Abstract
Mosquitoes, one of the deadliest animals on the planet, cause millions of fatalities each year by transmitting several human illnesses. Synthetic pesticides were previously used to prevent the spread of diseases by mosquitoes, which was effective in protecting humans but caused serious human health problems, environmental damage, and developed mosquito pesticide resistance. This research focuses on exploring new, more effective, safer, and environmentally friendly compounds to improve mosquito vector management. Phytochemicals are possible biological agents for controlling pests and many are target-specific, rapidly biodegradable, and eco-friendly. The potential of extracts of Lantana camara, Melia azedarach, Nerium oleander, Ricinus communis, and Withania somnifera against 3rd instar Culex pipiens (Common house mosquito) larvae was evaluated. Methanol extracts had more toxic effects against Cx. pipiens larvae (95–100%, 24 h post-treatment) than aqueous extracts (63–91%, 24 h post-treatment). The methanol extracts of Nerium oleander (LC50 = 158.92 ppm) and Ricinus communis (LC50 = 175.04 ppm) were very effective at killing mosquito larvae, 24 h after treatment. N. oleander (LC50 = 373.29 ppm) showed high efficacy in aqueous plant extracts. Among the different extracts of the five plants screened, the methanol extract of R. communis recorded the highest ovicidal activity of 5% at 800 ppm concentration. Total developmental duration and growth index were highly affected by R. communis and M. azedarach methanol extracts. In field tests it was clear that plant extracts decreased mosquito larval density, especially when mixed with mosquito Bti briquette, with stability up to seven days for N. oleander. GC–MS results showed that the methanol extract had a higher number of chemical compounds, particularly with more terpene compounds. A high-performance liquid chromatography (HPLC) technique was used to detect the existence of non-volatile polyphenols and flavonoids. All five methanol extracts showed high concentrations of active ingredients such as gallic acid, chlorogenic acid (more than 100 μg/ml) and the rosmarinic acid was also found in all the five extracts in addition to 17 active polyphenols and flavonoids presented at moderate to low concentrations. Molecular modeling of 18 active ingredients detected by the HPLC were performed to the vicinity of one of the fatty acid binding proteins of lm-FABP (PDB code: 2FLJ). Rutin, Caffeic acid, coumaric acid and rosmarinic acid which presented densely in R. communis and N. oleander showed multiple and stable intermolecular hydrogen bonding and π–π stacking interactions. The inhibition ability of the fatty acid binding protein, FABP4, was evaluated with remarkable receptor inhibition evident, especially with R. communis and N. oleander having inhibitory concentrations of IC50 = 0.425 and 0.599 µg/mL, respectively. The active phytochemical compounds in the plants suggest promising larvicidal and ovicidal activity, and have potential as a safe and effective alternative to synthetic insecticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Mosquitoes spread many harmful diseases to humans and animals, including malaria, dengue fever, yellow fever, filariasis, Japanese encephalitis, chikungunya, and Streptococcus epidermidis in livestock1. Although no region of the world is free of vector-borne diseases, mosquito-borne diseases have a disproportionate impact including economic (loss of commercial production and employment), disease, death, and poverty (resulting from reduced productivity). It particularly affects poorer people (e.g. without healthcare, mosquito nets, drugs, or employment protection should they fall ill) and is also a particular problem in poor countries within tropical and subtropical climates2.
Synthetic insecticides were developed to kill mosquitoes and control vector-borne disease, and are have been very effective. However, mosquitoes have adapted resistance to many of these insecticides, and some of these have shown significant risk to the environment and human health3. Plants produce secondary metabolites like alkaloids, carbohydrates, flavonoids, saponins, tannins, and terpenoids, that they use as natural defenses against insects and bacteria. These compounds can be extracted and consequently be used by humans for anti-microbial, insecticide and pharmacological uses. Pesticides derived from plants that do not harm the environment have recently received increased attention for industrial, medical, and agricultural use. Natural insecticides tend to be less deleterious in human health, can be biologically sourced, and tend to cause less harm to non-target species and the environment4,5,6,7,8.
In addition to insecticides, a variety of extracts and chemicals from several plant groups have been studied as potential new larvicides9. Plant extracts or essential oils contain a variety of phytochemicals such as tannins10, essential oils11, isoflavonoids12, and stimulants13, which can kill mosquito larvae. The effects range from oviposition inhibition, developmental toxins, hatching inhibition, adulticides, ovicides, and emergence inhibitors4,14. Extracts from plants have traditionally been used throughout the world both to treat diseases and as insecticides. For example, the roots of Lantana camara has been used to treat skin rashes, rheumatism, and malaria. Extracts from its flowers have been used as a mosquito repellent, and its leaves have shown larvicidal activity15 as well as being used as an antibacterial and antihypertensive drug16.
Five plants were evaluated for larvicideal and ovicidal activity in this study: Lantana camara, Nerium oleander, Ricinus communis, Melia azedarach, and Withania somnifera. The effect of L. camara extract on the mortality and sub-lethal effects of the mosquito Cx. pipiens has previously been scientifically evaluated. This includes the effect of extract in an acetone solution on Cx. pipiens larvae17, and the ability of essential oils extracted from L. camara leaves to kill Cx. pipiens larvae18. Comparative studies have also been done by Mondal, et al.19, finding that the ethanolic leaf extract of L. camara was better at killing Cx. quinquefasciatus mosquito larvae than Cx. pipiens larvae.
N. oleander (Apocynaceae) is a low ornamental shrub of the Dogbane family that grows naturally in subtropical regions of the Mediterranean and is native to north-central Morocco. It has been used in medicine as an antibacterial, anti-inflammatory, antinociceptive, antioxidant, hepatoprotective, antitumor, and cytotoxic compound20,21 and has been extensively studied for its benefits in health and cytotoxicity. Extracts of N. oleander have been tested on 3rd and 4th larval stages of Cx. pipiens, and methanol extracts of N. oleander has shown positive effects on destroying Anopheles spp larvae. N. oleander leaf extract was shown to kill both the eggs and adults of the mosquito Aedes aegypti (a dengue vector)20,21. Raveen, et al.22 also evaluated acetone extracts from N. oleander flowers (pink, red, and white) against larvae of Aedes aegypti, A. stephensi, and Cx. pentamer mosquitoes.
R. communis L. (Euphorbiaceae) is a plant widely distributed throughout the tropics and warm temperate regions of the world. Researchers have written much about how R. communis can help with various health problems, including protecting the liver, reducing inflammation, increasing urine production, fighting cancer, killing bacteria and viruses, lowering blood sugar, killing fungi and insects, healing wounds, and stopping the growth of asthma and alleviating asthmatic conditions23.
Other activities of various phytochemical compounds include preventing cancer cell growth by interfering with DNA non-replication, and stimulating the activities of enzymes24. Some phytochemicals may also have antibacterial and antioxidant properties25.
In this work, we hypothesize that extracts of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera contain bioactive phytochemical compounds with lethal effects against the Cx. pipiens mosquito; namely by inhibiting larvae growth and killing the mosquito eggs. Cx. pipiens is important as it is a major vector of the West Nile virus, which kills both humans and animals (especially horses), as well as infecting various animals which may act as hosts, particularly birds.
Therapeutic targets of macro-molecules such as proteins have been developed following the full sequencing of the human genome. This has been aided by the extensive development of molecular structure visualization tools, such as x-ray diffraction (XRD), proton and carbon nuclear magnetic resonance (NMR), Fourier transform infra-red (FTIR), and other structure-identifying tool kits that lead to more success in identifying both protein–ligand and protein complex structure26. Effective and rapid structure identification has been invaluable to computer-aided drug design, and consequently, molecular modeling. This has presented a theoretical-based simulation between drug and host protein, defining a specific area called the binding pocket. The interactions between the drug and the host protein can be described using classical and advanced calculation. Currently most research articles on drug-protein interactions detail the use of one or other artificial intelligence applications that can describe ligand–protein and protein–protein interactions27. Drug design, therapeutic chemistry, and synthetic chemistry are fields of research that now depend to a great extent on complex computer aided molecular modelling28. Molecular docking analysis has been used in the elucidating the structure and possible synthesis of structures such as: PI3k29, carbonic anhydrase30, EGFR analogues31, acetylcholinesterase32, topoisomerase33, Fatty acid binding protein, and m-tor inhibitors34. Such studies are necessary to produce the most powerful candidate for a drug from a database of various candidates selected to satisfy the purpose.
Within this study activated polyphenols and poly flavonoids were extracted from the plants with methanol, and analyzed with molecular docking analyses. Docking of the polyphenols and flavonoids was examined on one of the most important insect proteins, 2FLJ. The expectation was that the molecular modelling provides a convenient rationalization about the mechanism of protein inhibition caused by the active ingredients, when they bind to the fatty acid binding protein active site; consequently causing severe perturbation to insect bio-chemistry or growth enzymes.
Materials and Methods
Plant materials and analysis
Plant collection
Leaves of the study plants, L. camara, M. azedarach, and N. oleander, R. communis, and W. somnifera, were collected from different locations in agricultural land around the villages of Qalyubiya Governorate, Egypt, between March and June 2023 (Table 1). These plants are local and widespread in the agricultural governorates of Egypt’s Nile Delta. Identification of the plants was done by Dr. Ahmed Mubarak of the Department of Botany and Plant Taxonomy (Faculty of Science, Banha University, Egypt) according to the Egyptian flora reference35. The study plant specimens were deposited in an herbarium of the botany department, Faculty of Science, with respective voucher numbers for L. camara (B112), M. azedarach (B33), N. oleander (B89), R. communis (B22), and W. somnifera (B315).
Plant extraction
The plant materials were shade-air-dried at room temperature until all water content removed and the dry weight was contracted. The dried tissues were ground in a stainless-steel electric mixer and transferred into airtight containers to protect them from humidity. Exactly 25 gm of plant powder was placed in a Soxhlet apparatus for 4–7 h (methanol was used as solvent). After filtration, the insoluble fibers were removed and the filtrate reconcentrated using rotary evaporator (at low temperature between 38 and 40 °C) utile all solvents were disposed. The solid residue was collected cautiously and re-dissolved in a definite volumes and stored in dark bottles32.
The aqueous plant-extract was prepared using the same protocol with distilled water instead of methanol. The extraction solutions were concentrated using a freeze-drying lyophilization and the residue was then stored in dark bottles36.
Mosquito larvicidal assay
Rearing of Culex pipiens
The larvae of Cx. pipiens were cultivated in an insectary, where they were kept at a temperature of 27 ± 2 °C and a relative humidity of 75 ± 5%. The larvae were exposed to a consistent photoperiod of 12 h light, 12 h darkness. The were provided with a diet consisting of fish food (Tetramin) and ground bread at a ratio of 3:1. Subsequently, the pupae were transferred from the enamel pans to a container containing dechlorinated water and then placed in screened enclosures of 35 × 35 × 40 cm, where the adult individuals ultimately emerged. The female mosquitoes were provided with regular blood feeds from a hamster rat, and both male and female the adult mosquitoes were provided with a 10% sugar solution. Larvae and pupae, representing two distinct stages of development, were consistently accessible for experimentation and housed within the same laboratory facility37.
Larvicidal activity
The plant extracts by methanol and water of B. glabra, D. regia, L. camara, and P. orientalis were evaluated for the action on the 3rd larval instar of Cx. pipiens under laboratory conditions. The 3rd larval instar was treated with the following concentrations of active compound: 62.5, 125, 250, 500, 1000, and 1500 ppm (1 g/1000 mL of distilled water). Twenty larvae per concentration were transferred to a glass beaker containing 250 mL of distilled water. Three replicates were used for each concentration. Mortalities were recorded 24 h and 48 h after the initial exposure i.e. post treatment (PT)38.
Ovicidal test
The technique of Su and Mulla39 was used to evaluate ovicidal activity. Mosquito larvae and egg rafts were obtained from the Medical Insect Research Lab, Faculty of Science, Benha University. Each of the 130 eggs (on the egg raft) was collected and placed in separate ovicidal cups containing varying concentrations of plant extracts (20, 40, 60, 80, and 100 ppm). In parallel, a control cup was maintained with regular water mixed with acetone. There were three iterations of the experiment. Following the treatment, eggs of each concentration were moved to water cups that were kept until their hatchability could be evaluated. The percent egg mortality was determined based on unhatched eggs 4 days (96 h) post-treatment40:
Effect of the sublethal concentrations on survival and larval longevity
In this test, 25 mosquito larvae in the 3rd instar were exposed to different concentrations of plant extracts in a 100-mL water solution, from all five plants (L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera) to determine LC50. The mosquitoes were left for 48 h, with 15 groups (375 larvae) being treated and three groups (75 larvae) being applied with dechlorinated water as a control. Mortality was assessed after 48 h by counting the total number of moribund and dead larvae, according to the WHO38. Any live larvae at this time were removed with a pipette and transferred on a wire gauze to plastic cups containing 100 mL of distilled water. The larvae were then fed a small portion of dry bread until they reached the pupation stage and reached adulthood.
Field evaluation of larvicides
L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera extracts were tested on larval and pupal mosquito populations in standing water pools (average 2.50 m × 1.25 m and 0.35 m deep) in a field evaluation. This was done at Kafer Saad village, Qalyubiya Governorate, Egypt, using LC95 X2 concentration, where the water level was relatively stable with a high mosquito density. Dechlorinated water was used at the control site only. Three replicates were used for each treatment. Mosquitoes for each site were sampled before treatment and post-treatment daily, for a week. Using a larval dipper (450 mL) at each larvicide pond site, we collected fourth instar larvae within water from the pond, for counting and sample examination.
We also tested strains of the bacterial larvicide (Bti Dunks, Summit, USA, 7000 ITU; International Toxic Unit/mg) in combination with plant extract on Cx. pipiens larvae in test pools. Half a bacterial briquette (equivalent to 6 g) was ground and mixed with each plant extract and added to a pool to examine the effect on mosquito larvae.
Phytochemical identification and in silico analysis
GC/MS analysis
For the biochemical analyses of the plant leaf methanol and aqueous extracts, Thermo Scientific Trace GC Ultra/ISQ Single Quadrupole MS and TG-5MS fused silica capillary columns, 0.1 mm, 0.251 mm, and 30 m thick, were used. Analysis was done using an electronic ionizer with 70 eV ionization energy. A helium carrier gas was used (flow rate: 1 mL/min). The MS transmission line and injector were both set to 280 °C. The oven was pre-heated and adjusted to the temperature of 35 °C, then increased to 150 °C at a rate of 7 °C per min, then 270 °C at a rate of 5 °C per minute (pausing for two minutes), and lastly to 310 °C by increasing at a rate of 3.5 °C per minute (maintaining this temperature for 10 min). Relative peak area was employed to quantify all the different chemical components discovered. The presence of the detected compounds and their concentrations were checked by comparing the retention times and spectral data fragmentation with those in the NIST and Willy libraries on the GC/MS instrument. Identification was done using the aggregate spectrum of user-generated reference libraries. To evaluate peak homogeneity, single-ion chromatographic reconstructions were performed. To verify GC retention times, co-chromatographic analysis of reference substances was used whenever possible41.
Molecular docking study
Source of the objective protein
Binding capabilities of the detected polyphenols in the methanolic extracts on the lm-FABP binding site were assessed. This was to determine the ability of the polyphenols to form stable and successive interactions with the residue of the target protein, and consequently to propose the mechanism of enzyme inhibition. The three-dimensional structure of the fatty acid binding protein (FABP) of Cx. pipiens did not exist in the protein data bank, so the well-known crystal structure of the fatty acid binding protein in locust flight muscle in complex with oleate lm-FABP was instead used. This structure has been used as an equivalent structure for Cx. pipiens in many research articles42. Thus the protein lm-FABP (PDB code: 2FLJ) was downloaded from the protein data bank (https://www.rcsb.org/structure/2FLJ) in PDB format, all water and hetero-molecules were removed where chain a, and b constrained.
Energy minimization
Phenolic active ingredients from the alcoholic extracts were automatically identified by the HPLC. A database set of 18 candidates of the polyphenolic active ingredients were selected for this study. The structure of the target compounds was drawn using CAMBRIDGESOFT CHEMOFFICE 2015 Professional 15.0.0 software after recalling their SMILES from the PubChem database. All the investigated compounds were saved as “Mol format” after fulfilling the “energy minimization” step using the default function “Amber12: EHT forcefield”, until gradient convergence of 0.01 kcal/mol was achieved. The energy minimization step was assessed by Avogadro and the molecular simulations were done using Molecular Operating Environment MOE_2009, installed on a 64-bit operating computer [Intel (R) Core (TM) i5-2400 CPU @ 2.40 GHz, 8 GB RAM].
Docking procedure
The protein structure model of lm-FABP (PDB code: 2FLJ) was downloaded, and the candidate ligands and target protein were prepared as follows: (i) The reference drug, oleate, was colored green to be easily differentiated; (ii) The protein binding site was produced automatically from the “surfaces and maps” option and accordingly, the co-crystallized ligand’s binding site; (iii) Similarly, the pocket site was created, separated, and saved in MDB format.
After the “energy minimization” step was done, all the investigated candidates were docked at the active site pocket using the “compute” option with the defaults of “rotate bonds” to produce flexible ligand with rigid receptor docking fulfillment. The scoring energy function was adjusted to be “London G” with a “triangle matcher” replacement set. The default “thirty conformers” was chosen as the total number of conformers and the best five scoring energy values were automatically selected. One of the best five conformers was chosen to represent ligand-docking and the results showed two- and three-dimensional receptor interactions. The docking results of all the tested compounds presented in the extract were listed in one table, regarding the predilection of the number of interactions, scoring energy (kcal/mol), RMSD (Å), and the bond length (Å). Three- and two-dimensional docking interactions were then determined. As with the co-crystallized ligand, all the tested ligands were marked in green color. Inter-molecular hydrogen bonding and π-π staking (aromatic) were labeled in magenta and yellow color respectively, and loops, helical structure, etc. were colored automatically, with images rendered for better presentation.
Statistical analysis
SPSS V23 (IBM, USA) software was used for doing Probit analyses, to calculate the lethal concentration (LC) values, and for the one-way analysis of variance (ANOVA) (Post Hoc/Turkey's HSD test). The significant levels were set at P < 0.05.
Results
Mosquito larvicidal activity
In the first part of the study, the larvicidal activity of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera extracts on 3rd instar Cx. pipiens was evaluated. All the tested plant extracts in this study showed that methanol extracts had more toxic effects against Cx. pipiens larvae (95–100%, 24 h post-treatment) than aqueous extracts (63–91%, 24 h post-treatment). The mortality percent (MO%) reached 100% for Lantana camara, Melia azedarach, Nerium oleander, and Ricinus communis, and 95% (MO%) for Withania somnifera, 24 h post-treatment (PT) with 1600 ppm methanol extracts (Table 2) with LC50 (50%, median lethal concentration) = 225.64, 203.35, 158.92, 175.04, and 336.23 ppm, respectively (Table 3). With aqueous extracts, the mortality was 87, 91, 80, 85, and 63% for L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera, respectively, with LC50 = 432.96, 373.29, 515.34, 467.02, and 898.56 ppm, respectively (Table 3). The results showed that methanol extracts of N. oleander (LC50 = 158.92 and 99.63 ppm) and R. communis (LC50 = 175.04 and 107.29 ppm) are very effective at killing mosquito larvae 24 and 48 h post-treatment, and M. azedarach (LC50 = 373.29 and 262.24 ppm) showed high efficacy within aqueous plant extracts (Table 4 and Fig. 1).
Ovicidal activity
The egg hatchability of the Cx. pipiens was tested with different concentrations of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera extracts in both methanol and water (Table 5 and Fig. 2). Percent hatchability, as expected, was inversely proportional to the concentration of the extracts. Among the five plant extracts tested for ovicidal activity against Cx. pipiens, the methanol extracts of R. communis (0.6%) and M. azedarach (6.4%) had the highest ovicidal activity at 800 ppm, followed by L. camara, N. oleander, and W. somnifera.
Sublethal effect of plant extracts on mosquito larvae survival.
After exposure, the LC50 values of extracts in L. camara (225.64 ppm), M. azedarach (203.35 ppm), N. oleander (158.92 ppm), R. communis (175.04 ppm), and W. somnifera (336.23 ppm) were shown to significantly affect the survival percentage until adulthood in Cx. pipiens larvae. The control group did not experience any mortality. The percentage of mosquito larvae that survived and turned into pupae was much lower in all plant extracts after 48 h, with 64, 52, 28, 40, and 60% survival respectively, relative to the control group (Fig. 3a).
The rate of pupae that successfully transformed into adults was considerably lower after treatment with plant extracts compared to the control group (Fig. 3b). Overall, the survival rates of larvae and adult emergence after 48 h of exposure to LC50 concentrations of N. oleander (28% and 19%) and R. communis (40% and 33.3%) were significantly reduced (F = 13.242; df. = 2, 57; P < 0.001). These rates were much lower than the 96% survival rate seen in the control group.
Larvicidal Field Evaluation
Field evaluation of larvicides of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera extracts was performed using LC95 X2 (2878.4, 2277.2, 1688, 1881.9 and 4688 ppm, respectively) in larval breeding site ponds at Kafer Saad village. Larval density was measured before and after adding the larvicides (or dechlorinated water in the control location). Lower larval densities were found 24 h after treatment with 76, 80, 90.7, 82, and 84.7% larval reduction for L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera respectively. With N. oleander extract this effect lasted four days (Fig. 4a). With larvicide extract mixed with the bacterial larvicide Bti briquette the % hile the larval reduction in the ponds 24 h after treatment reached 82, 88, 98, 87.3, and 95.3% respectively. N. oleander extract with the ti briquette lasted seven days post-treatment (Fig. 4b).
Field evaluation for larvicidal efficacy of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera extracts (a) and plant extracts with Bti briquettes (b) treated at a dose of LC95 X2 (2878.4, 2277.2, 1688, 1881.9 and 4688 ppm) and half of a Bti briquette, respectively, in larval breeding sites.
Biological characteristics of the plant extracts
GC–MS data analysis
The five extracts were subjected to metabolomic analysis, using GC–MS analysis to identify the range of compounds L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera leaves. The compounds include terpenes, fatty acids, esters, ketones, alkanes, steroids, aliphatic amines, and phenols. The analysis was conducted using only the methanol solvent.
L. camara extract contained 16 different compounds (Table 6), with the highest concentrations being 1-Dodecanamine, n,n-dimethyl- (32.98%), 1-Dodecanamine, n,n-dimethyl- (18.39%), and benzene, (chloromethyl)- (12.81%). M. azedarach extract contained 15 compounds (Table 7), with the highest concentrations being tributyl acetylcitrate (37.39%), hexadecanoic acid, and ethyl ester (17.34%). The N. oleander extract contained 19 compounds (Table 8) with the highest concentrations being tributyl acetylcitrate (36.13%), mome inositol (12.11%), and squalene (9.98%). R. communis extract contained 14 compounds (Table 9), with the highest concentrations being bis(2-ethylhexyl) phthalate (48.29%) and hexadecanoic acid, methyl ester (13.33%). W. somnifera extract contained 17 different compounds (Table 10), with the highest concentration compounds being linoleic acid ethyl ester (20.14%), pentadecanoic acid, 14-methyl ester (14.10%), and isochiapin b (11.23%).
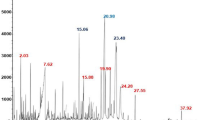
HPLC analysis and non-volatile constituents determination
One of the most important analyses to identify polyphenols and flavonoids is high-performance liquid chromatography (HPLC). The methanol extract of N. oleander, R. communis, L. camara, M. azedarach, and W. somnifera were analyzed, and 18 phenolic and flavonoid standards were used. The results of the HPLC peaks are presented in Figs. 5, 6, 7, 8 and 9 and the corresponding concentrations are listed in Table 11 and Fig. 10. All the five methanolic extracts had the polyphenols: gallic acid at concentrations of 114.75, 577.48, 168.4, 102.55 and 182 µg/mL respectively; chlorogenic acid at concentrations of 1007, 754.39, 115.7, 91.49 and 323.3 µg/mL respectively. Varied concentrations of catechin, methyl gallate caffeic acid methyl gallate, caffeic acid, syringic acid, rutin, ellagic acid, coumaric acid, vanillin, ferulic acid, naringenin, rosmaricic acid, daidzein, quercetin, cinnamic acid, kaempferol and hesperetin were also found.
Fatty acid binding protein (FABP4)
The inhibition activity of the methanolic extracts of Nerium oleander, Ricinus communis, Lantana camara, Melia azedarach, and Withania somnifera were tested with FABP4 protein. The inhibition results showed that all tested extracts are capable of inhibiting the FABP4 enzyme with different IC50s of 0.599, 0.425, 12.76, 1.47 and 4.55 µg/mL, respectively (Table 12 and Fig. 11).
Docking study
The target protein Lm-FABP, PDB:2FLJ was selected to perform a docking study using the detected polyphenols and flavonoids including gallic acid, chlorogenic acid, catechin, methyl gallate, caffeic acid, syringic acid, rutin, ellagic acid, coumaric acid, ferulic acid, naringenin rosmarinic acid, daidzein, quercetin, and cinnamic acid. These were all docked to the active site of the target protein with results presented in Table 13. Co-crystallized ligand, OLA, (Fig. 12) was used as a reference to use to compare and evaluate and compare the effectiveness of the active-ingredients presented in each extracts. Glutin, a low-molecular weight citrus flavonoid glycoside, had five electrostatic forces, a hydrogen bond, and multiple hydroxyl groups (Fig. 13) with arginine amino acid (Arg128), glutamine (Gln34), and aspartic acid (Asp75) in addition to one pi-pi stacking with the residue lysine (Lys60). Caffeic acid, coumaric acid, rosmarinic acid, and cinnamic acid had two dipole–dipole interactions and one dispersion force from Vander Waals forces (Figs. 14 and 15). Gallic acid, chlorogenic acid, methyl gallate, syringic acid, ferulic acid, naringenin, and daidzein had two intermolecular hydrogen bond between a hydroxyl group and a corresponding amino acid residue (Figs. 16 and 17). Catechin and quercetin had only one hydrogen bond (Fig. 18).
Discussion
Plant extracts and essential oils (EOs) contain several significant natural constituents that are effective in managing or eliminating pests and consequently associated diseases. They also undergo natural degradation processes43. Such biopesticides are being increasingly applied and are projected to surpass synthetic chemical pesticides soon, with an average yearly increase in usage of 9–20%44. The distinctive attributes of biopesticides, such as their low toxicity to non-target organisms and the environment, has contributed to their increased use in pest control.
All of the plant extracts we tested were very effective at killing mosquito larvae. According to our data, methanol extracts were more effective at killing larvae than aqueous extracts and produced high mortality. Methanol extracts of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera achieved 95–100% mortality among mosquito larvae treated in the lab after 24 h, while the mortality rate with water extracts reached only 63–91%. Plant extracts of N. oleander and R. communis were indicated to be the most effective against mosquito larvae in this study, confirming results of El-Akhal, et al.20, who tested extract effectiveness on various Culcidea mosquito larvae. El-Akhal, et al.20 found that the extract of N. oleander influenced the 4th instar larvae of Cx. pipiens, with an LC50 value of 57.57 mg/mL and an LC90 value of 166.35 mg/mL after 24 h of exposure. Also, acetone extracts from N. oleander flower were evaluated on larvae of Aedes aegypti, Anopheles stephensi, and Cx. quinquefasciatus and had LC50 values of 94.60, 101.21, and 121.79 mg/L, respectively22. Despite using extracts from flowers (whereas we used leaves) and using a broader range of species, the patterns in results with our study shows a similar efficacy of N. oleander.
Some researchers have studied N. oleander, commonly known as oleander, for its insecticidal properties, including its effects on mosquito larvae. Studies have shown the insecticidal properties of several toxic compounds found in N. oleander, such as oleandrin and nerin45. Research on the effectiveness of N. oleander leaf extracts against mosquito larvae has shown promising results. Other studies have demonstrated that these extracts have larvicidal activity against several species of mosquitoes, including Aedes aegypti and Culex quinquefasciatus, which are important vectors of diseases such as dengue fever, Zika virus, and West Nile virus. Methanol extract from oleander leaves has also previously shown efficacy in destroying Anopheles spp. larvae in vitro with an LC50 of 4–85 ppm21.
Oleander leaf extracts has been used for its larvicidal activity against Pine processionary moths, Thaumetopoea pityocampa with an LC50 value of 322.50 ppm and 190.00 ppm after 24 and 48 h post-treatment, respectively; using extract concentrations of 10, 25, 50, and 100 mg. Trogoderma granarium larvae also had a 10% mortality rate after 72 h at the 100 mg dose level46. Sotelo-Leyva, et al.47 evaluated the insecticidal activity of N. oleander against sugarcane aphid (Melanaphis sacchari) showing a 96% mortality rate at 72 h, and when 40% concentration N. oleander leaf extracts were used on Tribolium castaneum adult beetles there was 100% mortality. Leaf and stem extracts of 70% hydroethanolic from N. oleander has also been shown to prolong the first instar larval period of Pectinophora gossypiella48.
Our study showed that R. communis extract ranks second in its lethal effect on mosquito larvae after oleander extract, whether in methanol or aqueous extract. Many studies have confirmed the efficacy of R. communis extract in killing mosquito larvae in methanol, acetone, and aqueous extracts. The leaf extract of N. oleander has previously been tested49 against 4th instar larvae of Ae. aegypti at concentrations of 50–250 ppm with a mortality rate of 16.7%–92.7% giving an LC50 of 108.17 ppm. Also, in a study with 5% aqueous R. communis leaf extract, the extract killed 50% of Cx. pipiens larvae in less than 6 h for the L2 stage, less than 12 h for the L4, and 100% of mosquito larvae after 4 h50.
Waris et al.51 and Al‐Hakimi, et al.49 tested both leaf and seed extracts of R. communis and found significant mortality against Ae. aegypti at concentrations of 31.25, 62.5, 125, 250, 500 ppm, and against Anopheles culicifacies (a malaria vector) larvae at 2, 4, 8, 16, 32, and 64 ppm. After 24-h exposure, larvicidal activities were higher for the methanol extract of seeds than of leaves, with LC50 of 9.37 for seeds and 15.52 ppm for leaves on Ae. aegypti, and LC50 of 31.1 ppm for seeds and of 45.24 ppm for leaves on An. culicifacies. This was compared to a positive control of synthetic Temephos larvicide, which had LC50 of 106.24 ppm and LC90 of 175.73 ppm against Ae. aegypti. Kombieni, et al.44 has also found that that R. communis extract killed 75.8, 60.3 and 46.5% of Spodoptera frugiperda larvae at 250 g/L, 200 g/L, and 150 g/L of extract, respectively. Various solvents (aqueous, methanol, dichloromethane, and hexane) have been used to extract R. communis compounds from leaves and seeds to demonstrate larvicidal activity: severe toxicity on larval stages L2 and L4 of Cx. pipiens and the early IV instar larvae of Aedes aegypti and Anopheles culicifacies23,52.
Results in this study on the efficacy of Lantana camara extracts are similar to those found in other studies. Sharma et al.18 found leaf extract LC50 ranged from 47.47 to 52.06 ppm and LC90 ranged from 104.33 to 106.70 ppm on Cx. quinquefasciatus. Mondal, et al.19, found L. camara ethanol extract had an LC50 at 234.43 ppm, 131.82 ppm, and 89.12 ppm at 24 h, 48 h, and 72 h post-treatment intervals respectively for larvae of the same species, Cx. quinquefasciatus. Against Cx. pipiens53 (4th instar) it was extremely effective with an LC50 of only 29.3 ppm. Al-Solami17 illustrated increased mortality of Cx. pipiens larvae over time with an L. camara acetone extract which they tested over two and ten days, showing an LC50 of 140.1 and 51.3 ppm, respectively, and finally resulting in 98% mortality.
Studies using L. camara ethanol extract against the house fly (Musca domestica) gave an LC50 of 1462.7 ppm for leaves and 2101.8 ppm for stems54. Also a methanol extract from L. camara showed the highest mortality (74%), whereas the lowest mortality was found in ethyl acetate extract (26%) at 2% (w/w) concentration against Sitophilus zeamais54. Aisha, et al.55 showed that L. camara extract in essential oil against T. castaneum had an LC50 of 8.93 mg L. camara powder and LC90 of 13.54 mg/cm3. At 48 h exposure the LC50 was 7.92 mg/cm3 and LC90 was 10.47 mg/cm3.
Larval mortality occurred in all the pond studies when our plant extracts were added, both with and without Bti briquettes. Over 24 h, N. oleander was most effective at causing mortality, and was effective for up to 5 days. The next highest efficacy was with R. communis and W. somnifera. Combination of plant extracts with Bti briquettes (Mosquito unks) increased the larval reduction rate for all treatments, with up to nine days effect post-treatment for N. oleander. Previous studies we did with different natural extracts and essential oils showed no more than 5 days efficacy56.
Methanol extracts of L. camara, M. azedarach, N. oleander, R. communis, and W. somnifera had a higher number of organic compounds than aqueous extracts, with both a higher number of fatty acid and terpene compounds. It is believed that a group of secondary metabolites, including alkaloids, flavonoids, terpenoids, and phenolic compounds, are the compounds in R. communis extracts that kill the mosquito larvae. These compounds interfere with larval development or disrupt physiological processes, leading to death. The effectiveness of R. communis leaf extracts can vary depending on factors such as extraction method, extract concentration, mosquito species, and environmental conditions. More research is necessary to evaluate the human safety of these extracts and their potential environmental impacts, although they may provide a natural alternative to mosquito control.
Our findings align with prior research (Chengala et al.57) that endorses methanol as the preferred solvent for extracting useful metabolites from diverse medicinal and insecticidal plants. However, acetone is better at extracting polar phytocompounds like phenolics, being a polar solvent. This was shown with L. camara leaf58 extracts. Such extracts have been shown to reduce inflammation, fight cancer, reduce the growth and kill bacteria, fungi, insects and nematodes53.
The five plant leaf extracts that were analyzed had a high concentration of cedrol, caryophyllene, caryophyllene oxide, phytol, squalene, and caryophyllene; all of which are commonly found monoterpenes and sesquiterpenes. The observed insecticidal activity may be attributed to the main components, including caryophyllene (also known as isocaryophyllene), eucalyptol, and caryophyllene oxide. This finding aligns with the research conducted by Zoubiri and Baaliouamer59, who also reported insecticidal activity in b-caryophyllene and caryophyllene oxide. Caryophyllene oxide, spathulenol, and germacrene-D have been identified as having anti-carcinogenic, anti-inflammatory, insecticide, pesticide, and antibacterial effects59.
Plants also produce phenolic compounds, which are strong antioxidants60,61 .Plants exposed to high metal concentrations have shown a significant increase in the accumulation of phenolic compounds and peroxide activity. The primary reason for the antioxidant activity of phosphonates is mostly attributed to their redox properties, which enable them to function as reducing agents, hydrogen donors, and quenchers of singlet oxygen62. Phenolic compounds are commonly referred to as polyphenols, and include flavonoids, phenolic acids, intricate flavonoids, and vibrant anthocyanins. Phenolic metabolites are crucial in various biological activities, including attractants for pollinating invertebrates, coloration for concealment and defense against herbivores, to inhibit consumption by invertebrates, and for antibacterial and antifungal purposes63,64.
The efficacy of L. camara, M. azedarach, N. oleander, R. communis and W. somnifera leaf extracts can vary depending on factors such as the extraction method, concentration of the extract, mosquito species, and environmental conditions. Additionally, while these extracts may offer a natural alternative for mosquito control, further research is needed to assess their safety and potential ecological impacts. Biopesticides, despite their advantageous insecticidal properties, constitute only 5% of pesticides used globally43.
HPLC analysis revealed that N. oleander and R. communis leaf extracts had the highest percentage of gallic acid and chlorogenic acid, at concentrations exceeding 500 µg/mL reaching 1000 µg/mL in the case of N. oleander extract. Gallic acid and chlorogenic acid, was also present in the other plant extracts.
Lahlou et al.65 evaluated the in-vivo and in-vitro insecticidal and physiological effects of gallic acid on Cx. pipiens larvae under laboratory conditions. Gallic acid has been extensively in mosquito larvicides including in combination with the globally most used natural pesticide: the bacteria B. thuringiensis var. israelensis, to increase its potency as an anti-oxident in damaging the larvae central nervous system. Gallic acid also damages the central nervous and digestive systems of the cotton leaf worm, Spodoptera littorales at low concentrations66. Upon ingestion, the phenolic compound causes acute toxicity and paralysis to this economically important agricultural pest. Gallic acid was also found to display low genotoxicity potential in multiple assays and was successfully used as potential anti-malarial candidate67.
Another phenolic product is chlorogenic acid; a member of hydroxycinnamic acids. It has been extensively studied and used in several applications, including food, medical, and pesticide formulations. Synthetic insecticidal analogs, based on the parent chlorogenic acid scaffold, are commercially available for broad spectrum insect control. So, they are applied in the fight against mosquitoes, as they do not constitute a substantial threat to human life. Chlorogenic acid is a strong inhibitor of acetylcholine esterase (AChE) activity68. AChE is responsible for the termination of excitatory transmission in the nerve synapse.
HPLC confirmed a high percentage of rutin (a flavanol glycoside) in all five extracts, but particularly in N. oleander, R. communis and M. azedarach. Rutin has shown fast and effective larvicidal effects, as well as a possible chemical for deterring egg-laying. Rutin showed larval mortality of 10.05–82.52%. It possesses a wide range of pharmacological activities including anti-inflammatory, anti-carcinogenic, antiviral, and anti-bacterial activities.
HPLC confirmed an abundance of caffeic acid, rosmarinic acid and coumaric acid, such plant-derived products are known for their eco-friendliness, biodegradability, and availability in nature69 as well as for environmentally friendly mosquito control strategies70. Other secondary metabolites in the plants included catechin, methyl gallate, syringic acid, ellagic acid, ferulic acid, naringenin, daidzein and quercetin, which also enhance the pest control activity in the extracts. Indeed, the synergistic effects of the secondary plant metabolites as larvaecides likely increases the potency of the extracts, whilst still being composed of biodegradable and environmentally friendly chemicals, suggesting them to be ideal substitutes for toxic synthetic chemical compounds.
Fatty acid binding proteins (FABPs) are a collection of intracellular binding proteins, which bond to the hydrophobic lipids and water-insoluble materials for many purposes such as synthesis of phospholipids, and lipid metabolism. In-vivo studies conducted on measuring the FABP4 levels in mice show that down-regulation of FABP4 are associated with many metabolic diseases42,71. In our study the enzyme inhibition activity of the methanolic plant extracts was assessed. The inhibitory concentration of N. oleander and R. communis was IC50 = 0.599 µg/mL and 0.425 µg/mL respectively. This is very close to commonly used positive control reference drugs: IC50 = 0.599 µg/mL for Orlistat, and IC50 = 0.235 µg/mL for Cobimetinib. IC50 values of L. camara, M. azedarach, and W. somnifera extracts were higher i.e. less potent.
The high enzyme inhibition of N. oleander and R. communis may be due to the presence of both volatile and non-polar substances (detected by GC/MS) or non-volatile, polar substances (detected by HPLC). Extracts N. oleander and R. communis contain very high quantities of natural phenolic acids such as Gallic acid and chlorogenic acid, and the flavanols kaempferol and rutin. Earlier in-vitro study to evaluate the inhibition ability of methanolic and aqueous acacia extracts were done by our team showing that acacia methanolic extract had IC50 of 0.681 µg/mL, and aqueous extract had IC50 of 2.311 µg/mL, with a positive control of Orlistat with IC50 of 0.535 µg/mL72.
Fatty acid binding proteins (FABPs) are low-molecular weight single chain polypeptides. Their biological function is to solubilize and shield sensitive hydrophobic and water-insoluble retinoids, fatty acids, and bile acids constituents transported into the cytosol or any organelles in the cell for purposes such as phospholipid synthesis, lipid metabolism and mitochondrial beta oxidation. FABP synthesis is extensive in both animal (vertebrate and invertebrate) and insect kingdoms73,74. Most FABPs share the same amino acid sequence such that they have a 70% similarity, and their three-dimensional stereo-structure are all restricted to the β-barrel structure with a ligand binding cavity75,76. The first insect FABP discovered was in the flight muscle of the desert locust Schistocerca gregaria77. FABPs from various insects affect the physiological metabolism through modifying intracellular fatty acid components, modulating sleep, long-term memory reinforcement, lipid accumulation, and a role in feeding and social caste divisions78.
In this study, lm-FABP (PDB code: 2FLJ) was used as the target protein, and the docking with the ligands of the 18 detected polyphenols and flavonoids modeled. Rutin (a flavanol) showed the highest number (five) of electrostatic forces and one additional dipole–dipole interaction between the target protein and rutin. Two such interactions were possible, with the same amino acid residue with a co-crystallized ligand. The root mean square deviation (RMSD = 1.55) of the interaction was less than 1.7, meaning a possibility of the co-crystallized ligand being replaced78. The N. oleander extract had high efficacy, may is probably due to having a high rutin concentration, with rutin being confirmed (in the docking model) to be an effective FABP inhibitor.
Caffeic acid, coumaric acid and rosmarinic acid all make two types of intermolecular hydrogen bonds with different amino acids, with bond lengths ranging from 1.88 to 2.40 Å and scoring energy ranging from (− 4.47 kcal/mol) to (− 6.78 kcal/mol) kcal/mol, and one dispersion force (from Van der Waal forces). Furthermore, gallic acid, chlorogenic acid, methyl gallate, syringic acid, ellagic acid, ferulic acid, naringenin and daidzein all have two interactions with at least one residue, similar to the co-crystallized ligand. Quercetin and daidzein each had a only one hydrogen bond, and thus a limited connection to the target protein.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Cheng, S.-S. et al. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresour. Technol. 100, 452–456 (2009).
Hemalatha, P. et al. Larvicidal activity of Lantana camara aculeata against three important mosquito species. J. Entomol. Zool. Stud. 3, 174–181 (2015).
Jirakanjanakit, N. et al. Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J. Econ. Entomol. 100, 545–550 (2014).
Baz, M. M., Selim, A., Radwan, I. T., Alkhaibari, A. M. & Khater, H. F. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Sci. Rep. 12, 4406 (2022).
Radwan, I. T., Baz, M. M., Khater, H. & Selim, A. M. Nanostructured lipid carriers (NLC) for biologically active green tea and fennel natural oils delivery: Larvicidal and adulticidal activities against Culex pipiens. Molecules 27, 1939 (2022).
Baz, M. M. et al. Novel pesticidal efficacy of Araucaria heterophylla and Commiphora molmol extracts against camel and cattle blood-sucking ectoparasites. Plants 11, 1682 (2022).
Baz, M. M., Selim, A. M., Radwan, I. T. & Khater, H. F. Plant oils in the fight against the West Nile Vector, Culex pipiens. Int. J. Trop. Insect Sci. 42, 2373–2380 (2022).
Mohammed, S. H. et al. Acaricide resistance and novel photosensitizing approach as alternative acaricides against the camel tick, Hyalomma dromedarii. Photochem. Photobiol. Sci. 22, 87–101 (2023).
Baz, M. M., Hegazy, M. M., Khater, H. F. & El-Sayed, Y. A. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens Say (Diptera: Culicidae). Pak. Vet. J. 41 (2021).
Yang, Y.-C., Lee, E.-H., Lee, H.-S., Lee, D.-K. & Ahn, Y.-J. Repellency of aromatic medicinal plant extracts and a steam distillate to Aedes aegypti. J. Am. Mosq. Control Assoc. 20, 146–149 (2004).
Cavalcanti, E. S. B., Morais, S. M. d., Lima, M. A. A. & Santana, E. W. P. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Memórias do Instituto Oswaldo Cruz 99, 541–544 (2004).
Joseph, C., Ndoile, M., Malima, R. & Nkunya, M. Larvicidal and mosquitocidal extracts, a coumarin, isoflavonoids and pterocarpans from Neorautanenia mitis. Trans. R. Soc. Trop. Med. Hyg. 98, 451–455 (2004).
Chowdhury, N., Ghosh, A. & Chandra, G. Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement. Altern. Med. 8, 1–8 (2008).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils: A review. Food Chem. Toxicol. 46, 446–475 (2008).
Begum, S. et al. Leishmanicidal triterpenes from Lantana camara. Chem. Biodivers. 11, 709–718 (2014).
Baz, M. M. et al. Evaluation of four ornamental plant extracts as insecticidal, antimicrobial, and antioxidant against the West Nile vector, Culex pipiens (Diptera: Culicidae) and metabolomics screening for potential therapeutics (2023).
Al-Solami, H. M. Larvicidal activity of plant extracts by inhibition of detoxification enzymes in Culex pipiens. J. King Saud Univ.-Sci. 33, 101371 (2021).
Sharma, M., Alexander, A., Nakhate, K. T. & Nagwanshi, K. K. Evaluation of the mosquito larvicidal potential and comparative assessment of the juice of Lantana camara Linn and Ocimum gratissimum Linn. Exp. Parasitol. 249, 108521 (2023).
Mondal, S., Ghosh, S., Maity, S., Ghosal, G. & Sultana, A. Comparative study on larvicidal potentials of three medicinal plants on larvae of Culex quinquefasciatus Say, 1823 mosquitoes. Int. J. Mosq. Res. 10, 54–61 (2023).
El-Akhal, F., Guemmouh, R., Ez Zoubi, Y. & El Ouali Lalami, A. Larvicidal activity of Nerium oleander against larvae West Nile vector mosquito Culex pipiens (Diptera: Culicidae). J. Parasitol. Res. 2015, 1–5 (2015).
Behravan, M., Vaezi-Kakhki, M. R. & Baharshahi, A. Comparing larvicidal effect of methanol extract of the aerial parts of henbane (Hyoscyamus niger L.) and oleander (Nerium oleander L.) plants on Anopheles spp larvae (Diptera: Culicidae) in vitro. Zahedan J. Res. Med. Sci. 19, 8631 (2017).
Raveen, R. et al. Bioefficacy of Nerium oleander Linnaeus (Apocynaceae) floral extracts on the larva of three vector mosquitoes of medical importance. Int. J. Mosq. Res. 4, 65–77 (2017).
Sogan, N. et al. Larvicidal activity of Ricinus communis extract against mosquitoes. J. Vector Borne Dis. 55, 282–290 (2018).
Majrashi, T. A. et al. Insight into the biological roles and mechanisms of phytochemicals in different types of cancer: Targeting cancer therapeutics. Nutrients 15, 1704 (2023).
Prakash, B., Kumar, A., Singh, P. P. & Songachan, L. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. Functional and Preservative Properties of Phytochemicals 1–45 (2020).
Bolje, A. & Gobec, S. Analytical techniques for structural characterization of proteins in solid pharmaceutical forms: An overview. Pharmaceutics 13, 534 (2021).
Dhakal, A., McKay, C., Tanner, J. J. & Cheng, J. Artificial intelligence in the prediction of protein–ligand interactions: Recent advances and future directions. Brief. Bioinform. 23, 476 (2022).
Pinzi, L. & Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 20, 4331 (2019).
Radwan, I. T. et al. Design, synthesis, docking study, and anticancer evaluation of novel bis-thiazole derivatives linked to benzofuran or benzothiazole moieties as PI3k inhibitors and apoptosis inducers. J. Mol. Struct. 1265, 133454 (2022).
Zain-Alabdeen, A. I. et al. Synthesis and anticancer activity of new benzensulfonamides incorporating s-triazines as cyclic linkers for inhibition of carbonic anhydrase IX. Sci. Rep. 12, 16756 (2022).
Mohareb, R. M., Bagato, N. M. A. & Radwan, I. T. Design, synthesis, molecular docking, and biological studies of new heterocyclic compounds derived from β-diketones as novel EGFR and pim-1 inhibitors endowed with antitumor activity. Anti-Cancer Agents Med. Chem. 22, 2558–2576 (2022).
Radwan, I. T. et al. Effect of nanostructure lipid carrier of methylene blue and monoterpenes as enzymes inhibitor for Culex pipiens. Sci. Rep. 13, 12522 (2023).
Elanany, M. A., Osman, E. E. A., Gedawy, E. M. & Abou-Seri, S. M. Design and synthesis of novel cytotoxic fluoroquinolone analogs through topoisomerase inhibition, cell cycle arrest, and apoptosis. Sci. Rep. 13, 4144 (2023).
Zhang, B. et al. Design, synthesis and biological evaluation of substituted 2-(thiophen-2-yl)-1, 3, 5-triazine derivatives as potential dual PI3Kα/mTOR inhibitors. Bioorg. Chem. 95, 103525 (2020).
Boulos, L. Flora of Egypt Checklist. Revised Annotated Edition 410 (Al-Hadara Publishing, 2009).
Kumar, J., Ramlal, A., Mallick, D. & Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 10, 1185 (2021).
Maysa, M. H. & Baz, M. M. Efficacy of some plant wastes and cigarette filter residues as alternative agents for controlling Culex pipiens (Diptera: Culicidae). Int. J. Mosq. Res. 10, 1–7 (2023).
WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides (World Health Organization, 2005).
Su, T. & Mulla, M. S. Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 14, 204–209 (1998).
Elango, G., Rahuman, A. A., Kamaraj, C., Bagavan, A. & Zahir, A. A. Efficacy of medicinal plant extracts against malarial vector, Anopheles subpictus Grassi. Parasitol. Res. 108, 1437–1445. https://doi.org/10.1007/s00436-010-2192-4 (2011).
Ashmawy, A., Mostafa, N. & Eldahshan, O. GC/MS analysis and molecular profiling of lemon volatile oil against breast cancer. J. Essential Oil Bear. Plants 22, 903–916 (2019).
Thompson, B. R., Mazurkiewicz-Munoz, A. M., Suttles, J., Carter-Su, C. & Bernlohr, D. A. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J. Biol. Chem. 284, 13473–13480 (2009).
Marrone, P. G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 75, 2325–2340 (2019).
Kombieni, E. et al. Insecticidal activity of Ricinus communis L seed extract against Spodoptera frugiperda JE Smith under laboratory and field conditions. Int. J. Biol. Chem. Sci. 17, 760–772 (2023).
Shridhar, N. Nerium oleander toxicity: A review. Int. J. Adv. Acad. Stud 4, 23–32 (2022).
Semi̇z, G. Larvicidal activity of Nerium oleander L. leaf extract against Pine Processionary Moth (Thaumetopoea wilkinsoni Tams.). J. Entomol. Zool. Stud. 5, 79–81 (2017).
Sotelo-Leyva, C. et al. Insecticidal Cmpounds in Ricinus communis L. (Euphorbiaceae) to Control Melanaphis sacchari Zehntner (Hemiptera: Aphididae). Fla. Entomol. 103, 91–95, 95 (2020).
Moustafa, H. Z., Al Shater, H. & Yousef, H. Toxicity of Nerium oleander extracts against Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). Int. J. Adv. Res. Biol. 5, 163–168 (2018).
Al‐Hakimi, A. N., Abdulghani, M. A., Alhag, S. K., Aroua, L. M. & Mahyoub, J. A. Larvicidal activity of leaf extract of Nerium oleander L. and its synthesized metallic nanomaterials on dengue vector, Aedes aegypti. Entomol. Res. 52, 148–158 (2022).
RIBEIRO, I. A. T. d. A. Óleos essenciais de Croton rudollphianus e Algrizea macrochlamys no combate à doenças negligenciadas: Esquistossomose e dengue. (2020).
Waris, M. et al. Evaluation of larvicidal efficacy of Ricinus communis (Castor) and synthesized green silver nanoparticles against Aedes aegypti L. Saudi J. Biol. Sci. 27, 2403–2409 (2020).
Aouinty, B., Chennaoui, M., Mahari, S., Rihane, A. & Mellouki, F. Larvicidal effects of aqueous extract from Ricinus communis L. leaves against mosquito Culex pipiens: Mortality and histopathology of treated larvae. JMES 9, 619–623 (2018).
Nawaz, H., Shad, M. A., Rehman, N., Andaleeb, H. & Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 56, e17129 (2020).
Fouda, M. A., Hassan, M. I., Shehata, A. Z., Hasaballah, A. I. & Gad, M. E. Larvicidal and Antifeedant activities of different extracts from leaves and stems of Lantana camara (Verbenaceae) against the housefly, Musca domestica L. Egypt. Acad. J. Biol. Sci. F 9, 85–98 (2017).
Aisha, K. et al. Extraction, chemical composition and insecticidal activities of Lantana camara Linn. leaf essential oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis. Molecules 29, 344 (2024).
Radwan, I. T. et al. Design, synthesis, docking study, and anticancer evaluation of novel bis-thiazole derivatives linked to benzofuran or benzothiazole moieties as PI3k inhibitors and apoptosis inducers. J. Mol. Struct. 1265, 133454. https://doi.org/10.1016/j.molstruc.2022.133454 (2022).
Chengala, L. & Singh, N. Botanical pesticides—a major alternative to chemical pesticides: A review. Int. J. Life Sci 5, 722–729 (2017).
Prabha, S. et al. Biopesticides—An alternative and eco-friendly source for the control of pests in agricultural crops. Plant Arch. 16, 902–906 (2016).
Zoubiri, S. & Baaliouamer, A. GC and GC/MS analyses of the Algerian Lantana camara leaf essential oil: Effect against Sitophilus granarius adults. J. Saudi Chem. Soc. 16, 291–297 (2012).
Dawood, A. S., Chua, L. S., Tan, T. S. & Alshemary, A. F. Apoptotic mechanism of lantadene A from Lantana camara leaves against prostatic cancer cells. Egypt. J. Chem. 64, 7603–7610 (2021).
Reyad, A. M., Karam, Y. A., Radwan, T. E., Hassan, G. M. & Hemida, K. A. Multidrug-resistant Staphylococcus bacteria isolated from pregnant women and the antimicrobial effect of Lantana camara L. different extracts. Egypt. J. Exp. Biol. (Botany) 17, 33 (2021).
Qureshi, H. et al. Isolation of natural herbicidal compound from Lantana camara. Int. J. Environ. Anal. Chem. 101, 631–638 (2021).
Negi, G. C. et al. Ecology and use of Lantana camara in India. Bot. Rev. 85, 109–130 (2019).
Abdalla, A. I., Kehail, M., Abdelrahim, Y. M. & Ibrahim, N. A. Phytochemical screening of Calotropis procera ait flower parts and their larvicidal potentialities against Anopheles and Culex Larvae, Gezira State, Sudan. Int. J. Biol. Res. 2, 88–92 (2017).
Lahlou, R. A. et al. Thymus hirtus Willd. Ssp. algeriensis Boiss. and Reut: A comprehensive review on phytochemistry, bioactivities, and health-enhancing effects. Foods 11, 3195 (2022).
Youssefi, M. R. et al. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the West Nile vector Culex pipiens. Molecules 24, 1867 (2019).
Srivastava, A. K., Kumar, A. & Misra, N. On the inhibition of COVID-19 protease by Indian herbal plants: An in silico investigation. arXiv preprint. arXiv:2004.03411 (2020).
El Zayyat, E. A., Soliman, M. I., Elleboudy, N. A. & Ofaa, S. E. Bioefficacy of some Egyptian aromatic plants on Culex pipiens (Diptera: Culicidae) adults and larvae. J. Arthropod-Borne Dis. 11, 147 (2017).
Matiadis, D. et al. Curcumin derivatives as potential mosquito larvicidal agents against two mosquito vectors, Culex pipiens and Aedes albopictus. Int. J. Mol. Sci. 22, 8915 (2021).
Ramzi, A. et al. Synergistic effect of bioactive monoterpenes against the mosquito, Culex pipiens (Diptera: Culicidae). Molecules 27, 4182 (2022).
Adida, A. & Spener, F. Adipocyte-type fatty acid-binding protein as inter-compartmental shuttle for peroxisome proliferator activated receptor γ agonists in cultured cell. Biochim. Biophys. Acta (BBA) 1761, 172–181 (2006).
Baz, M. M. et al. Larvicidal activity of Acacia nilotica extracts against Culex pipiens and their suggested mode of action by molecular simulation docking. Sci. Rep. 14, 6248 (2024).
Ali, M. M., Ramadan, M. A., Ghazawy, N. A., Afify, A. & Mousa, S. A. Photochemical effect of silver nanoparticles on flesh fly larval biological system. Acta Histochem. 124, 151871 (2022).
Haunerland, N. H. & Spener, F. Fatty acid-binding proteins–insights from genetic manipulations. Prog. Lipid Res. 43, 328–349 (2004).
Zimmerman, A. & Veerkamp, J. New insights into the structure and function of fatty acid-binding proteins. Cell. Mol. Life Sci. CMLS 59, 1096–1116 (2002).
Marcelino, A. M. C., Smock, R. G. & Gierasch, L. M. Evolutionary coupling of structural and functional sequence information in the intracellular lipid-binding protein family. Proteins 63, 373–384 (2006).
Haunerland, N. H., Andolfatto, P., Chisholm, J. M., Wang, Z. & Chen, X. Fatty-acid-binding protein in locust flight muscle: Developmental changes of expression, concentration and intracellular distribution. Eur. J. Biochem. 210, 1045–1051 (1992).
Smith, A. F., Tsuchida, K., Hanneman, E., Suzuki, T. C. & Wells, M. A. Isolation, characterization, and cDNA sequence of two fatty acid-binding proteins from the midgut of Manduca sexta larvae. J. Biol. Chem. 267, 380–384 (1992).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, investigation, data curation, formal analysis, resources, writing-original draft preparation, M.M.B., I.T.R, A.S., A.M.A., M.H.A., S.M.A., H.S.G., M.E.G.; editing and writing-review, M.M.B., I.T.R, A.S., M.H.A., A.M.A., S.M.A., H.S.G., M.E.G.; project administration, A.S.; funding achievement, M.M.B., I.T.R, A.S., A.M.A., S.M.A., M.H.A., H.S.G., M.E.G. All authors have read and approved the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical consideration
The study was carried out according to the guidelines of the declaration of Benha University and approved by the Ethics Committee of the Faculty of Science, Benha University (Code: BUFS-REC-2024-241Ent).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baz, M.M., Selim, A.M., Radwan, I.T. et al. Evaluating larvicidal, ovicidal and growth inhibiting activity of five medicinal plant extracts on Culex pipiens (Diptera: Culicidae), the West Nile virus vector. Sci Rep 14, 19660 (2024). https://doi.org/10.1038/s41598-024-69449-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69449-6
- Springer Nature Limited