Abstract
Adipocyte-cancer cell interactions promote tumor development and progression. Previously, we identified adipsin (CFD) and its downstream effector, hepatocyte growth factor (HGF), as adipokines that enhance adipocyte-breast cancer stem cell interactions. Here, we show that adipsin-dependent adipocyte maturation and the subsequent upregulation of HGF promote tumor invasion in breast cancers. Mature adipocytes, but not their precursors, significantly induced breast tumor cell migration and invasion in an adipsin expression-dependent manner. Promoters of tumor invasion, galectin 7 and matrix metalloproteinases, were significantly upregulated in cancer cells cocultured with mature adipocytes; meanwhile, their expression levels in cancer cells cocultured with adipocytes were reduced by adipsin knockout (Cfd KO) or a competitive inhibitor of CFD. Tumor growth and distant metastasis of mammary cancer cells were significantly suppressed when syngeneic mammary cancer cells were transplanted into Cfd KO mice. Histological analyses revealed reductions in capsular formation and tumor invasion at the cancer-adipocyte interface in the mammary tumors formed in Cfd KO mice. These findings indicate that adipsin-dependent adipocyte maturation may play an important role in adipocyte-cancer cell interaction and breast cancer progression.
Similar content being viewed by others
Introduction
Breast cancer is the most frequently diagnosed cancer and one of the major causes of cancer death globally, with around 685,000 patients dying from this disease each year1. Breast cancer development, progression, and therapeutic resistance are at least partly attributed to the presence of cancer stem cells (CSCs) in breast tumor tissues2,3,4. Indeed, CD44+/CD24−/low human breast CSCs in human breast tumors have an extremely higher ability to drive tumor formation in a mouse xenograft model compared to the remaining non-tumorigenic cancer cells within the same breast tumor5. We previously analyzed the expression profile of 466 microRNAs (miRNAs) in human breast CSCs isolated from surgical specimens of breast cancer patients and found that 37 miRNAs are differentially expressed between breast CSCs and non-tumorigenic cancer cells6. Among the downregulated miRNAs in the human breast CSCs, both miR-200c and miR-183 suppress the protein expression of a stem cell self-renewal gene BMI1 and/or an epithelial–mesenchymal transition (EMT) regulator, ZEB16,7. The findings are not limited to the human breast CSCs, and similar molecular mechanisms also regulate normal mammary stem cells, colorectal stem cells, and colorectal CSCs8. miR-142 and miR-150 are upregulated in human breast CSCs and participate in WNT signaling pathway regulation9. Using a breast cancer patient-derived tumor xenograft (PDX) mouse model, we then compared gene and miRNA expression profiles between CSCs at the primary site and those at distant metastasized sites, and found that downregulation of miR-93, which targets WASF3, and upregulation of S100A10 promote CSC properties of cancer cells specifically at distant metastasized sites10,11.
CSCs maintain their specific properties by residing in and interacting with niches, which are anatomically distinct regions within the tumor microenvironment12. In the mammary tissue, mammary adipose tissues form an indispensable niche for normal mammary epithelial stem cells and breast CSCs13. Using human mammary adipose tissue-derived stem cells (ADSCs) as a model, we identified adipsin (complement factor D, CFD), also known as a component of the complement alternative pathway, as a niche factor that mediates adipocyte-CSC interactions in breast cancer13,14. Together with its downstream effector hepatocyte growth factor (HGF), adipsin enhances the proliferation and CSC properties of breast cancer cells14,15. Adipsin is predominantly secreted from adipocytes and is induced during adipogenesis by PPARγ, the master regulator of adipocyte biology16,17,18. In the complement system, adipsin functions as a serine protease that activates the alternative complement pathway, in which it cleaves complement factor B into Ba and Bb, and then a C3 convertase—composed of Bb and complement component 3b (C3b)—cleaves C3 into C3a and C3b. The molecules in the adipsin/C3a pathway also play roles in various other processes, such as immune regulation, cell migration, insulin tolerance, adipocyte differentiation, and homing of hematopoietic stem cells13.
It is becoming increasingly evident that obesity characterized by overaccumulation of mature adipocytes promotes multiple cancers, including post-menopausal breast, colorectal, endometrial, kidney, esophageal, pancreatic, liver, and gallbladder cancers19,20. In addition to the adipokine-mediated enhancement of CSC properties, multiple other mechanisms are involved in tumor promotion by adipocytes13. However, the roles of adipocyte maturation in tumor progression remain to be elucidated. The present study shows that adipsin-dependent adipocyte maturation promotes invasion and metastatic progression of breast cancer cells.
Results
Adipocyte maturation promotes migration and invasion of mammary cancer cells
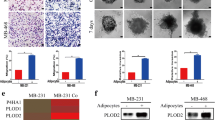
Adipocytes are a major component of the tumor microenvironment in breast cancers13,21,22. To investigate the roles of mammary adipocytes—and in particular the adipokine adipsin—in tumor invasion and migration, transwell migration/invasion assays were performed using murine and human breast cancer cells and adipocytes (Fig. 1A, left). When murine mammary adipocyte precursors (i.e., ADSCs) were cultured in lower wells, both wild-type (WT) and adipsin knockout (Cfd KO) ADSCs induced comparable levels of invasion of murine mammary tumor EO771 cells (Fig. 1B, Supplemental Fig. 1A). Essentially similar results were obtained for human breast tumor MDA-MB-231 cells: human mammary ADSCs in lower wells induced comparable levels of cancer cell invasion, irrespective of the presence of danicopan, a selective small-molecule competitive inhibitor of adipsin, in the culture medium23 (Fig. 1D, Supplemental Fig. 1B).
Mature adipocytes promote tumor invasion in a CFD dependent manner. (A) Schematic illustration of the transwell migration/invasion assays in which adipocytes and cancer cells were cultured in the lower well and upper chamber, respectively. Mature adipocytes were prepared by inducing the differentiation of confluent ADSCs for 8 days (Middle and right). Adipocyte precursors (ADSCs) were seeded the day before the analysis (Left). For invasion assays, upper chambers were prepared by coating the upper surface of the filter membrane with DMEM with 10% Matrigel, while the filter membrane remained uncoated for migration assays. Upper chambers seeded with breast cancer cells were put in the wells and migration/invasion abilities of breast cancer cells were evaluated. (B, C) Invasion ability of murine breast cancer EO771 cells cocultured with WT or Cfd KO murine ADSCs (B) or mature adipocytes (C). Left: representative images of the invaded cells. Black arrows: pore in the transwell membrane, white arrows: transmigrated cells. Scale bars, 50 μm. Data are presented as mean ± SD. n = 4, *p < 0.05. (D, E) Invasion ability of human breast cancer MDA-MB-231 cells cocultured with human ADSCs (D) or mature adipocytes (E) supplemented with or without 10 μM danicopan, a competitive inhibitor for CFD (CFD Inh). Left: representative images of the invaded cells. Black arrows: pore in the transwell membrane, white arrows: transmigrated cells. Scale bars, 50 μm. Data are presented as mean ± SD. n = 4, *p < 0.05.
Because in breast cancers, mammary tumor cells locally infiltrate surrounding adipose tissue enriched with mature adipocytes, we investigated the effects of adipocyte maturation on tumor invasion abilities of mammary cancer cells. Murine mammary ADSCs were cultured in the lower wells and induced to differentiate for 8 days before evaluating the invasion or migration abilities of mammary cancer cells (Fig. 1A, middle and right). Coculture with WT mature adipocytes significantly increased the number of invaded EO771 and MDA-MB-231cells, making it 15 and 8 times higher than induced by WT ADSCs, respectively (Supplemental Fig. 1A). The enhancement of tumor invasion by mature adipocytes was adipsin-dependent because: (i) coculture with Cfd KO mature adipocytes significantly reduced the number of invaded EO771 cells (Fig. 1C, Supplemental Fig. 1A); and (ii) addition of danicopan significantly reduced the invasion of human breast cancer MDA-MB-231 cells cocultured with WT mature adipocytes (Fig. 1E, Supplemental Fig. 1B). The suppressive effects of danicopan on the invasion ability of tumor cells were mostly indirect because the addition of danicopan to the cell culture had little impact on the invasion of MDA-MB-231 cells (Supplemental Fig. 1C). Similarly, migration of mammary cancer cells was promoted by WT mature adipocytes, but much weaker by Cfd KO mature adipocytes in transwell migration assays (Supplemental Fig. 2). These results suggest that adipsin and maturation of adipocytes promote mammary cancer cell invasion and migration.
Adipsin-dependent secretion of HGF from mature adipocytes promotes cancer cell invasion
Adipokines secreted from adipocytes mediate the adipocyte-cancer cell interactions13. Because the maturation of adipocytes significantly promoted cancer cell migration and invasion in an adipsin-dependent manner (Fig. 1), adipokine expression profiles of WT and Cfd KO mature adipocytes were analyzed. Comparison of the adipokine expression profiles of WT and Cfd KO mature adipocytes revealed that HGF and MCP-1 were among the adipokines whose expression levels were reduced by Cfd KO in mature adipocytes (Fig. 2AB, Supplemental Fig. 3AB). Therefore, we investigated whether the adipokines HGF and MCP-1 can rescue the reduced ability of Cfd KO mature adipocytes to promote cancer cell invasion. The addition of HGF, but not MCP-1, in the culture medium significantly increased the number of invaded EO771 cells, which barely express Hgf, in the coculture with Cfd KO mature adipocytes (Fig. 2BC, Supplemental Fig. 3C). Moreover, the inhibition of HGF using a specific blocking antibody significantly reduced the number of invaded MDA-MB-231 cells cocultured with mature adipocytes (Fig. 2D), while adding HGF to the culture medium significantly rescued the reduction in the number of invaded MDA-MB-231 cells incubated with danicopan (Fig. 2E). These observations suggest that HGF is an important mediator of adipocyte-cancer cell interaction and promotes breast cancer cell invasion.
HGF rescued the reduction of tumor invasion caused by Cfd deficiency in adipocytes. (A) Secretion levels of HGF in the culture medium of WT and Cfd KO mature adipocytes. Secretion profile of 111 murine cytokines was measured utilizing the Proteome Profiler Mouse XL Cytokine Array Kit (Supplementary Fig. 3A). Density of the spots corresponding to the indicated cytokine were quantified using the Image J software. Data are presented as mean ± SD. n = 2 (B). Relative expression level of Hgf mRNA in WT and Cfd KO mature adipocytes and EO771 cells. Data are presented as mean ± SD. n = 4 for adipocytes, n = 3 for EO771, *p < 0.05, **p < 0.01. (C) HGF rescued the reduction of tumor invasion caused by Cfd KO in mature adipocytes. Murine breast cancer EO771 cells were cocultured with WT or Cfd KO murine mature adipocytes supplemented with or without HGF (50 ng/mL) in the culture medium. Left: representative images of the invaded cells. Black arrows: pore in the transwell membrane, white arrows: transmigrated cells. Scale bars, 50 μm. Data are presented as mean ± SD. n = 4, *p < 0.05. (D) Reduction of tumor invasion by the inhibition of HGF. MDA-MB-231 cells were incubated with or without anti-HGF neutralizing antibody (100 ng/ml, AMG 102, rilotumumab) in the culture medium. Left: representative images of the invaded cells. Black arrows: pore in the transwell membrane, white arrows: transmigrated cells. Scale bars, 50 μm. Data are presented as mean ± SD. n = 4, *p < 0.05. (E) HGF rescued the reduction of tumor invasion by the inhibition of CFD from mature adipocytes. MDA-MB-231 cells were cocultured with human mature adipocytes supplemented with or without HGF (50 ng/mL) and danicopan (10 μM) in the culture medium. Left: representative images of the invaded cells. Black arrows: pore in the transwell membrane, white arrows: transmigrated cells. Scale bars, 50 μm. Data are presented as mean ± SD. n = 4, *p < 0.05.
Suppression of tumor growth, invasion, and metastasis in Cfd KO mice
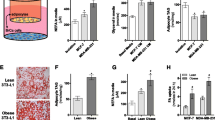
Because cancer cell migration and invasion were regulated by mature adipocytes in an adipsin expression-dependent manner, their ability to promote tumor progression was evaluated in vivo by inoculating syngeneic mammary cancer EO771 cells into the mammary fat pad region of WT or Cfd KO C57BL/6 mice. EO771 cells barely expressed adipsin (Fig. 3A) and adipsin was undetectable in the serum of EO771 tumor bearing Cfd KO mice (Fig. 3B). Consistent with our previous findings that growth of the breast cancer PDX cells coinjected with ADSCs is suppressed by CFD knockdown in the ADSCs14, growth of EO771 tumors was significantly slower in Cfd KO mice than in WT mice (Fig. 3C).
Suppression of tumor growth in Cfd KO mice. (A) Relative expression level of Cfd mRNA in murine mature adipocytes and EO771cells. Expression levels were normalized to Actb mRNA expression levels. Data are presented as mean ± SD. n = 4, **p < 0.01. (B) Concentration of adipsin (CFD) in the serum of WT or Cfd KO mouse bearing EO771 tumor. Data are presented as mean ± SD. n = 7 (WT) and 6 (KO), **p < 0.01. (C) The capacity of breast cancer EO771 cells (1 × 105 cells/site) to form tumors upon in vivo transplantation into the mammary fat pad region of C57BL/6 mice was suppressed by Cfd KO in the mouse. Tumor growth was monitored by caliper measurements twice a week. Data are presented as mean ± SD. n ≥ 3, *p < 0.05.
The adipsin/C3a/C3a receptor 1 (C3aR1) pathway activates cancer cells and promotes immune reactions24. Histological analysis revealed that at the tumor invasion front facing the mammary adipose tissue (tumor-adipose tissue interface), a significantly thicker fibrotic capsule25,26 with inflammatory cells and tumor cell invasion was formed in the WT mice compared to Cfd KO mice (Fig. 4A). Consistently, lung metastases were significantly less frequently observed in Cfd KO mice than in WT mice (Fig. 4B). These results suggest that the lack of adipsin in the tumor-bearing mice caused a reduction in tumor growth and metastasis.
Suppression of tumor progression in Cfd KO mice. (A) Histological analysis of the tumor invasion fronts. At the tumor-mammary adipose tissue interfaces, much thicker fibrotic capsules with inflammatory cells and tumor cell invasion were formed for the tumors grown in WT mice compared to those grown in Cfd KO mice. Upper panels, H&E (hematoxylin and eosin), silver and Masson’s trichrome staining images are presented. Dotted lines: border between tumor and mammary adipose tissue, white arrows: thick fibrotic capsules. Lower panels, thickness of the fibrotic capsule at the tumor invasion front facing the mammary adipose tissue (tumor-adipose tissue interface). n of the analyzed mice ≥ 3, **p < 0.01. (B) Number of mice bearing lung metastases. The presence of lung metastasis was histologically evaluated. White arrows: tumor metastases. n = 11 (WT) and 10 (KO), *p < 0.05.
Adipsin-dependent adipocyte maturation induces MMP9 and Lgals7 expression in cancer cells
Finally, we investigated the molecular mechanisms involved in the promotion of tumor invasion at the tumor-adipose tissue interface. Comparison of the gene expression profiles between the tumors formed in WT and Cfd KO mice revealed that the expression level of Lgals7 (gene encoding galectin 7), which induces tumor growth and spontaneous metastases of breast cancer cells27,28, was significantly reduced in the tumors formed in Cfd KO mice compared to those in WT mice (Fig. 5A). Additionally, the expression level of Lgals7 mRNA, which was hardly detectable in EO771 cell monoculture, was significantly lower in EO771 cancer cells cocultured in vitro with Cfd KO mature adipocytes compared to those cocultured with WT mature adipocytes (Fig. 5B).
Cfd KO in adipocytes reduces Lgals7 expression in tumor cells. (A) Reduction of Lgals7 expression in the tumors grown in Cfd KO mice. Left: volcano plot showing the standardized mean difference and the adjusted p-value for the differentially expressed genes (DEGs) in the tumors grown in WT mice versus those grown in Cfd KO mice. The red dot indicates the gene with the largest negative standardized mean difference. Right: Lgals7 mRNA expression levels of the tumors formed in the WT or Cfd KO mice. FDR, false discovery rate. Data are presented as mean ± SD. n = 4, *p < 0.05. (B) Reduction of Lgals7 mRNA expression level in the tumor cells cocultured with Cfd KO mature adipocytes and in EO771 cells. Left: Schematic illustration of the coculture method. EO771 cells were cocultured with mature adipocytes grown in the upper chamber for 2 days. Right: Lgals7 mRNA expression level of the EO771 cells cocultured with WT or Cfd KO mature adipocytes. Data are presented as mean ± SD. n = 3, *p < 0.05, **p < 0.01.
Using this coculture system (Fig. 5B), we then analyzed the activation of matrix metalloproteinases (MMPs), which mediate cancer invasion through the digestion of dense extracellular matrices (ECMs). The expression level of Mmp9 (gene encoding gelatinase B) mRNA, which was barely expressed in EO771 cell monoculture, was significantly lower when EO771 cells were cocultured with Cfd KO mature adipocytes than when cocultured with WT mature adipocytes (Fig. 6A). The expression level of Mmp2 (gene encoding gelatinase A) mRNA was also lower, but that difference was not significant (Fig. 6A). The results of gelatin zymography confirmed these findings by showing that the activities of MMPs in the EO771 cells cocultured with Cfd KO mature adipocytes were lower than those cocultured with WT mature adipocytes, and the difference in MMP9 activities was significant (Fig. 6B).These results indicate that adipocyte-cancer cell interactions induce the expression of molecules that promote tumor invasion—such as MMPs and LGALS7—in mammary cancer cells, and that adipsin plays a major role in this stimulation (Fig. 6C).
Cfd KO in adipocytes reduced MMP9 expression in tumor cells. (A) Mmp2 and Mmp9 mRNA expression levels in EO771 cells or those cocultured with WT or Cfd KO mature adipocytes. Data are presented as mean ± SD. n = 3, *p < 0.05, **p < 0.01. (B) Activity of MMPs derived from EO771 cells cocultured with WT or Cfd KO mature adipocytes. Left, gelatin zymography; Right, densities of the bands for MMP2 and MMP9 were measured. Data are presented as mean ± SD. n = 3, *p < 0.05. (C) Visual abstract of the core findings of the study: mature adipocyte-cancer cell interaction promotes tumor invasion and metastasis at least partly by upregulating Lgals7 and MMP expression in the cancer cells.
Discussion
Adipocytes are integral components of CSC niches, especially in breast cancers, and secrete adipsin that enhances CSC properties and cell growth13,14,15. Multiple mechanisms have been proposed by which cancer-associated adipocytes promote cell growth, angiogenesis, anti-apoptotic effects, invasion, migration, and metastasis13,21,22. Our present study found that after maturation, adipocytes are stronger inducers of breast cancer cell invasion and uncovered the importance of two adipokines, adipsin and HGF, in adipocyte-induced tumor invasion.
Mammary adipocytes can be divided into three categories: mature adipocytes, preadipocytes, and ADSCs. Enhancement of CSC properties is mediated by cocultured ADSCs in breast cancer PDX cells13,14,15. In contrast, although breast cancer cell invasion was significantly induced by cocultured mature adipocytes, it was only weakly induced by ADSCs in vitro. Because the expression level of adipsin is much higher in mature adipocytes than in ADSCs16,17,18, it is possible that a much higher exposure to adipsin and subsequent changes in adipokine secretion, including HGF, are required to induce tumor invasion compared to inducing CSC properties. Considering that various mechanisms, including secretion of adipokines, metabolic reprogramming, and exosome trafficking, are proposed by which adipocytes promote the malignant progression of cancer cells21,29, further studies are required to determine why the maturation of adipocytes is required to promote tumor invasion efficiently.
Obesity, characterized by an increase in adipose mass and an alteration of adipose tissue, is an independent risk factor for cancer progression. Previous studies have shown that adipocytes and adipose tissues can promote tumor growth and progression. For example, mature adipocytes promote cancer growth30, and pancreatic tumor growth is enhanced in obese mice in an osteopontin-dependent manner31. Adipocytes promote tumor invasion and metastasis by upregulating the generation of glycerol-3-phosphate in cancer cells as a precursor for membrane and signaling components32, and enhance the mesenchymal phenotype of cancer cells by adipocyte-derived CXCL3 and further activation of FAK pathway in cancer cells33. We showed here that mature adipocytes have a significantly higher ability to promote tumor invasion than ADSCs and propose that adipsin and HGF secreted from mature adipocytes in an autocrine regulatory manner promote tumor invasion15 (Fig. 6C). MCP-1 (CCL2) is associated with tumor development, including tumor invasion and metastasis, angiogenesis, and immune cell infiltration34,35. MCP-1 is overexpressed in breast cancer cells and promotes tumor invasion35; however, MCP-1 failed to rescue the reduced ability of Cfd KO mature adipocytes to promote invasion. This may have been caused by the relatively low expression levels of its receptor, C–C motif chemokine receptor 2 (CCR2) in breast cancer cells35, and/or the differential roles of HGF and MCP-1 in the adipsin/C3a/C3aR1 pathway.
MMP9 is a member of the matrix metalloproteinase family of enzymes that play a crucial role in the breakdown of ECM components. MMP9 plays an important role in the onset, progression, and metastasis of various cancers, including gastric, lung, colon, and breast cancers36. Activation of the enzymatic activity of MMP9 is mediated by proteases, such as serine proteinases or other matrix metalloproteinases, including MMP2 and plasmin, and by the suppression of tissue inhibitors of metalloproteinases (TIMPs). While adipsin is a serine protease, its only known substrate is factor B in a complex with C337. Therefore, activation of the enzymatic activity of MMP9 is less likely to be mediated by adipsin itself. On the other hand, our finding suggests that adipsin upregulates the transcription of MMP9 mRNA. Previous results have shown that tumor necrosis factor-alpha (TNF-α)38, interleukin-1 (IL-1)39, and direct cell–cell contacts between cancer cells and stromal cells, influence MMP9 expression and activation. Molecular mechanisms by which adipsin upregulates MMP9 activities remain to be clarified because our coculture system was not suitable for the evaluation of the effects of direct cell–cell contacts, and the results of the cytokine array did not support the upregulation of those cytokines. Therefore, further studies are required to delineate the molecular mechanisms by which adipsin activates MMPs.
LGALS7 (galectin-7) is a member of the galectin family implicated in epithelial stratification and cell migration. It is a β-galactoside-binding protein involved in various cellular functions, including cell adhesion, apoptosis, immune response, and cancer progression. LGALS7 may contribute either to neoplastic transformation or tumor progression through the impacting of cell growth, cell proliferation, angiogenesis, apoptosis, and/or cell migration40. Cellular stress, such as exposure to certain cytotoxic agents or environmental stressors, can induce the expression of LGALS7. Indeed, activation of the p53 or TNFα pathways induces the binding of p53 (irrespective of its WT or mutants) and nuclear factor-kappa B (NF-κB) to the Lgals7 promoter and enhances LGALS7 expression41. We speculate that the significant upregulation of LGALS7 in the tumors grown in WT mice compared to those in Cfd KO mice (Fig. 5) may be caused by differences in adipokines secreted from the adipocytes (Fig. 2A, Supplemental Fig. 3AB) and/or differences in immune responses within the tumor microenvironment (Fig. 4A).
Xenotransplantation of human tumor tissue into highly immunodeficient mice led to the establishment of PDX models which reflect the heterogenous nature of human cancer tissues containing CSCs. We have previously shown that the growth of the xenograft tumor formed by the co-injection of PDX cells and human ADSCs is significantly reduced by CFD knockdown in ADSCs. However, in that model, it is difficult to eradicate the tumor-promoting and immune-modulating effects of murine adipsin supplied to the systemic blood circulation. In the present study, we employed a syngeneic tumor model which enables the analysis of immune function and its interaction with tumor cells, both in WT and Cfd KO settings. Indeed, using syngeneic mammary tumor cells, we found that fibrogenic and immune reactions, and tumor cell invasion were significantly reduced at the tumor-adipose tissue interface in the Cfd KO mice. However, further analyses are required to delineate the systemic effect of Cfd KO on tumor development and progression in tumor-bearing mice.
In conclusion, our study proposes that adipsin expressed by adipocytes is an important mediator of adipocyte-cancer cell interaction and promotes multiple aspects of tumor initiation and progression. Therefore, adipsin will be an attractive target to attach the obesity-related cancers, including breast cancers.
Materials and methods
Ethics statements
Human adipose tissues were obtained from surgical specimens of breast cancer patients admitted to the Division of Breast and Endocrine Surgery of Kobe University Hospital. The research was pre-approved by Kobe University’s Institutional Review Board (permission number: 1299 and 1481) and Fujita Health University’s Institutional Review Board (permission number: HM22-323), and was conducted in accordance with recognized ethical guidelines. All patients included in the study provided written informed consent. Animal experiments were performed with the approval of Fujita Health University’s Animal Care and Use Committee (permission numbers: APU23019, APU22102) and carried out according to the Animal Experiment Regulations of Fujita Health University.
Establishment and differentiation of ADSCs
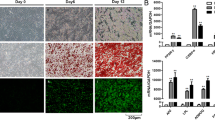
Murine ADSCs and human ADSCs were established from the mammary fat pads of WT and Cfd KO mice, and from a surgical specimen of a breast cancer patient, respectively14. Briefly, adipose tissue was minced into small pieces with a razor blade, put in medium 199 (Thermo Fisher Scientific) containing 1 mg/mL collagenase I (Worthington Biochemical) and 10 unit/mL DNase I (Sigma), and incubated in a shaking water bath at 37 °C for 1 h. After filtration, the cells were collected by centrifugation and the precipitates were washed twice using Dulbecco’s modified Eagle’s medium/nutrient mixture F12 (DMEM/F12) medium (Gibco, Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco, Thermo Fisher Scientific). The cells were resuspended and cultured in DMEM F12 10% FBS at 37 °C with 5% CO2. ADSCs grown to confluency were induced to terminally differentiate into mature adipocytes using Osteogenic/Adipogenic Base Media with Adipogenic Supplement (100×) (CCM007 and CCM011, StemXVivo, R&D systems) for 8 days according to the manufacturer’s protocol.
Transwell migration and invasion assay
Transwell migration and invasion assays were undertaken using 24-well transwell inserts with 8 µm pore size (Corning). For invasion assays, the upper surface of the filter membrane was coated with 60 μL of DMEM with 10% Matrigel (Corning), while the filter membrane remained uncoated for migration assays. Twenty thousand cells in DMEM were added to the upper chamber, and DMEM containing 10% murine or human serum (for EO771 or MDA-MB-231 cells, respectively) was added to the lower chamber, where adipocytes were cultured. Danicopan, a competitive inhibitor of CFD catalytic activity (10 μM, S0803, Selleck), HGF (50 ng/mL, 100-39H PeproTech), HGF neutralizing antibody (100 ng/ml, AMG 102, rilotumumab), or MCP1 (10 nM, Tonbo Biosciences) was added to the medium in the lower chamber to evaluate their effects on cancer cell invasion and migration.
After incubation at 37 °C for 24 h, cells on the upper side of the filter membrane were removed using a cotton swab. The cells that had invaded and were attached to the bottom side of the filter membrane were fixed in 10% formaldehyde and stained with 1% crystal violet. The number of cells was counted under a microscope at 4× magnification.
Syngeneic tumor transplantation
EO771 cells (1 × 105 cells/site) were injected into the 4th mammary fat pad region of 9–12-week-old female WT or Cfd KO C57BL/6 mice. Tumor growth was monitored by caliper measurements twice a week by using the formula: ab2/2 [a, length; b, width]. After 21 days, tumors were harvested, and tissue samples were soaked into RNA later (Thermo Fisher Scientific) and 75% of formaldehyde. Histological analyses were performed using formaldehyde-fixed and paraffin-embedded tissues. Molecular analyses of the primary tumor and metastatic organs were performed by quantitative real-time PCR and next generation RNA sequencing. Blood serum adipsin levels of the tumor-bearing mice were evaluated by PicoKine ELISA (Boster) according to the manufacturer’s protocol.
Next generation RNA sequencing
RNA was extracted from fresh frozen tumor tissue samples using the Pure Link RNA mini Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Next generation RNA sequencing was performed by Rhelixa, Japan.
Gelatin zymography
ADSCs were seeded on an Atelocollagen, permeable membrane for 6-well culture plate (KOKEN) and differentiated using Osteogenic/Adipogenic Base Media with Adipogenic Supplement (100×) (CCM007 and CCM011, StemXVivo, R&D systems). The permeable membranes were set into the wells of a 6-well plate and cocultured with cancer cells. After 24 h, the permeable membrane culturing adipocytes was removed, and the cancer cells were starved for 48 h. Culture supernatant was collected and centrifuged at 1500×g for 5 min to remove debris, followed by concentration using an Amicon Ultra-4 centrifugal filter unit (10 kDa MWCO (molecular weight cutoff membrane), UFC801024, MilliporeSigma). The total amount of protein was measured using the Bradford Ultra assay (Expedeon) according to the manufacturer’s protocol, and equal amounts of protein were loaded into an SDS-PAGE gel containing gelatin. After electrophoresis, the gel was washed for 30 min twice in washing buffer (2.5% Triton X‐100, 5 mmol/L CaCl2, 50 mmol/L Tris‐HCl [pH 7.5]) and incubated in incubation buffer (1% Triton X‐100, 5 mmol/L CaCl2, 50 mmol/L Tris‐HCl [pH 7.5]) overnight at 37 °C. The gel was stained with Coomassie brilliant blue solution to visualize the MMP activity.
Semi-quantitative real-time PCR
First-strand cDNA extracted from samples was synthesized from total RNA (1 μg) using a MultiScribe Reverse Transcriptase Kit (Thermo Fisher Scientific). The primer sequences were: mouse Actb, forward GTTGGAGCAAACATCCCCCA and reverse ACGCGACCATCCTCCTCTTA; mouse Mmp2, forward ACAAGTGGTCCGCGTAAAGT and reverse AAACAAGGCTTCATGGGGGC; mouse Mmp9, forward CGGTCCTCACCATGAGTCC and reverse ACAAGTATGCCTCTGCCAGC; mouse Lgals7, forward CAACACACTCTTGGTCCCCT and reverse GTTAAAGTGCAAGGCGGCAT; mouse Cfd; forward GGCTGTATGTGCAGCACAG and reverse GAGGCATTCTGGGATAGCTTA; mouse Hgf; forward GGCTGTATGTGCAGCACAG and reverse GAGGCATTCTGGGATAGCTTA; human LGALS7 forward GCTGAGAATTCGCGGCTTG and reverse GCTCCTTGCTGTTGAAGACC. TaqMan probe is used for human ACTB (Hs01060665_g1, ThermoFisher).
Cytokine array
The mammary ADSCs were seeded at a density of 3 × 104 cells/cm2 in DMEM/F12 containing 10% FBS and penicillin/streptomycin and cultured to 100% confluency. The cells were maintained for 48 h, and then induced to differentiate using a mouse-specific MesenCult Adipogenic Differentiation Kit (Catalog #05507, STEMCELL Technologies) according to the manufacturer’s protocol. The medium was refreshed every 2 to 3 days, and on day 10 when the cells were terminally differentiated, the medium was replaced with DMEM/F12 containing 1% FBS and cultured for 3 days. Supernatant of the culture medium centrifuged at 300×g for 10 min at 4 °C was applied to the membrane for the assessment of the secretion profile of 111 murine cytokines, utilizing the Proteome Profiler Mouse XL Cytokine Array Kit (Catalog # ARY028, R&D Systems), according to the manufacturer’s instruction. Images of the membrane were taken using the Fusion Solo S (Vilber, Collégien) and signal intensities were quantified using the Image J software42.
Statistical analysis
Data are presented as means ± standard deviation (SD). Comparisons between continuous data normally distributed with equal variance or unequal variances between groups were performed using unpaired two-tailed Student’s t-tests. Differences in the frequency of animals developing lung metastases following the injection of equal numbers of cancer cells were tested for statistical significance using the Chi-squared test. Sample sizes and P-values are indicated in the figures or figure legends. P values < 0.05 were deemed statistically significant.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was pre-approved by Kobe University’s Institutional Review Board (permission number: 1299 and 1481) and Fujita Health University’s Institutional Review Board (permission number: HM22-323).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arnold, M. et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66, 15–23. https://doi.org/10.1016/j.breast.2022.08.010 (2022).
Lobo, N. A., Shimono, Y., Qian, D. & Clarke, M. F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699. https://doi.org/10.1146/annurev.cellbio.22.010305.104154 (2007).
Shimono, Y., Mukohyama, J., Nakamura, S. & Minami, H. MicroRNA regulation of human breast cancer stem cells. J. Clin. Med. https://doi.org/10.3390/jcm5010002 (2015).
Zheng, Q., Zhang, M., Zhou, F., Zhang, L. & Meng, X. The breast cancer stem cells traits and drug resistance. Front. Pharmacol. 11, 599965. https://doi.org/10.3389/fphar.2020.599965 (2020).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 100, 3983–3988. https://doi.org/10.1073/pnas.0530291100 (2003).
Shimono, Y. et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603. https://doi.org/10.1016/j.cell.2009.07.011 (2009).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495. https://doi.org/10.1038/ncb1998 (2009).
Hisamori, S. et al. Upregulation of BMI1-suppressor miRNAs (miR-200c, miR-203) during terminal differentiation of colon epithelial cells. J. Gastroenterol. 57, 407–422. https://doi.org/10.1007/s00535-022-01865-9 (2022).
Isobe, T. et al. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife https://doi.org/10.7554/eLife.01977 (2014).
Shibuya, N., Kakeji, Y. & Shimono, Y. MicroRNA-93 targets WASF3 and functions as a metastasis suppressor in breast cancer. Cancer Sci. 111, 2093–2103. https://doi.org/10.1111/cas.14423 (2020).
Yanagi, H. et al. Upregulation of S100A10 in metastasized breast cancer stem cells. Cancer Sci. 111, 4359–4370. https://doi.org/10.1111/cas.14659 (2020).
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells?. Cell Stem Cell 16, 225–238. https://doi.org/10.1016/j.stem.2015.02.015 (2015).
Khaledian, B., Thibes, L. & Shimono, Y. Adipocyte regulation of cancer stem cells. Cancer Sci. 114, 4134–4144. https://doi.org/10.1111/cas.15940 (2023).
Goto, H. et al. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 38, 767–779. https://doi.org/10.1038/s41388-018-0477-8 (2019).
Mizuno, M. et al. Adipsin-dependent secretion of hepatocyte growth factor regulates the adipocyte-cancer stem cell interaction. Cancers (Basel) https://doi.org/10.3390/cancers13164238 (2021).
Cook, K. S. et al. Adipsin: A circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 237, 402–405. https://doi.org/10.1126/science.3299705 (1987).
Rosen, E. D. et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617. https://doi.org/10.1016/s1097-2765(00)80211-7 (1999).
Aaron, N. et al. Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells. Elife https://doi.org/10.7554/eLife.69209 (2021).
Berger, N. A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 1311, 57–76. https://doi.org/10.1111/nyas.12416 (2014).
Pati, S., Irfan, W., Jameel, A., Ahmed, S. & Shahid, R. K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel) https://doi.org/10.3390/cancers15020485 (2023).
Wu, Q. et al. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 12, 95. https://doi.org/10.1186/s13045-019-0778-6 (2019).
Bernard, J. J. & Wellberg, E. A. The tumor promotional role of adipocytes in the breast cancer microenvironment and macroenvironment. Am. J. Pathol. 191, 1342–1352. https://doi.org/10.1016/j.ajpath.2021.02.006 (2021).
Boyer, D. D. et al. Danicopan, an oral complement factor D inhibitor, exhibits high and sustained exposure in ocular tissues in preclinical studies. Transl. Vis. Sci. Technol. 11, 37. https://doi.org/10.1167/tvst.11.10.37 (2022).
Ajona, D., Ortiz-Espinosa, S. & Pio, R. Complement anaphylatoxins C3a and C5a: Emerging roles in cancer progression and treatment. Semin Cell Dev. Biol. 85, 153–163. https://doi.org/10.1016/j.semcdb.2017.11.023 (2019).
Jackson, T. L. & Byrne, H. M. A mechanical model of tumor encapsulation and transcapsular spread. Math. Biosci. 180, 307–328. https://doi.org/10.1016/s0025-5564(02)00118-9 (2002).
Boulter, L., Bullock, E., Mabruk, Z. & Brunton, V. G. The fibrotic and immune microenvironments as targetable drivers of metastasis. Br. J. Cancer 124, 27–36. https://doi.org/10.1038/s41416-020-01172-1 (2021).
Demers, M. et al. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am. J. Pathol. 176, 3023–3031. https://doi.org/10.2353/ajpath.2010.090876 (2010).
Campion, C. G., Labrie, M., Lavoie, G. & St-Pierre, Y. Expression of galectin-7 is induced in breast cancer cells by mutant p53. PLoS One 8, e72468. https://doi.org/10.1371/journal.pone.0072468 (2013).
Duong, M. N. et al. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 8, 57622–57641. https://doi.org/10.18632/oncotarget.18038 (2017).
Manabe, Y., Toda, S., Miyazaki, K. & Sugihara, H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 201, 221–228. https://doi.org/10.1002/path.1430 (2003).
Fukusada, S. et al. Osteopontin secreted from obese adipocytes enhances angiogenesis and promotes progression of pancreatic ductal adenocarcinoma in obesity. Cell Oncol. (Dordr.) 47, 229–244. https://doi.org/10.1007/s13402-023-00865-y (2024).
Mukherjee, A. et al. Adipocytes reprogram cancer cell metabolism by diverting glucose towards glycerol-3-phosphate thereby promoting metastasis. Nat. Metab. 5, 1563–1577. https://doi.org/10.1038/s42255-023-00879-8 (2023).
He, X. et al. CSF2 upregulates CXCL3 expression in adipocytes to promote metastasis of breast cancer via the FAK signaling pathway. J. Mol. Cell Biol. https://doi.org/10.1093/jmcb/mjad025 (2023).
Ling, Z. et al. Targeting CCL2-CCR4 axis suppress cell migration of head and neck squamous cell carcinoma. Cell Death Dis. 13, 158. https://doi.org/10.1038/s41419-022-04610-5 (2022).
Dutta, P., Sarkissyan, M., Paico, K., Wu, Y. & Vadgama, J. V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 170, 477–486. https://doi.org/10.1007/s10549-018-4760-8 (2018).
Zeng, Y., Gao, M., Lin, D., Du, G. & Cai, Y. Prognostic and immunological roles of MMP-9 in pan-cancer. Biomed. Res. Int. 2022, 2592962. https://doi.org/10.1155/2022/2592962 (2022).
Rosen, B. S. et al. Adipsin and complement factor D activity: An immune-related defect in obesity. Science 244, 1483–1487. https://doi.org/10.1126/science.2734615 (1989).
Lee, I. T., Lin, C. C., Wu, Y. C. & Yang, C. M. TNF-α induces matrix metalloproteinase-9 expression in A549 cells: Role of TNFR1/TRAF2/PKCα-dependent signaling pathways. J. Cell Physiol. 224, 454–464. https://doi.org/10.1002/jcp.22142 (2010).
Cheng, C. Y., Kuo, C. T., Lin, C. C., Hsieh, H. L. & Yang, C. M. IL-1β induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 160, 1595–1610. https://doi.org/10.1111/j.1476-5381.2010.00858.x (2010).
Kaur, M., Kaur, T., Kamboj, S. S. & Singh, J. Roles of galectin-7 in cancer. Asian Pac. J. Cancer Prev. 17, 455–461. https://doi.org/10.7314/apjcp.2016.17.2.455 (2016).
Advedissian, T., Deshayes, F. & Viguier, M. Galectin-7 in epithelial homeostasis and carcinomas. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18122760 (2017).
Anna, K. Semi-automated analysis of dot blots using ImageJ/Fiji. F1000Research 9, 1385. https://doi.org/10.12688/f1000research.27179.1 (2020).
Acknowledgements
The authors wish to thank Yusuke Akama (TechnoPro, Japan) and Lisa Thibes (Department of Biochemistry, Fujita Health University School of Medicine) for excellent technical assistances; and Dr. Johannes M. Dijkstra (Fujita Health University, Office of Research Administration) for the critical reading of the manuscript to increase the scientific clarity and readability. Mind the Graph platform (www.mindthegraph.com) was used to depict the graphic in the Fig. 6C under the CC BY-SA license (https://creativecommons.org/licenses/by-sa/4.0/deed.en).
Funding
This work was supported by: (1) grants-in-aid from the Japan Society for the Promotion of Science (JSPS KAKENHI) (18K07231, 21H02769 and Japan-Belgium Research Cooperative Program to Y.S.; 22K15532 to B.K.); (2) the Princess Takamatsu Cancer Research Fund (to Y.S.); (3) the Fujita Health University (to Y.S. and B.K.); 4) an extramural collaborative research grant of Cancer Research Institute, Kanazawa University (to Y.S.); 5) the Promotion and Mutual Aid Corporation for Private Schools of Japan (to Y.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.H., E.M., N.A. and Y.S.; Methodology, J.Y., T.H., E.M., T.W., E.S. and Y.S.; Formal Analysis, J.Y., T.H., E.M., B.K., F.S., K.U., E.S., N.A. and Y.S.; Investigation, J.Y., T.H., E.M., B.K., F.S., M.Mi., K.U., E.S., N.A. and Y.S.; Resources, J.Y., T.H., E.M., B.K., M.Mi., M.Ma., K.U., E.S., K.I., K.K., N.A. and Y.S.; Writing—Original Draft Preparation, J.Y., T.H. and Y.S.; Writing—Review and Editing, Y.S.; Visualization, J.Y., T.H., B.K. and Y.S.; Supervision, K.I., K.K., N.A. and Y.S.; Project Administration, Y.S.; Funding Acquisition, J.Y., B.K. and Y.S. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yoshida, J., Hayashi, T., Munetsuna, E. et al. Adipsin-dependent adipocyte maturation induces cancer cell invasion in breast cancer. Sci Rep 14, 18494 (2024). https://doi.org/10.1038/s41598-024-69476-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69476-3
- Springer Nature Limited