and uniform measurements, which is essential for the index’s validity in evaluating the degree of obesity and associated health risks.
Diagnosis of MetS
The criteria for diagnosing MetS were established according to the guidelines of the National Cholesterol Education Program (NCEP) and its Adult Treatment Panel III. An individual is considered to have MetS if they exhibit three or more of the following conditions: a fasting blood glucose (FBG) level exceeding 100 mg/dL or are undergoing medication for diabetes; a low level of high-density lipoprotein cholesterol (HDL-C), defined as less than 50 mg/dL for women and less than 40 mg/dL for men, or are receiving treatment for reduced HDL-C levels; elevated triglycerides (TG) with a plasma concentration above 150 mg/dL or are on medication for high TG levels; an increased WC, which is more than 88 cm for women and more than 102 cm for men; high blood pressure, with readings above 130/85 mmHg or are on treatment for elevated blood pressure24,25.
Diagnosis of CVD
The diagnosis of CVD was ascertained through self-reports by medical practitioners, gathered via personal interviews that employed a standardized medical questionnaire. Participants were asked, “Has a doctor or health professional ever informed you that you have conditions such as angina, coronary heart disease (CHD), heart failure (HF), a heart attack, or a stroke?” A positive reply to any of these questions led to the classification of the individual as having CVD26. This approach to identifying CVD relies on direct medical confirmation from healthcare providers, ensuring that the reported cases are based on professional medical assessments. The use of a consistent inquiry form across the survey helps maintain the uniformity and reliability of the data collected, which is vital for accurate CVD prevalence analysis.
Assessment of mortality
For the purpose of determining the mortality rates among the participants in our study during the subsequent timeframe, we utilized the NHANES public-use linked mortality database, which was current up to December 31, 2019. This database has been meticulously integrated with the National Death Index (NDI) by the National Center for Health Statistics (NCHS) through a sophisticated probabilistic matching methodology. To identify deaths caused by specific illnesses, we leveraged the International Statistical Classification of Diseases, 10th Revision (ICD-10). Deaths resulting from cardiovascular disorders, including but not limited to rheumatic heart disease, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, pericardial conditions, and acute myocarditis, as well as HF, were classified under the ICD-10 codes I00 to I09, I11, I13, and I20 through I5127. This systematic classification allows for a precise and comprehensive analysis of cardiovascular-related mortality within the study population.
Potential confounders
Demographic variables were considered as confounding factors in our analysis, including gender (male or female), age, ethnicity (White, Black, Mexican, or other), educational attainment (below high school, high school or equivalent, and above high school), and marital status (married, never married, or other). Lifestyle factors such as smoking habits (non-smoker or smoker), alcohol intake (non-drinker or drinker), and the family income-to-poverty ratio (IPR) were also examined. Physiological measurements like systolic blood pressure (SBP) and diastolic blood pressure (DBP) were incorporated into the study. A thorough examination of medical histories was conducted, with an emphasis on conditions including diabetes, hypertension, and chronic kidney disease (CKD). The study also evaluated a range of blood markers, including TG, total cholesterol (TC), HDL-C, and low-density lipoprotein cholesterol (LDL-C), along with liver function tests such as glutamic pyruvic transaminase (ALT) and glutamic oxaloacetic transaminase (AST), FBG, and hemoglobin A1c (HbA1c). To assess renal function, the estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI 2021 creatinine formula. In addition to this, the level of physical activity was divided into three categories: mild, moderate, and vigorous.
Statistical analysis
The statistical analysis was conducted using R software, specifically version 4.1.1. The criterion for statistical significance was set at a P value of less than 0.05, considering both directions of the test (two-tailed). To ensure the generalizability of the study findings and to prevent any bias due to overrepresentation, sampling weights were incorporated into the analysis. Data that was continuous was reported as the mean with 95% confidence intervals (CI). Categorical data was summarized by the number of occurrences and their respective percentages.
For a more nuanced analysis, multivariate logistic and Cox regression models were employed to assess the influence of the WWI on CVD prevalence and mortality rates within the MetS cohort. We used logistic regression to analyze the relationship between WWI and CVD (including stroke, heart attack, angina, HF, and CHD), and Cox regression to analyze the relationship between WWI and mortality (including all-cause and CVD mortality). The selection of covariates for this study was informed by existing literature on MetS survival studies25. Given the strong correlation between WWI and BMI, BMI was not included as a covariate in the regression models. Additionally, a separate analysis was conducted to examine the relationship between BMI and MetS prognosis, highlighting the superiority of WWI as an obesity indicator for evaluating patient outcomes.
Specifically, crude model represented the unadjusted analysis. Model l incorporated basic adjustments for age, gender, and ethnicity. The fully adjusted model 2 considered a comprehensive set of variables including age, gender, ethnicity, marital status, education level, IPR, smoking habits, alcohol consumption, presence of diabetes, hypertension, CKD, SBP, DBP, eGFR and serum levels of TG, TC, HDL-C, LDL-C, ALT, AST, FBG, and HbA1c.
To ensure the robustness of the association of WWI with CVD and mortality in the MetS population, four sets of sensitivity analyses were conducted. Initially, we included physical activity in the analysis based on model 2. Subsequently, model 2 without a history of diabetes and model 2 without other comorbidities were performed. Finally, participants who passed away within the first two years post-interview were excluded from the analysis. Subgroup analyses were conducted and are reported, stratified using a comprehensive model 2 that included all adjustments. The potential nonlinear associations were assessed utilizing restricted cubic spline (RCS) curves.
The diagnostic and predictive capabilities of the WWI for CVD and mortality were compared using the area under the receiver operating characteristic (ROC) curve (AUC). This method is a standard approach in evaluating the performance of a diagnostic test or a predictive model, with the AUC providing a single value that represents the overall accuracy of the test or model. A higher AUC value indicates a better predictive or diagnostic ability. Kaplan–Meier (KM) curves were utilized to compare survival patterns among different quartiles of the WWI and groups stratified by the WWI cutoff value within the MetS population.
Ethical approval
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board (ERB). All participants provided written informed consent.
Results
Demographic characteristics
During the study period, a total of 12,641 participants diagnosed with MetS were included in the research. The mean age of the MetS population was 52 years, with 30.6% being over the age of 65. Additionally, the proportion of females was slightly higher than that of males, accounting for 54.9% (n = 6940). Nearly half of the MetS population were non-Hispanic whites, representing 46.2% (n = 5841). Approximately 17.2% (n = 2175) of the overall MetS population suffered from CVD. Over a median follow-up period of 100 months, there were 2464 (19.5%) recorded deaths, of which 677 (5.4%) were attributed to cardiovascular causes. (Fig. 2). Table 1 presented the baseline demographics categorized into quartiles based on WWI (WWI-Q1 [9.22,11.06]; WWI-Q2 (11.06,11.53]; WWI-Q3 (11.53,12.00]; WWI-Q4 (12.00,14.79]). The mean WWI value was 11.45. Except AST, all variations were observed across the quartiles in terms of age, gender, ethnicity, education, marital status, smoking status, alcohol consumption, IPR, SBP, DBP, eGFR, ALT, FBG, HbA1c, TG, TC, HDL-C, LDL-C, diabetes, hypertension and CKD, all of which were statistically significant (p-value < 0.05). When comparing the lowest WWI quartile to the higher ones, there was a progressive increase in the likelihood of experiencing CVD (Q1: 323 cases, 8.12%; Q2: 478 cases, 12.86%; Q3: 595 cases, 16.88%; Q4: 779 cases, 23.41%; p-value < 0.0001), CVD mortality (Q1: 94 deaths, 2.19%; Q2: 139 deaths, 3.36%; Q3: 206 deaths, 5.33%; Q4: 238 deaths, 6.50%; p-value < 0.0001), and all-cause mortality (Q1: 352 deaths, 8.04%; Q2: 525 deaths, 13.52%; Q3: 714 deaths, 18.21%; Q4: 873 deaths, 23.71%; p-value < 0.0001). This indicated a clear trend of escalating health risks with increasing WWI scores in MetS. Demographic data, categorized by the presence or absence of CVD, whether the death was CVD-related, and whether death was due to all causes, were further detailed in Supplemental Tables 1–3.
Association between WWI and the risk of CVD in MetS population
Table 2 delineated the correlation between the WWI and the propensity for CVD. Our research disclosed a positive correlation between an elevated WWI and an increased risk of CVD. This relationship held true in both the crude analysis (odds ratio [OR] = 1.941, 95% CI 1.774–2.125, p-value < 0.0001) and the minimally adjusted model (OR = 1.362, 95% CI 1.216–1.524, p-value < 0.0001). After comprehensive adjustment, the link between WWI and CVD risk was still evident (OR = 1.320, 95% CI 1.087–1.602, p-value = 0.005), indicating that each increment in WWI was correlated with a 32.0% rise in the risk of CVD. When WWI was stratified into quartiles, the fully adjusted models revealed that individuals in the top WWI quartile had a notably higher risk of CVD by 83.3% compared to those in the lowest quartile (OR = 1.833, 95% CI 1.276–2.633, p-value = 0.001). For HF, our study established a significant positive association with WWI and the likelihood of HF. In both the unadjusted and minimally adjusted models, there was an escalated risk of HF among those with higher WWI (crude model: OR = 2.163, 95% CI 1.876–2.494, p-value < 0.0001; minimally adjusted model: OR = 1.543, 95% CI 1.284–1.855, p-value < 0.0001). Upon full adjustment, each unit increment in WWI was associated with a 69.5% increase in the risk of HF (fully adjusted model: OR = 1.695, 95% CI 1.289–2.230, p-value < 0.001). When WWI was divided into quartiles, participants in the highest quartile had a substantial 190.9% increase in risk compared to those in the lowest quartile (OR = 2.909, 95% CI 1.490–5.677, p-value = 0.002). This study did not find any significant associations between WWI and the risks of stroke, myocardial infarction, angina, or CHD. Additionally, there was no non-linear relationship between the WWI and CVD as well as HF, as seen in Supplementary Fig. 1. The relationship between BMI and CVD was presented in Supplementary Table 4 (BMI-Q1: [16.8,28.59]; BMI-Q2: (28.59,31.8]; BMI-Q3: (31.8,36.2]; BMI-Q4: (36.2,44.87]).
Association between WWI and mortality in MetS population
Table 3 delineated the correlation between the WWI and the risk of CVD mortality within the MetS population. The crude hazard ratio (HR) was 2.388 with a 95% CI of 2.114 to 2.697, achieving statistical significance (p-value < 0.0001). After less adjustment, the HR was 1.575 with a 95% CI of 1.351 to 1.837, still indicating significance (p-value < 0.0001). Upon full adjustment, the HR was 1.511 with a 95% CI of 1.163 to 1.963, maintaining its statistical relevance (p-value = 0.002). The fully adjusted models indicated that individuals in the highest WWI quartile faced a significantly elevated risk of CVD mortality by 108.8% compared to the lowest quartile (OR = 2.088, 95% CI 1.279–3.409, p-value = 0.003). Furthermore, Table 3 also presented the link between WWI and the risk of all-cause mortality in the MetS population. The unadjusted HR was 2.248 with a 95% CI of 2.105 to 2.401, which was highly significant (p-value < 0.0001). With less adjustment, the HR was 1.461 with a 95% CI of 1.351 to 1.580, again significant (p-value < 0.0001). After full adjustment, the HR was 1.228 with a 95% CI of 1.066 to 1.414, which was statistically significant (p-value = 0.004). The fully adjusted models showed that the top WWI quartile was associated with a notably increased risk of all-cause mortality by 39.4% relative to the lowest quartile (OR = 1.394, 95% CI 1.070–1.816, p-value = 0.014). Additionally, there was no non-linear relationship between the WWI and mortality, as seen in Supplementary Fig. 1. The relationship between BMI and mortality was presented in Supplementary Table 4.
Subgroup and sensitivity analysis
In subgroup analyses, the associations between the WWI and CVD, HF, CVD mortality, and all-cause mortality were consistently observed across both age groups and different genders, with the exception of the association with CVD mortality in the male population. Compared to the other two ethnic groups, the aforementioned relationships exhibited stronger associations among non-Hispanic whites and other racial groups. Regardless of smoking or drinking status, a positive correlation between the WWI and HF was observed. Furthermore, the associations of WWI with the other three adverse outcomes were significant only among those who drank and smoked, and not statistically significant among non-smokers and non-drinkers. For diabetes, the correlation between the WWI and CVD mortality was observed in both diabetic and non-diabetic populations. However, the relationship between WWI and both CVD and all-cause mortality was only evident in the diabetic group. Conversely, the association between WWI and HF was consistently present in the non-diabetic group. Ultimately, the associations between the WWI and the four adverse outcomes of MetS were only observed in patients with healthy kidney function, while no such relationships were detected in those with CKD (Fig. 3).
Stratified analyses of weight-adjusted waist circumference index (WWI). (A) associations between WWI and cardiovascular disease (CVD); (B) associations between WWI and heart failure (HF); (C) associations between WWI and CVD mortality; (D) associations between WWI and all-cause mortality. DM diabetes; CKD chronic kidney disease.
To validate the robustness of the principal outcomes, a series of sensitivity analyses were conducted. Initially, physical activity was included in the analysis based on model 2, the association between the WWI and CVD, HF, CVD mortality, and all-cause mortality remained statistically significant throughout the observation period (the baseline data of physical activity were presented in Supplementary Table 5). The findings were equally robust when using model 2 without diabetes mellitus or without all other comorbidities. Furthermore, after the exclusion of participants who passed away within the first two years post-survey, the relationships between WWI and the aforementioned outcomes were consistently observed within the revised MetS cohort (Table 4).
Receiver operating characteristic of the WWI to CVD and mortality
The ROC curve analysis assessed the efficacy of the WWI in predicting four adverse outcomes in MetS, as illustrated in Fig. 4. Notably, the AUC for each outcome surpassed the threshold of 0.5, signifying their predictive value for adverse conditions. It is noteworthy that WWI demonstrated superior predictive accuracy for these MetS-related adverse outcomes compared to BMI. Among the four adverse outcomes, the AUC for the association between the WWI and CVD was 0.689, with a cutoff value of 11.71. For the relationship between WWI and HF, the AUC was 0.717, with a cutoff value of 11.85. The AUC for WWI in relation to CVD mortality was 0.695, also with a cutoff value of 11.71. Lastly, the AUC for WWI in relation to all-cause mortality was 0.688, with a cutoff value of 11.74.
Receiver operating characteristic (ROC) curve analysis of the weight-adjusted waist circumference index (WWI) and body mass index (BMI). (A) ROC of WWI to cardiovascular disease (CVD); (B) ROC of WWI to heart failure (HF); (C) ROC of WWI to CVD mortality; (D) ROC of WWI to all-cause mortality; (E) ROC of BMI to CVD; (F) ROC of BMI to HF; (G) ROC of BMI to CVD mortality; (H) ROC of BMI to all-cause mortality. Sens sensitivity; Spec specificity; PPV positive predictive value; NPV negative predictive value; AUC area under curve.
Survival patterns of MetS population in different levels of WWI
The KM curves revealed that individuals in the MetS cohort with WWI values in the lower quartile had significantly better overall and cardiovascular disease-specific survival rates compared to those in the upper quartiles. Similarly, the group with lower WWI values, as determined by the ROC curve cutoff, demonstrated improved survival. However, stratification by BMI yielded divergent results. Surprisingly, the group with a lower BMI, whether divided by quartiles or by the binary cutoff, showed a reduced overall and cardiovascular-specific survival rate (Fig. 5).
Kaplan–Meier curve analysis of the weight-adjusted waist circumference index (WWI) and body mass index (BMI). (A) cardiovascular-specific survival with different quartiles of WWI; (B) overall survival with different quartiles of the WWI; (C) cardiovascular-specific survival grouped by cutoff value of WWI to cardiovascular disease (CVD) mortality; (D) overall survival grouped by cutoff value of WWI to all-cause mortality; (E) cardiovascular-specific survival with different quartiles of BMI; (F) overall survival with different quartiles of the BMI; (G) cardiovascular-specific survival grouped by cutoff value of BMI to CVD mortality; (H) overall survival grouped by cutoff value of BMI to all-cause mortality. WWIQ quartiles of the WWI; BMIQ quartiles of the BMI; WWIHL WWI high or low; BMIHL BMI high or low.
Discussion
This comprehensive study involving 12,641 participants diagnosed with MetS provided a detailed examination of the demographic characteristics, associations between WWI and various health outcomes, and the predictive accuracy of WWI in relation to CVD and mortality. The strong correlations were been found between higher WWI and increased risk of CVD and HF, even after comprehensive adjustments. Elevated WWI values were associated with higher risks of CVD mortality and all-cause mortality, with individuals in the top quartile showing notably higher risks. Subgroup and sensitivity analyses confirmed the robustness of the observed associations, with some variations noted across different demographic subgroups. The study did not find significant associations between WWI and the risks of stroke, myocardial infarction, angina, or CHD. The ROC curve analysis demonstrated the predictive value of WWI for adverse outcomes in MetS, which was better than BMI. KM curves showed better survival rates for individuals with lower WWI values, while stratification by BMI revealed unexpected results, with lower BMI associated with reduced survival rates. The study was the first time to conclude that WWI was a significant predictor of CVD and mortality in the MetS population, which was better than BMI to be an indicator for clinical practice and public health strategies.
The WWI has demonstrated significant associations with a range of cardiovascular conditions and adverse health outcomes. A thorough analysis of 21,040 individuals revealed a pronounced link between elevated WWI and the risk of CVD, particularly pronounced in younger adults below the age of 50. This underscored the potential of WWI in early intervention for CVD within this demographic28. This association was further supported by a long-term cohort study of 26,822 participants, which indicated that higher WWI values were associated with increased risks of cardiovascular and all-cause mortality29. Additional research had identified WWI as a significant prognostic indicator for HF30, and a cross-sectional analysis of 23,389 subjects suggested that an increased WWI could independently predict stroke risk31. Moreover, WWI’s connection to left ventricular function pointed to its importance as a prognostic factor for cardiometabolic risk32. These collective findings underscored the utility of WWI as a key predictor for a variety of cardiovascular events and mortality across diverse populations and age groups. Furthermore, a robust correlation existd between WWI and metabolic disorders. In the adult population of the United States, for each unit increase in WWI, there was a 14% increase in the risk of diabetes among the study participants33. The predictive power of WWI for newly diagnosed diabetes has been noted in specific populations, such as in rural Chinese adults34. Recent studies have also affirmed WWI’s role as an independent factor in identifying patients at higher risk for diabetic kidney disease35. Our study introduced novel insights by connecting WWI with CVD and mortality in individuals with MetS, thereby broadening the application of WWI and offering innovative metrics for assessing and monitoring risk in this patient cohort. This advancement in understanding WWI’s predictive capabilities marked a significant step forward in the early identification and management of cardiometabolic risks.
Traditionally, obesity has long been recognized as a significant risk factor for CVD and mortality36,37. However, the so-called “obesity paradox” in mortality rates may be due, in part, to the limitations of current obesity assessment tools14,15. Our research also noted a correlation between lower BMI and decreased survival rates. While BMI was a general indicator of metabolic health, it did not precisely measure central abdominal and visceral fat accumulation, which were critical for cardiovascular and cerebrovascular health38. The distribution of fat varies among individuals, and BMI’s inability to account for this variation limited its reliability as a cardiovascular disease predictor. The WWI offered a more precise method for detecting central obesity. Evidence suggested that higher WWI values correlate with adverse changes in body composition, such as increased fat levels, reduced muscle mass, and lower bone density39. Recent research highlighted the WWI as a strong predictor of various health conditions, outperforming traditional obesity measures40,41. The WWI had shown greater consistency and reliability in disease prediction across diverse ethnicities and demographic contexts, especially in multiethnic and multicenter studies. Thus, the WWI may be a more effective metric for assessing obesity-related health risks in MetS than BMI, providing a more nuanced and accurate reflection of an individual’s cardiovascular and metabolic health.
Several potential mechanisms could explain the correlation observed between the WWI and CVD and mortality in patients with MetS. One possibility was that an elevated WWI signifies adipose tissue dysfunction, particularly an increase in visceral fat. This surplus of visceral fat could stimulate the substantial release of inflammatory cytokines such as interleukin-6 and tumor necrosis factor, which are known to initiate inflammatory processes—a factor implicated in the development of CVD42. In obesity, adipose tissue was also known to produce higher levels of reactive oxygen species (ROS). Increased ROS could deplete nitric oxide (NO), leading to the formation of harmful hydrogen peroxide when ROS interacts with NO. This chain of events could culminate in endothelial cell dysfunction43,44. Endothelial dysfunction has been directly linked to the advancement of CVD in numerous studies45,46. This complex interplay of biological factors highlighted the intricate link between adipose tissue health, as indicated by WWI, and the risk of CVD and mortality among MetS patients. The WWI, therefore, served not only as a measure of obesity but also as a potential indicator of the underlying physiological processes that contribute to cardiovascular risk and mortality in MetS.
One of the study’s key strengths was its use of data from the NHANES, renowned for employing a stratified, multi-stage probability sampling approach. This rigorous sampling technique enhanced the study’s credibility and ensures that the findings were generalizable to the wider population. However, the study was not without its limitations. Firstly, due to the cross-sectional design used in the relationship between WWI and CVD, we could not infer causality from the observed associations. The potential causal role of WWI in the development of CVD in patients with MetS required further verification through future longitudinal and interventional studies. Additionally, the reliance on a single baseline measurement of WWI restricted our insight into the impact of changes in this index over time on the risk of CVD and mortality in MetS patients. It was important to consider the dynamic nature of obesity metrics and their potential influence on health outcomes. Finally, while the study controlled for several known confounding factors, we recognized that there may be residual confounders that were not addressed, including unmeasured environmental factors. These limitations should be taken into account when interpreting the study’s results and when designing future research in this area.
Conclusion
Our research indicated that the WWI served as a significant indicator of the risk for CVD and mortality among MetS patients in the United States. Consequently, we recommended the regular assessment of WWI in MetS individuals as a potentially advantageous method for evaluating their risk of CVD and the likelihood of mortality. This approach could be integral to enhancing preventive and treatment strategies tailored to the needs of this patient population.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm. We obtained data from 11 cycles of NHANES spanning from 1999 to 2020, collecting all data from demographic information, physical examination, laboratory tests, and survey questionnaires.
References
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365(9468), 1415–1428 (2005).
Saklayen, M. G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 20(2), 12 (2018).
Samson, S. L. & Garber, A. J. Metabolic syndrome. Endocrinol. Metab. Clin. North Am. 43(1), 1–23 (2014).
Beltran-Sanchez, H., Harhay, M. O., Harhay, M. M. & McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 62(8), 697–703 (2013).
Shin, D., Kongpakpaisarn, K. & Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 259, 216–219 (2018).
Li, W., Qiu, X., Ma, H. & Geng, Q. Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front. Endocrinol. (Lausanne) 13, 1029736 (2022).
Chen, T., Wang, J., Liu, Z. & Gao, F. Effect of supplementation with probiotics or synbiotics on cardiovascular risk factors in patients with metabolic syndrome: A systematic review and meta-analysis of randomized clinical trials. Front. Endocrinol. (Lausanne) 14, 1282699 (2023).
Li, W. et al. Association between metabolic syndrome and mortality: Prospective cohort study. JMIR Public Health Surveill. 9, e44073 (2023).
Xiong, L. et al. 17-year follow-up of association between telomere length and all-cause mortality, cardiovascular mortality in individuals with metabolic syndrome: Results from the NHANES database prospective cohort study. Diabetol. Metab. Syndr. 15(1), 247 (2023).
Fahed, G. et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 23(2), 786 (2022).
Collaboration NCDRF: Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387(10026), 1377–1396 (2016).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the american heart association. Circulation 143(21), e984–e1010 (2021).
Dwivedi, A. K., Dubey, P., Cistola, D. P. & Reddy, S. Y. Association between obesity and cardiovascular outcomes: Updated evidence from meta-analysis studies. Curr. Cardiol. Rep. 22(4), 25 (2020).
Tutor, A. W., Lavie, C. J., Kachur, S., Milani, R. V. & Ventura, H. O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 78, 2–10 (2023).
Lavie, C. J., De Schutter, A., Patel, D., Artham, S. M. & Milani, R. V. Body composition and coronary heart disease mortality–an obesity or a lean paradox?. Mayo Clin. Proc. 86(9), 857–864 (2011).
Ford, E. S., Maynard, L. M. & Li, C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 312(11), 1151–1153 (2014).
Liu, B. et al. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011–18: Population based study. BMJ 372, n365 (2021).
Nomura, S. O., Karger, A. B., Weir, N. L., Duprez, D. A. & Tsai, M. Y. Free fatty acids, cardiovascular disease, and mortality in the multi-ethnic study of atherosclerosis. J. Clin. Lipidol. 14(4), 531–541 (2020).
Skidmore, P. M. et al. Plasma free fatty acid patterns and their relationship with CVD risk in a male middle-aged population. Eur. J. Clin. Nutr. 64(3), 239–244 (2010).
Shin, H. I. & Jung, S. H. Body fat distribution and associated risk of cardiovascular disease in adults with cerebral palsy. Front. Neurol. 12, 733294 (2021).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 8(1), 16753 (2018).
Kim, N. H., Park, Y., Kim, N. H. & Kim, S. G. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing 50(3), 780–786 (2021).
Wang, X., Yang, S., He, G. & Xie, L. The association between weight-adjusted-waist index and total bone mineral density in adolescents: NHANES 2011–2018. Front. Endocrinol. (Lausanne) 14, 1191501 (2023).
National Cholesterol Education Program Expert Panel on Detection E, Treatment of high blood cholesterol in A: Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation 106(25), 3143–3421 (2002).
Wei, X., Min, Y., Song, G., Ye, X. & Liu, L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc. Diabetol. 23(1), 134 (2024).
Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 22(1), 279 (2023).
Liu, C., Liang, D., Xiao, K. & Xie, L. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc. Diabetol. 23(1), 171 (2024).
Fang, H., Xie, F., Li, K., Li, M. & Wu, Y. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: A cross-sectional study. BMC Cardiovasc. Disord. 23(1), 435 (2023).
Han, Y. et al. The weight-adjusted-waist index predicts all-cause and cardiovascular mortality in general US adults. Clinics (Sao Paulo) 78, 100248 (2023).
Zhang, D. et al. Association between weight-adjusted-waist index and heart failure: Results from national health and nutrition examination survey 1999–2018. Front. Cardiovasc. Med. 9, 1069146 (2022).
Ye, J. et al. Association between the weight-adjusted waist index and stroke: A cross-sectional study. BMC Public Health 23(1), 1689 (2023).
Cai, S. et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens. Res. 46(1), 253–260 (2023).
Zheng, D. et al. Association between the weight-adjusted waist index and the odds of type 2 diabetes mellitus in United States adults: A cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1325454 (2023).
Yu, S. et al. Weight-adjusted-waist index predicts newly diagnosed diabetes in Chinese rural adults. J. Clin. Med. 12(4), 1620 (2023).
Wang, Z. et al. The relationship between weight-adjusted-waist index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne) 15, 1345411 (2024).
Ortega, F. B., Lavie, C. J. & Blair, S. N. Obesity and cardiovascular disease. Circ. Res. 118(11), 1752–1770 (2016).
Vinneau, J. M., Huibregtse, B. M., Laidley, T. M., Goode, J. A. & Boardman, J. D. Mortality and obesity among U.S. Older adults: The role of polygenic risk. J. Gerontol. B Psychol. Sci. Soc. Sci. 76(2), 343–347 (2021).
Neeland, I. J. et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 7(9), 715–725 (2019).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle 14(5), 2196–2203 (2023).
Xie, F., Xiao, Y., Li, X. & Wu, Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: Results from the national health and nutrition examination survey 2013–2014. Front. Cardiovasc. Med. 9, 948194 (2022).
Kim, J. Y. et al. Associations between weight-adjusted waist index and abdominal fat and muscle mass: Multi-ethnic study of atherosclerosis. Diabetes Metab. J. 46(5), 747–755 (2022).
Huby, A. C. et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132(22), 2134–2145 (2015).
Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T. & Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104(22), 2673–2678 (2001).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114(12), 1752–1761 (2004).
Singhal, A. Endothelial dysfunction: Role in obesity-related disorders and the early origins of CVD. Proc. Nutr. Soc. 64(1), 15–22 (2005).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766 (2021).
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
The National Natural Science Foundation of China [82370346] provided funding for this work.
Author information
Authors and Affiliations
Contributions
Z.T. conceived and designed the study and wrote the manuscript. P.Z. analyzed the data. G.M. took the quality control of data and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tao, Z., Zuo, P. & Ma, G. Association of weight-adjusted waist index with cardiovascular disease and mortality among metabolic syndrome population. Sci Rep 14, 18684 (2024). https://doi.org/10.1038/s41598-024-69486-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69486-1
- Springer Nature Limited
Keywords
This article is cited by
-
Weight-Adjusted Waist Index: an anthropometric measure for frailty in older adults
European Geriatric Medicine (2024)