Abstract
Due to the many applications of manganese oxides in water treatment, this research aimed to synthesize α-Mn2O3 nanoparticles through a green method and investigate the dye adsorption capacity of them. The α-Mn2O3 nanoparticles were successfully synthesized using KMnO4 and aqueous extract of Pyracantha angustofolia fruits under hydrothermal conditions and calcination. The products were identified using Fourier transform infrared (FTIR), X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), and scanning electron microscopy (SEM) analyses. The adsorption capacity of methyl orange (MO) and Congo red (CR) dyes were evaluated at different concentrations (25, 50, and 75 ppm) using α-Mn2O3 nanoparticles. Results revealed the spherical and porous structure of α-Mn2O3 nanoparticles with a specific surface area of 21.7 m2.g-1. Dye removal significantly increased with pH decrement. The adsorption capacity for MO and CR was 73.07 and 70.70 mg.g−1, respectively. The adsorption data of both dyes followed a pseudo-second-order kinetic model. The best fitted models for MO and CR adsorption were the Langmuir isotherm and the Dubinin–Radushkevich, respectively. In addition, a possible adsorption mechanism was proposed for both dyes. The findings showed that α-Mn2O3 nanoparticles are very efficient adsorbents for removing anionic dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Organic dyes, a subgroup of synthetic dyes, are an essential source of water pollution. They are considered a destructive threat to the ecosystem, and the discharge of such pollution in surface water is hazardous for living organisms1. Even a small concentration of these pollutants in water is unacceptable due to their toxic effects2. Therefore, dye-contaminated wastewater is a critical environmental target, especially considering the scarcity of water resources3,4. Cationic and anionic organic dyes, which are dissociated into positive and negative ions in aqueous solutions, respectively, are widely used in industries such as textiles, dyes, leather, paper, cosmetics, and pharmaceuticals5,6,7,8,9. Congo red (CR) and methyl orange (MO) are important examples of anionic basic dyes that are mainly used as colouring agents in textile and paper industries10,11. Both colours are harmful to the environment and human health12. CR and MO are two classes of azo dyes, di- and mono-, respectively. Owing to toxic, mutagen, and harmful to the environment, both dyes have restricted in many countries13,14,15,16. Consequently, it is highly desirable to develop treatment techniques to eliminate them.
There are several methods to remove dyes from polluted waters, including coagulation, ion exchange, electrochemical processes, adsorption, photocatalytic and catalytic degradation, chlorination, membrane separation, and biological treatment17. However, methods with a broader application range that are environmentally friendly, cost-effective, and fast to remove such pollutants are being developed18. For example, chlorination is effective for a specific group of dyes that chlorine can destroy19. In addition, the biological treatment of dyes is a time-consuming process, and more than just using this method is needed due to their low efficiency in removing dyes20. Among these techniques, the adsorption method is preferred due to its simplicity, high efficiency, and economic aspects21,22. This method can effectively remove a wide range of dyes, and the sorbents used in this process are often reusable, making it a sustainable option. Several factors, such as pH and temperature of the dye solution, dye concentration, and the type and dosage of adsorbent, affect the adsorption efficiency23,24.
Researchers are motivated to develop nano-sorbents using simple, economical, and fast methods. Nanoadsorbents have a high adsorption capacity due to their high surface area to volume25. In recent years, the production of metal oxide nanoparticles through green synthesis has attracted the attention of researchers due to the importance of environmental issues. Metal oxide nanoparticles produced by this method have a high capacity to adsorb water pollutants. Sridevi et al.26 synthesized polypyrrole-coated Fe2O3 nanoparticles using Tabebuia aurea leaf for the removal of an agricultural herbicide. Also, green synthesized Fe2O3 nanoparticles using Acacia falcata leaf extract demonstrated efficient adsorption of Cr (VI) ions27. Not only green synthesis is an eco-friendly technique, but also the use of plant extract in this method strengthens the pollutant adsorption capacity of nanoadsorbents due to the presence of carbon in the structure of the synthesized nanoparticles28. Manganese oxide nanoparticles are also synthesized via green methods and used for dye removal through photocatalyst29 and adsorption30. Manganese trioxide (Mn2O3) is one of the common forms of manganese oxide that has various applications in industries. Researchers are trying to improve the properties of Mn2O3 by controlling the shape, size, and performance of synthesized nanoparticles through different synthesis methods31,32,33. Incorporation of synthesized Mn2O3 nanoparticles in specific composites has shown the photocatalytic activity of these particles at neutral pH34,35. Therefore, developing a simple and environmentally friendly method to produce Mn2O3 nanoparticles is crucial. Various techniques, including co-precipitation, hydrothermal treatment, reflux treatment, molecular sieve techniques, and green methods36, have been used to produce MnO-based adsorbent catalysts with high stability and excellent catalytic performance in the oxidation of aromatic compounds. Among these methods, the green synthesis of manganese oxide nanoparticles has been considered as a novel approach due to its cost-effectiveness, simplicity, and non-toxic nature37,38,39.
Manganese oxides are attractive metal oxides for researchers due to their structural and functional diversity. The development of α-Mn2O3 production methods is always of interest to researchers due to the wide applications of α-Mn2O3. This research used Pyracantha angustofolia extract as a reducing agent in the production process of α-Mn2O3 nanoparticles for the first time. Based on the green approach, potassium permanganate, and Pyracantha angustofolia fruit extract were the only materials used during the synthesis of α-Mn2O3. In addition, the effect of calcination temperature was investigated, and finally, the optimal temperature was determined to obtain magnesium trioxide. The synthesized α-Mn2O3 nanoparticles were fully characterized by Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET) analyses. The capability and capacity of fabricated α-Mn2O3 nanoparticles to remove MO and CR dyes were studied. The dye removal efficiency was analyzed by considering initial pH, process duration, and dye concentration. Finally, kinetic models and adsorption isotherms were investigated to understand the adsorption mechanism of dyes through nanoparticles.
Results and discussion
Structural analysis of products
The purity and phase characterization of the products were investigated using XRD analysis. As presented in Fig. 1a, the XRD pattern of the as-synthesized sample matches with the MnCO3 XRD pattern based on JCPDS file 089-4836 due to the existence of seven main peaks at positions (2\(\uptheta \)) of 24.3, 31.4, 37.4, 41.4, 45.1, 51.6 and 59.8°, which correspond to planes (012), (104), (110), (202), (116), (122), respectively40,41,42. It has long been known that plant extracts are able to reduce metal ions. Various biomolecule of plant extracts acts as reducing agents during metal oxide synthesis. According to thermodynamics data of different forms of manganese oxides43, a probable mechanism of synthesized MnCO3 and Mn2O3 nanoparticles may be indicated by the following Equations:
The first formed atoms operate as a nucleation core, followed by growing nucleus to form combined crystallites leading to nanoparticles.
The manganese carbonate peaks at XRD patterns of as-synthesized sample are broad. However, after calcination at 300 °C, the the sharp and narrow peaks of pure MnCO3 appeared. The carbonate peaks ‘ intensity decreased considerably, and the low-intense peaks of Mn2O3 came to exist by increasing the calcination temperature to 430 °C. The samples calcined at 480 °C showed the peaks of both MnO and Mn2O3 oxides. Nonetheless, the pure Mn2O3 phase was not formed at this temperature. Two extra peaks are also observed in samples after 600 °C calcination, observable in Fig. 1b. These peaks disappeared by calcination at 650 °C. This temperature was sufficient for carbon dioxide’s omitting.
In brief, the XRD patterns verified the formation of pure green synthesized Mn2O3 nanoparticles after calcination at the temperature of 650 °C for 2.5 h. The peaks of pure α-Mn2O3 detected at nine main positions (2θ) of 23.2°, 32.9°, 38.2°, 45.8°, 49.4°, 55.2°, 60.6°, 64.2°, and 65.8°, which can be attributed to the (211), (222), (400), (332), (431), (440), (611), (541) and (622) plans, respectively44,45,46.
The KMnO4 as a manganese source and the bio-compounds of the Pyracantha angustofolia extract as reducing agents are the reactants of the green synthesis approach. The functional groups of flavonoids, antioxidants, alkaloids, and other phytochemicals of the plant, such as –C–O–C–, –C–O–, –C=C–, O–H, and –C=O–, play essential role in the formation of Mn2O3 nanoparticles which were discussed by various researches47,48. The Pyracantha angustofolia extract comprises high content of carbon, oxygen, and hydrogen elements and their derivatives, such as phenolic compounds, proteins, and hydrocarbons that were released into the extract during its preparation process. There are a large number of hydroxyl groups in these compounds, which act as a reducing agent. These compounds reduce the Mn7+ of KMnO4 to Mn2+. The produced Mn2+ can react with the CO2 that is created in the reaction solution due to the presence of Pyracantha angustofolia angustofolia extract49. In addition, the biomolecules of the extract can increase the durability of nanoparticles by attaching to nanoparticles and also act as a modifier to control the morphology and growth of the crystals during the nanoparticle’s formation50. To better understand of production process, Fig. 2 shows the production process of MnCO3, and α-Mn2O3 nanoparticles and color changing of product in every step.
The other forms of manganese oxide would be produced at a higher calcination temperature. For instance, by increasing the temperature to 800–900 °C, the other forms like MnO2 and Mn3O4 would be produced. However, the objective of this research was the Mn2O3 nanoparticles synthesis.
The crystallite size of the MnCO3 and α-Mn2O3 were estimated using the Deby-Scherrer equation from the XRD data. This equation can be represented by:
where D displays the mean crystallite size (nm), k is a shape factor (usually set to 0.9 for spherical crystallites), λ denotes the incident wavelength (1.54 Å), β is the full width at half maximum (FWHM) of the peak (rad), and θ represents the Bragg angle in this equation51,52,53. The average crystallite size of the MnCO3 and α-Mn2O3 were obtained 17.00 ± 8.92, and 26.32 ± 9.38 nm, respectively. It is concluded that the calcination process resulted in the conversion of manganese carbonate to Mn2O3; however, it had minimal impact on the crystallite size.
The cell parameter was calculated to be 9.405 Å using Unitcell program54 (Version 2017, http://ccp14.cryst.bbk.ac.uk/ccp/ccp14/ftp-mirror/crush/pub/minp/UnitCell/), which is comparable with other reported studies55.
To understand the surface functionality of the green synthesized α-Mn2O3 nanoparticles, the FTIR analysis was carried out. Figure 3 shows the FTIR spectrum of the Pyracantha angustofolia extract, as-synthesized, and α-Mn2O3 nanoparticles in a wavenumber of 4000 to 400 cm−1. The broad absorption band that was observed at 3432 cm−1 designates to the stretching vibration of the hydroxyl group in the water molecule that adsorbed by the powder during the preparation process.
The FTIR spectrum of the pyracantha extract shows the absorption bands related to O–H, C=C, C-N, C-O, and aromatic ring groups due to presence of multiple phytochemicals, such as polysaccharides, phenolic, proteins, and flavonoids compounds56. The sharp and broad peaks at wave numbers 1733, and 1625 cm−1 refer to the C=O group, and the C–O stretching was detected at wave number 1080 cm−157. The C-H bending and C-N stretching can observe at wave number 1425, and 1038 cm−1, respectively58. The FTIR spectrum of manganese carbonate exhibits two distinct types of peaks. The first set of peaks corresponds to the carbonate composition including the two specific bands of \({\text{CO}}_{3}^{2-}\) vibration was detected at 1433 and 859 cm−159. The second set of peaks overlaps with the results of the plant extract, indicating the adsorption of functional groups of the Pyracantha angustofolia extract onto the manganese product, namely, the C–H stretching and bending modes at 2926 and 1425 cm−1, respectively, C–O peak at 1625 cm−1, and the C–N stretching. The observed absorption band at 1612 cm−1 is related to the vibration of the adsorbed H2O.
Upon calcination, the intensity of the peaks that were previously common with those of the plant extract reduced. Conversely, new peaks indicative of the formation of Mn2O3 have emerged. The characteristic peaks at 582, and 523 cm−1 referred to the stretching vibration of Mn–O and Mn–O–Mn bonds which confirmed the creation of Mn2O3 nanoparticles60,61. The combined attaching vibrations of the Mn atoms with –OH groups created the weak peak at 1046 cm−162,63. The vibration of the adsorbed H2O at 1612 cm−1 also showed a lower intensity after calcination.
Shape and size distribution, and elemental composition of produced α-Mn2O3 nanoparticles
The FE-SEM analysis examined the topographic of the green synthesized α-Mn2O3 nanoparticles. SEM images and elemental composition of pure α-Mn2O3 nanoparticles are shown at various magnifications in Fig. 4. The green Mn2O3 nanoparticles appeared in spherical shape with uniform distribution. As observed in Fig. 4, the green Mn2O3 nanoparticles formed the nanocluster in size of \(\sim \)100 to 200 nm. The aggregations of nanoparticles can be attributed to the high surface energy of nanoparticles64. To estimate the particle size of the green Mn2O3 nanoparticles, the 100 particles were selected based on SEM images, and their sizes were measured using ImageJ software. The gathered data was analyzed by Origin software. The particle size distribution of Mn2O3 nanoparticles is presented in Fig. 5. The spherical shape of Mn2O3 nanoparticles appeared in a mean size of 60.67 nm. In addition, the particle size result shows the homogeneous and monodispersed synthesized nanoparticles due to low standard deviation (SD = 6.88). It seems that the biomolecules of Pyracantha angustofolia extract not only conducted manganese ions to form Mn2O3 but also could control the morphology and size of the formed nanoparticles. The energy-dispersive spectroscopy (EDS) analysis confirmed the elemental composition of α-Mn2O3 nanoparticles. The weight percent of Mn and O elements were 63.34 and 27.49, but the atomic percent were obtained 32.36 and 46.20, respectively, which is similar to Jassa et al. report.
It concludes that the formation of the spherical nanoparticles results from the presence of the plant biomolecules such as phenolic and flavonoid compounds65,66. In addition to clean syntheses compared with chemical methods, the green method helps to control size and morphology of nanoparticles, creating monodispersed nano-scaled particles. One of the natural and non-toxic agents for decreasing the size and stabilizing the nanoparticles is the plant extracts due to existing of biomolecules such as flavonoids, phenols, saponins, alkaloids, etc. This bio-compounds affect the morphology, size, and aggregation of nanoparticles67.
Since the chemical synthesis of Mn2O3 led to a large particle size in recent studies, our result confirms the vital role of plant extract in controlling the size of nanoparticles in the reaction solution. Gomaa et al. synthesized Mn2O3 nanoparticles using a chemical route (mixing of ascorbic acid anhydrous with Manganese (II) acetate tetrahydrate) under hydrothermal conditions and produced Mn2O3 particles as a hierarchical triangular prism shape nano-bar. The crystallite size of Mn2O3 was about 91 nm68. The cubic Mn2O3 was fabricated by chemical precipitation method using MnSO4⋅H2O, ethanol, (NH4)2SO4 by He et al.69. Their result showed that the particle size of synthesized Mn2O3 was in the micron range. In the other research, the chemically synthesized porous Mn2O3 nanoparticles were obtained in a particle size of 250 nm for supercapacitor electrode70.
The Effect of Plant Extract in the Formation of a Mesoporous Structure
To determine the surface area (m2.g−1), pore diameter (nm), and pore volume (cm3.g−1) of the MnCO3, and α-Mn2O3 nanoparticles, the Brunauer–Emmett–Teller (BET) analysis was performed with adsorption and desorption of N2 gas by nanoparticles. Figure 6 demonstrated the nitrogen adsorption/desorption isotherm curves of the as-prepared MnCO3, and Mn2O3 nanoparticles, respectively. The isotherm curves closely match a typical type IV isotherm graph with a type H3 hysteresis loop, which reveals the presence of mesoporous structure of the MnCO3, and Mn2O3 nanoparticles71,72. Table 1 presents the result of BET analysis of the produced MnCO3, and Mn2O3 nanoparticles. In addition, the pore size distribution of MnCO3 and Mn2O3 nanoparticles obtained from desorption isotherms are shown in Fig. 6. As observed, the surface area of nanoparticles decreased after calcination and formation of Mn2O3 nanoparticles while the mean pore diameter and pore volume of the green synthesized Mn2O3 nanoparticles increased and decreased, respectively. The small pores in the structure of nanoparticles might join together to form the new pores in a large size during the calcination leading to increasing the pore diameter. Therefore, the larger pore size of Mn2O3 nanoparticles are due to the interconnected pores of MnCO3 nanoparticles during the calcination process. The elimination of CO2 gas during calcination also can increase the pore diameter. This phenomenon was reported by Cheung et al. for MnCO373 and kim et al. for TiO2 nanoparticles74. The surface area of green synthesized Mn2O3 nanoparticles was obtained 21.70 m2.g−1. Pudukudy et al.51 produced Mn2O3 nanoparticles using chemical synthesis with almost the same surface area and reported that the synthesized Mn2O3 has a higher surface area than the commercial powder Mn2O3. Based on the BET result, the green fabricated α-Mn2O3 nanoparticles are classified under mesoporous material due to low pore diameters.
Adsorption study of MO and CR dyes
Effect of pH
The final adsorption percentage of the MO and CR dyes are presented in Fig. 7 at different pHs (2, 7, and 12). With a rise in pH to seven, the uptake of CR neared zero, while MO experienced a removal of approximately 65%. At a pH of 12, the adsorption of MO increased by around 5%, whereas CR exhibited decrease in adsorption. As it shown in Fig. 7, the pH of 2 was found the optimum pH for two anionic dyes in all experiments.
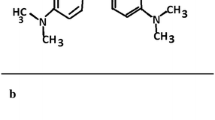
The pH is the key factor in the adsorption process. In a low pH (acidic solution), the dye solution would be positively charged due to the existence of protons. The protons can change the surface charge of nanoparticles to positive. The positively charged adsorbent can attract the negative anionic dye molecules electrostatically. At a high pH (base solution), OH- ions in the reaction solution can influence the surface charge of the adsorbent nanoparticles and make them negatively charged. The electrostatic interaction between the negatively charged surface and negative anionic dye molecules decreased the adsorption. However, 45% of CR dye was still removed in pH equal to 12, implying an additional mechanism than electrostatic force for uptake of CR. Increasing the removal percentage of MO at pH 12 compared to pH equal to 7 also revealed that there are other mechanisms for dye removal besides electrostatic force, which play a more prominent role in CR adsorption compared to MO. Comparing the structures of the two dyes reveals that CR has more potential for establishing hydrogen bonds due to its greater number of hydrogen bond-forming heads, while MO has fewer. A probable mechanism of dye adsorption is presented in Fig. 8.
Furthermore, the FTIR spectrum also demonstrated that Mn2O3 possesses hydroxyl groups, and even its structural oxygen can form hydrogen bonds. The adsorbed hydroxyl groups in the basic environment can also participate in the hydrogen bonding formation. Therefore, by comparing the results, it may be suggested that adsorption is not only driven by electrostatic force but also involves other mechanisms including the formation of hydrogen bonds. The pH of 2 was considered as an optimum pH for all tests of anionic dyes adsorption due to a higher removal percentage.
The pHPZC was determined to investigate the role of electrostatic force in adsorption process. Figure 9 demonstrates the result of the pH drift test for determining the pHPZC. It shows that the pHPZC is ~ 7.7 for the Mn2O3 nanoparticles.
To better understand of dye removal process and explain the dye adsorption, the FTIR spectra of the synthesized α-Mn2O3 nanoparticles before and after adsorption of CR and MO dyes were studied (Fig. 10). As mentioned above, in the FTIR spectra of α-Mn2O3 nanoparticles before adsorption, an intense and sharp peak was observed at about 3443 cm−1 which corresponded to the elongation of OH and showed the presence of free hydroxyl groups on the surface of α-Mn2O3 nanoparticles. However, the intensity of this peak differs from α-Mn2O3 nanoparticles after dye adsorption, especially CR dye. The difference of the FTIR spectra of α-Mn2O3 nanoparticles before and after dye adsorption is obvious at 582, and 523 cm−1 referred to the stretching vibration of Mn–O and Mn–O–Mn bonds. As observed, the sharpness and intensity of these peaks were changed remarkably after dye adsorption with no chang in the positions of the peaks, which confirms the inclusion of CR and Mo dyes in the surface of α-Mn2O3 nanoparticles after adsorption process. Moreover, a new peak at 421 cm−1 wavenumber come to exist after adsorption of two dyes which is attributed to C–O or C–H groups. in brief, the adsorption of both CR and MO causes the creation of new C–O and C–H bonds and decrement of intensities of the peaks in 400–450 cm−1 wavenumbers. In addition, adsorption of CR is accompanied by involving the OH groups too. These results confirm the previous discussion about the mechanism of the adsorption of CR and MO.
Adsorption kinetics
To determine the dye removal capacity of Mn2O3 nanoparticles and the adsorption rate of the MO and CR dyes, the study of the adsorption kinetic is crucial. The adsorption kinetic can be influenced by various factors, such as the dye concentration, dye removal time, type of adsorbent, and the ratio of adsorbent to the dye solution. In this regard, the percentage removal of two anionic dyes was estimated using synthesized Mn2O3 nanoparticles as adsorbent under different dye concentrations (25, 50, and 75 ppm) in about 2 h in a certain period. According to Fig. 11, the quantity of adsorbed CR and MO dyes (qt) increased with increasing the decoloring time and initial dye concentration. As observed, the adsorption kinetic of the two dyes were distinctly different. The dye uptake of MO is higher than CR dye in the first 20 min as a result in removing dye about 95% for MO. Even though, the CR dye removal was about 80% in the first 20 min. Therefore, the low speed of CR dye uptake is obvious. The molecular mass of CR and MO are 695.66 and 327.33 g.mol−1, respectively. In addition, the volume per unit molecular structure of CR is greater than that of MO. The rapid uptake of MO can be attributed to a lower molecular weight than CR dye. The number of molecules of MO that can attach to nanoparticles' surface will be more than the molecules of CR dye due to its lighter and smaller molecules. As a result, the MO dye removal capacity of nanoparticles would be higher compared with CR in a same period. After equilibration of the adsorption process, the maximum dye removal capacity of synthesized nanoparticles was 70.70 and 73.07 mg. g−1 for CR and MO, respectively. Our results showed that the produced manganese oxide can highly adsorb anionic dyes.
In the dye adsorption studies for Mn2O3 reported by some researchers, the dye adsorption capacity is lower than that of the α-Mn2O3 nanoparticles produced in this study. In some studies, other metal oxides have been used in addition to α-Mn2O3 nanoparticles to increase the dye adsorption capacity. In this study, pure α-Mn2O3 was used to remove anionic dyes, and the adsorption results showed a high efficiency of these nanoparticles. Table 2 refers to some of these studies.
Three different kinetic models: pseudo-first order (PFO), pseudo-second order (PSO), and intraparticle diffusion (IPD)79,80,81 were studied to evaluate the kinetic model of MO and CR dyes adsorption onto green synthesized Mn2O3 nanoparticles. Evaluation of the kinetic models was carried out based on the regression coefficient, (\({R}^{2}\)), and comparing the quantity of dye adsorbed at the saturation state of nanoparticles (after about 2 h) and calculated adsorbed each dye (qe) from each kinetic model. The kinetic data were presented in Table 3. As shown in Fig. 12, the best fit of experimental data for both CR and MO dyes was obtained with the PSO model. Although the experimental data was well fitted with PSO models for both anionic dyes considering R-squared values, the calculated qe for MO dye was much closer to the experimentally determined value compared with qe of the CR dye. The calculated maximum adsorbed dye was found to be 78.74, and 72.97 mg. g−1, respectively, for CR and MO dyes.
The relative errors for the PSO model of CR and MO were obtained 11.4 and 0.1%, respectively. In the other two models, this relative error was more than 70%. Since both anionic dyes adsorption followed PSO model. This result reflects that the boundary layer resistance would not be the adsorption limiting step82.
Adsorption isotherms
Studying adsorption isotherms is crucial in the examination of adsorption processes due to their importance in realizing the adsorption behavior. The adsorption isotherms were studied to explain the correlation between the quantity of adsorbed dye onto nanoparticles (qe) and the concentration of dye solution in the equilibrium state (Ce) at the temperature of 25 \(^\circ{\rm C} \) and pH of 2 for about two hours of adsorption process. Langmuir, Freundlich, Dubinin–Radushkevich, and Temkin as isotherm models were studied to describe the adsorption mechanism of CR and MO dyes onto green Mn2O3 nanoparticles (Fig. 13).
The calculated parameters of each isotherm for CR and MO dyes are displayed in Table 4. Considering the calculated constant of isotherms, each dye follows a different isotherm. Considering the \({R}^{2}\) values, the adsorption of CR follows the Dubinin–Radushkevish isotherm, while the adsorption of MO adheres to the Langmuir isotherm model. This means that MO molecules homogeneously adsorb on the positively charged surface of nanoparticles without interactions with adjacent sites. Therefore, it can be implied that the adsorption of MO dye occurs uniformly onto the active sites of nanoparticles. In the Langmuir isotherm, the adsorbed MO dye using nanoparticles (Q0 = 78.74 mg.g−1) was estimated to be closer to the trial data (with about 7.7% relative error) than the adsorbed CR dye in value of 120.48 mg.g-1 (with about 70% relative error). Therefore, the parameters of the Langmuir isotherm are more fit with the experimental data of MO dye adsorption. The Dubinin–Radushkevish isotherm tries to describe the adsorption based on the filling of micropores volume of adsorbent and single dye molecule adsorption83. This model is superior to the Langmuir model due to considering the \({B}_{D}\) as a parameter of adsorption energy and \(\varepsilon \) (\(RTln(1+\frac{1}{{C}_{e}})\)) as an adsorption potential factor84. The maximum adsorbed CR was calculated to be 78.814 mg.g-1 with \({R}^{2}\) of 0.99. Consequently, it is clear that the adsorption of CR is more correlated to the surface area and porosity of green synthesized nanoparticle than that of MO.
The average free energy of adsorption can be measured using the following Eq. 6:
Physical adsorption leads to E < 8, while chemical ion-exchange or chemisorption has 8 < E < 1685. In this study the E for CR was estimated to be 1.23 kJ. mol−1 implying the physical nature of CR adsorption on the Mn2O3 nanoparticles. Dyes are mainly adsorbed by hydrophobic, electrostatic attraction, and hydrogen bonding86 which are all physical adsorption. Hydrophobic interactions are absent in this study. On the other hand, as mentioned in Sect. 3.5.1, since the CR was adsorbed at a pH of 12, it appears that hydrogen bonding is involved in adsorption process. In fact, the isotherm studies confirm the previous discussion about the various mechanisms of the adsorption of CR and MO on green synthesized Mn2O3.
Thermodynamic Studies
To evaluate the temperature effect on the adsorption of CR and MO dyes onto the Mn2O3 nanoparticles, the adsorption process was accomplished at three various temperatures (298, 318, and 338 K). Some vital thermodynamic data, such as entropy change (∆S°, kJ. kmol−1.K-1), enthalpy change (∆H°, kJ. kmol−1) , and Gibbs free energy change (∆G°, kJ. kmol−1) were accurately evaluated for two dyes. Studying these parameters is crucial in recognizing the feasibility and energetics of adsorption. In this research, thermodynamic parameters were calculated according to Eqs. (7) and (8)87,88,89.
where the \({q}_{e}\) and \({C}_{e}\), milligrams of dye adsorbed per one gram of adsorbent and dye solution concentration after reaching equilibrium, respectively. The R and T are the gas constant (8.314 j.mol−1.K−1) and the absolute temperature (K) of the dye solution, respectively. The thermodynamic parameters were obtained by line plotting of the ln \(\frac{{q}_{e}}{{C}_{e}}\) data versus \(\frac{1}{T}\) at various temperatures and the obtained parameters were presented in Table 5 for dye concentration of 75 ppm for 45 min. ∆G° of adsorption can help to evaluate the nature of the adsorption. It is known that ∆G° for physisorption ranges between −20 and 0 kj.mol−1 and ΔH° in the range of 2.1–20.9 kj.mol−1, while for chemisorption ∆G° and ΔH° are in the range of −20 and −80 kj.mol−1 and −80 to −400 kj.mol−1 respectively90. The thermodynamic parameters that are tabulated in Table 5 express the physisorption of both dyes on the Mn2O3 nanoparticles. This result confirms the previous discussion about the adsorption mechanism.
According to Table 5, the result indicates that increasing the temperature leads to slightly increases in the adsorbed quantities of CR and MO dyes after 45 min. However, this increas is not significant for MO dye. It is concluded that the adsorbed quantity of MO is not influenced by the temperature. In addition, the negative and positive signs of ∆G° and ∆H°, respectively indicat that the adsorption of both anionic dyes was spontaneous and endothermic. The positive quantity of ∆S° designates an irregular rise of the randomness at the nanoparticles–dye solution boundary over the adsorption process. The adsorption was endothermic due to positive values of enthalpies, and ∆G° declined with growing the temperature for both dyes. This means that the adsorption is more favorable at higher temperatures which is confirmed by increasing the qe by increasing the temperature. However, the results confirm that the adsorption of MO and CR was slightly influenced by the temperature. Additionally, the more negative ∆G0, larger qe, and smaller ∆H0 of MO adsorption reveal the more facile adsorption of this dye at pH equal to 2.
Reusability of α-Mn2O3 nanoparticles
From an economic point of view, the reusability of adsorbents is key factor. the reusability of α-Mn2O3 nanoparticles after adsorption of CR and MO dyes (with a concentration of 50 ppm) was investigated using a 0.1 M NaOH solution at identical temperature and time for the desorption studies. After the desorption, the collected adsorbent was washed with distilled water and dried for four hours at 100 °C. As observed in Fig. 14, the dye removal capacity of reused α-Mn2O3 nanoparticles was not changed significantly after three consecutive cycles, which implies that α-Mn2O3 nanoparticles have the acceptable reusability characteristic at the anionic dye adsorption process.
Materials and methods
Chemicals and reagents
The chemical materials were purchased from Sigma-Aldrich, included NaOH, HCL, and KMNO4, which were used without further purification. Congo red (CR) and methyl orange (MO) were obtained from Merck company. All solutions were made with distilled water.
Preparation of aqueous Pyracantha angustofolia extract
The 11-year-old pyracantha plants were originally provided from Hashempour Nursery Stock located in Garmdareh, Karaj city and cultivated in ornamental plant collection of University of Zanjan. The plants were identified as Pyracantha angustofolia by Dr. Mohsen Sanikhani head of the Department of Horticultural Science, University of Zanjan. The Pyracantha plant’s fresh fruits were harvested from the ornamental plant collection of University of Zanjan in autumn 2022. The first step involved drying the Pyracantha angustofolia fruits in a shaded environment. Subsequently, the extraction process was carried out as follows: the dried Pyracantha angustofolia fruits were pulverized and the resulting powder was immersed in distilled water with a ratio of 1–10 (g.ml−1) for four hours at room temperature. The biomolecules extraction was carried out at 70 °C using a heater stirrer for two hours. The supernatant was separated by centrifugation at a speed of 4000 rpm and filtered through Whatman paper to eliminate any residual Pyracantha angustofolia powder. The resulting filtrate, containing the aqueous Pyracantha angustofolia extract, was utilized for the synthesis of nanoparticles.
Synthesis of the α-Mn2O3 nanoparticles
The α-Mn2O3 nanoparticles were synthesized through the reduction of KMNO4 using Pyracantha angustofolia extract. The Pyracantha angustofolia extract and a 200 Mm KMNO4 solution were mixed dropwise in equal volumes. The resulting mixture was then poured into the Teflon chamber of a stain-less steel reactor and incubated at 160 °C for 15 h. The produced particles were collected using centrifuge at 4000 rpm for 15 min. The produced brown powder was washed with distilled water to eliminate unwanted ions and residual Pyracantha angustofolia extract three times. The precipitated nanoparticles were dried at 120 °C for two hours and then calcined at temperatures of 300, 400, 480, 600, and 650 °C. The obtained powders were stored in the sealed containers for further analysis. The synthesis of α-Mn2O3 Nanoparticles is shown in Fig. 15 step by step.
Characterization of the green synthesized α-Mn2O3 nanoparticles
The green synthesized α-Mn2O3 nanoparticles were characterized through various analyses including Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET). The XRD patterns were obtained with a Cu-Kα radiation source with a scan rate of 0.05os−1 in the 2θ range of 5°–80°. the XRD measurements were performed using an X-ray diffractometer (X‘Pert Pro, Panalytical, Holland) operated at 40 kV and 30 mA, with an exposure time of 300 s. Infrared absorption spectrum of specimens in the range of 500–4000 cm–1 was recorded using an FTIR spectrophotometer (THERMO, Nicolet is50, USA).
Brunauer–Emmett–Teller (BET) and Barret–Joyner–Halenda (BJH) analyses were conducted to determine the pore volume, pore radius, porosity, and surface area of nanoparticles. These analyses were performed using a Brunauer–Emmett–Teller Surface Area & Porosity Analyzer (BELSORP MINI X,Microtrac MRB, Japan, Osaka). The morphology of the synthesized nanoparticles was examined using a FE-SEM (TESCAN, MIRA III, Ceska Republika). Additionally, the energy-dispersive spectroscopy (EDS) analysis was used to obtain the elemental composition of the synthesized nanoparticles. The point zero charge (PZC) of the samples was measured using the pH drift method. pH drift method was applied to evaluate the pHPZC of the Mn2O3 sample. In brief, the initial pH of 20 ml of 0.05 M NaCl solution was adjusted between 2 and 12 using 0.1 M HCl and NaOH. Then, Mn2O3 nanoparticles, 0. 1 g, were added to each solution, and the change in pH was recorded after 24 h of maintenance on the shaker incubator. The pHPZC point is a pH that is not altered after 24 h.
Dye adsorption experiments
Batch adsorption experiments were conducted on MO and CR dyes under varying pH conditions (pH of 2, 7, and 12) and initial dye concentrations (25, 50 and 75 ppm). The experiments were carried out in sealed glass bottles, where 60 mL of dye solution and 0.06 g of the green synthesized Mn2O3 nanoparticles were used. Dye adsorption tests were accomplished using a heater stirrer at a constant speed of 130 rpm for 135 min. Samples were collected at specified time intervals, followed by centrifugation. The optical density of supernatants was determined with a UV–visible spectrophotometer. Calibration curves were obtained by preparing solutions of MO and CR dyes at specific concentrations, 5 ppm, 15 ppm, 25 ppm, 50 ppm, and 75 ppm, and measuring their absorbance at 464 nm, and 498 nm, respectively.
The percentage of dye removal was calculated using the following equation91:
where \({C}_{i}\) represents the initial dye concentration,\(and {C}_{t}\) the concentration of the dye solution after a specific adsorption time. The quantity of adsorbed dye was computed using Eq. 1092:
The \({q}_{t} (mg.{g}^{-1})\) is the milligrams of adsorbed dye (mg) per one gram of adsorbent at a certain time. W and Q are the weight of the α-Mn2O3 nanoparticles (g) and the volume of the treated solution (L), respectively. The definition of \({C}_{i}, and {C}_{t}\) are the same as Eq. 1.
Conclusions
Dye removal from polluted water with efficient and cost-effective adsorbents has always been of interest to researchers. Besides, facile, and eco-friendly synthesis of adsorbents is vital. For these reasons, the α-Mn2O3 nanoparticles were synthesized using Pyracantha angustofolia extract under hydrothermal conditions for the first time following calcination. Indeed, compared to chemical synthesis methods, our method substituted the expensive and hazardous chemical-reducing agent with cheap and safe plant extract.
The calcination and decomposition of as-synthesized MnCO3 were investigated at various temperatures to determine the optimum temperature for Mn2O3 formation. Based on the result of FTIR, XRD, SEM, and BET surface area analyses, the temperature of 650 °C was selected as the optimum temperature for pure α-Mn2O3 nanoparticles production. The SEM result verified the formation of the spherical Mn2O3 nanoparticles in a mean diameter of 60.68 nm which were monodispersed nanoparticles. The impact of pH value, temperature, and initial concentration of dye solution was evaluated on dye removal over time. The reduction in pH, and an increase in the initial dye concentration and temperature, enhanced the adsorption capacity of the synthesized nanoparticles for both CR and MO dyes. The maximum adsorbed CR and MO dyes were obtained 70.70 and 73.07 mg.g−1, respectively at an initial dye concentration of 75 ppm. The pseudo-second-order kinetic model was well fit with adsorption data. To understand the adsorption mechanisms, the adsorption isotherm models such as Langmuir, Freundlich, Dubinin–Radushkevich, and Temkin were studied. The result indicated that the MO and CR dyes removal followed Langmuir and Dubinin–Radushkevich, respectively, which revealed the monolayer adsorption for the MO adsorption. Based on thermodynamic studies, both dyes adsorption was endothermic and spontaneous with increasing randomness. Finally, proposed mechanisms were suggested for both dye adsorption. The comprehensive study of dye adsorption in this research led to the conclusion that the α-Mn2O3 nanoparticles would be promising candidate for wastewater treatment.
Data availability
All experimental results analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BET:
-
Brunauer–Emmett–Teller
- CR:
-
Congo red
- FTIR:
-
Fourier transform infrared spectroscopy
- FWHM:
-
Full width at half maximum
- IPD:
-
Intraparticle diffusion
- MO:
-
Methyl orange
- PFO:
-
Pseudo-first order
- PSO:
-
Pseudo-second order
- SEM:
-
Scanning electron microscopy
- UV:
-
Ultraviolet
- XRD:
-
X-ray diffraction
References
Hanafi, M. F. & Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today: Proc. 31, 141–150 (2020).
Robinson, T., McMullan, G., Marchant, R. & Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77(3), 247–255 (2001).
Saini, R. D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 9(1), 121–136 (2017).
Ali, S. S., Al-Tohamy, R. & Sun, J. Performance of Meyerozyma caribbica as a novel manganese peroxidase-producing yeast inhabiting wood-feeding termite gut symbionts for azo dye decolorization and detoxification. Sci. Total Environ. 806, 150665 (2022).
Lellis, B., Fávaro-Polonio, C. Z., Pamphile, J. A. & Polonio, J. C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 3(2), 275–290 (2019).
Almroth, B. C. et al. Assessing the effects of textile leachates in fish using multiple testing methods: From gene expression to behavior. Ecotoxicol. Environ. Saf. 207, 111523 (2021).
Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 97(9), 1061–1085 (2006).
Rauf, M. A. & Ashraf, S. S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 209, 520–530 (2012).
Mukherjee, A., Dhak, P., Hazra, V., Goswami, N. & Dhak, D. Synthesis of mesoporous Fe/Al/La trimetallic oxide for photodegradation of various water-soluble dyes: Kinetic, mechanistic, and pH studies. Environ. Res. 217, 114862 (2023).
Karim, S., Ahmad, N., Hussain, D., Mok, Y. S. & Siddiqui, G. U. Active removal of anionic azo dyes (MO, CR, EBT) from aqueous solution by potential adsorptive capacity of zinc oxide quantum dots. J. Chem. Technol. Biotechnol. 97, 2087–2097 (2022).
Wang, Z., Chen, Z. & Ye, C. Enhancing methyl orange adsorption capacity via functionalizing ZIF-8 with amphiphilic cetylpyridinium bromide. Taiwan Inst. Chem. Eng. 146, 104865 (2023).
Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 28, 10715–10739 (2021).
Haque, M. M. et al. Decolorization, degradation and detoxification of mutagenic dye Methyl orange by novel biofilm producing plant growth-promoting rhizobacteria. Chemosphere 346, 140568 (2024).
Ain, Q. U., Rasheed, U., Chen, Z., He, R. & Tong, Z. Activation of Fe3O4/bentonite through anchoring of highly dispersed and photo-reduced Cu ions for higher pH fenton-like degradation and effective adsorption of Congo red dye. J. Ind. Eng. Chem. 134, 327–342 (2024).
Dalvi, A., Hubale, V. & Sawant, V. Two dimensional Co-based metal organic framework for selective adsorption of Congo red from wastewater. Sep. Purif. Technol. 348, 127712 (2024).
Mukherjee, A., Dhak, P., Mandal, D. & Dhak, D. Solvothermal synthesis of 3D rod-shaped Ti/Al/Cr nano-oxide for photodegradation of wastewater micropollutants under sunlight: a green way to achieve SDG: 6. Environ. Sci. Pollut. Res., 1–16 (2023).
Khan, M. D., Singh, A., Khan, M. Z., Tabraiz, S. & Sheikh, J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J. Water Process. Eng. 53, 103579 (2023).
Ismail, M. et al. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 25, 3645–3663 (2019).
Bożęcka, A., Orlof-Naturalna, M. & Kopeć, M. Methods of dyes removal from aqueous environment. J. Ecol. Eng. 22, 111–118 (2021).
Katheresan, V., Kansedo, J. & Lau, S. Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 6, 4676–4697 (2018).
Chunfeng, W., Jiansheng, L. I., Lianjun, W., Xiuyun, S. U. N. & Huang, J. Adsorption of dye from wastewater by zeolites synthesized from fly ash: Kinetic and equilibrium studies. Chin. J. Chem. Eng. 17, 513–521 (2009).
Qin, W. H. et al. High capacity and selective adsorption of Congo red by cellulose-based aerogel with mesoporous structure: Adsorption properties and statistical data simulation. Int. J. Biol. Macromol. 259, 29137 (2024).
Gupta, V. K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manage. 90, 2313–2342 (2009).
Haleem, A., Shafiq, A., Chen, S. Q. & Nazar, M. A comprehensive review on adsorption, photocatalytic and chemical degradation of dyes and nitro-compounds over different kinds of porous and composite materials. Molecules 28, 1081 (2023).
Mukherjee, A., Goswami, N. & Dhak, D. Photocatalytic remediation of industrial dye waste streams using biochar and metal-biochar hybrids: A critical review. Chem. Afr. 6, 609–628 (2023).
Sridevi, H., Bhat, R. & Selvaraj, R. Removal of an agricultural herbicide (2, 4-Dichlorophenoxyacetic acid) using magnetic nanocomposite: A combined experimental and modeling studies. Environ. Res. 238, 117124 (2023).
Juturu, R., Murty, V. R. & Selvaraj, R. Efficient adsorption of Cr (VI) onto hematite nanoparticles: ANN, ANFIS modelling, isotherm, kinetic, thermodynamic studies and mechanistic insights. Chemosphere 349, 140731 (2024).
Selvaraj, R. et al. Machine learning and statistical physics modeling of tetracycline adsorption using activated carbon derived from Cynometra ramiflora fruit biomass. Environ. Res. 252, 118816 (2024).
Fardood, S. T. et al. Green synthesis and characterization of α-Mn2O3 nanoparticles for antibacterial activity and efficient visible-light photocatalysis. Sci. Rep. 14, 6755 (2024).
Patra, T. et al. Effect of calcination temperature on morphology and phase transformation of MnO2 nanoparticles: A step towards green synthesis for reactive dye adsorption. Chemosphere 288, 32472 (2022).
Martinez de la Torre, C., Grossman, J. H., Bobko, A. A. & Bennewitz, M. F. Tuning the size and composition of manganese oxide nanoparticles through varying temperature ramp and aging time. PLoS One 15, 0239034 (2020).
Vignesh, R., Prabha, C. N., Sivakumar, R. & Sanjeeviraja, C. Optical constants, optical dispersion and group index parameters of Mn2O3 thin films. Phys. B: Condens. 624, 413431 (2022).
Chen, F. et al. Controllable synthesis of novel nanoporous manganese oxide catalysts for the direct synthesis of imines from alcohols and amines. Chin. J. Chem. Eng. 27, 2438–2446 (2019).
Ghaffari, Y., Gupta, N. K., Bae, J. & Kim, K. S. One-step fabrication of Fe2O3/Mn2O3 nanocomposite for rapid photodegradation of organic dyes at neutral pH. J. Mol. Liq. 315, 113691 (2020).
Soufi, A. et al. One-step fabrication of coral-like α-Fe2O3/α-Mn2O3 nanocomposite via gel-thermal decomposition for enhanced removal of Acid Yellow 23 dye using Fenton-like reaction. Inorg. Chem. Commun. 154, 110995 (2023).
Mounika, T., Belagali, S. L. & Vadiraj, K. T. Manganese oxide nanoparticles synthesis route, characterization and optical properties. Mater. Today: Proc. 75, 72–76 (2023).
Ying, S. et al. Green synthesis of nanoparticles: current developments and limitations. Environ. Technol. Innov. 26, 102336 (2022).
Mahboub, S., Zerrouki, D. & Henni, A. Green synthesis and characterization of silver nanoparticles using Juniperus communis leaf extract: Catalytic activity in real-outdoor conditions and electrochemical properties. Appl. Organomet. Chem. 34, e5956 (2020).
Jadhav, V. et al. Role of Moringa oleifera on green synthesis of metal/metal oxide nanomaterials. J. Nanomater. 2022, 1–10 (2022).
Lu, H., Zhang, Y. & Liu, P. Mn2O3 microcubes with three-dimensional porous network structure as electrochemical sensing material for nitrite. J. Appl. Electrochem. 46, 1059–1065 (2016).
Pei, X. Y., Mo, D. C., Lyu, S. S., Zhang, J. H. & Fu, Y. X. Synthesis of MnCO3/multiwalled carbon nanotube composite as anode material for lithium-ion batteries. J. Nanosci. Nanotechnol. 19, 5743–5749 (2019).
Deng, Y. et al. Porous Mn2O3 microsphere as a superior anode material for lithium ion batteries. RSC Adv. 2(11), 4645–4647 (2012).
Jacob, K. T., Kumar, A., Rajitha, G. & Waseda, Y. Thermodynamic data for Mn3O4, Mn2O3 and MnO2. High Temp. Mater. Process. 30, 459–472 (2011).
Chandiran, K. et al. Long single crystalline α-Mn2O3 nanorods: facile synthesis and photocatalytic application. Mater. Res. Express 7, 074001 (2020).
Zhang, J. et al. Constructing three-dimensional Mn2O3 catalysts with various morphologies for catalytic oxidation of benzene. Appl. Organomet. Chem. 37, 7010 (2023).
Yang, G., Yan, W., Wang, J. & Yang, H. Fabrication and formation mechanism of Mn2O3 hollow nanofibers by single-spinneret electrospinning. Cryst. Eng. Comm. 16, 6907–6913 (2014).
Huang, J. et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18, 105104 (2007).
Shafey, A. M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 9, 304–339 (2020).
Du, W., Xu, X., Zhang, D., Lu, Q. & Gao, F. Green synthesis of MnOx nanostructures and studies of their supercapacitor performance. Sci. China. Chem. 58, 627–633 (2015).
Pandian, C. J., Palanivel, R. & Dhananasekaran, S. Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin. J. Chem. Eng. 23, 1307–1315 (2015).
Pudukudy, M., Yaakob, Z. & Rajendran, R. Facile synthesis of mesoporous α-Mn2O3 microspheres via morphology conserved thermal decomposition of MnCO3 microspheres. Mater. Lett. 136, 85–89 (2014).
Shabani, M., Saebnoor, E., Hassanzadeh-tabrizi, S. A. & Bakhsheshi-Rad, H. R. Novel synthesis of nickel ferrite magnetic nanoparticles by an in-liquid plasma. J. Mater. Sci. Mater. Electron 32, 10424–10442 (2021).
Aydoghmish, S. M., Hassanzadeh-Tabrizi, S. A. & Saffar-Teluri, A. Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Ceram. Int. 45, 14934–14942 (2019).
Holland, T. J. B. & Redfern, S. A. T. Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral. Mag. 61, 65–77 (1997).
Sharrouf, M., Awad, R., Roumie, M. & Marhaba, S. Structural, optical and room temperature magnetic study of Mn2O3 nanoparticles. Materials Sciences and Applications 6, 850 (2015).
Chang, R. Bioactive polysaccharides from traditional Chinese medicine herbs as anticancer adjuvants. J. Altern. Complement. Med. 8, 559–565 (2002).
Miao, X. et al. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B 227, 459–469 (2018).
Kaushik, N. et al. Antiproliferative activity of pyracantha and paullinia plant extracts on aggressive breast and hepatocellular carcinoma cells. Appl. Sci. 10, 7543 (2020).
Swathi, S. et al. Ethylene glycol assisted MnCO3electrocatalyst for water oxidation and hydrogen production application. Fuel 302, 121151 (2021).
Yang, S. et al. Manganese oxide nanocomposite fabricated by a simple solid-state reaction and its ultraviolet photoresponse property. Chem. Commun. 47, 2619–2621 (2011).
Chen, H. & He, J. Facile synthesis of monodisperse manganese oxide nanostructures and their application in water treatment. J. Phys. Chem. C 112, 17540–17545 (2008).
Bharath, G. et al. Synthesis of hierarchical Mn3O4 nanowires on reduced graphene oxide nanoarchitecture as effective pseudocapacitive electrodes for capacitive desalination application. Electrochim. Acta 337, 135668 (2020).
Amirtharaj, S. N. & Mariappan, M. Rapid and controllable synthesis of Mn2O3 nanorods via a sonochemical method for supercapacitor electrode application. Appl. Phys. A 127, 607 (2021).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS nano. 4, 4806–4814 (2010).
Ahmad, T. et al. Green synthesis of stabilized spherical shaped gold nanoparticles using novel aqueous Elaeis guineensis (oil palm) leaves extract. J. Mol. Struct. 1159, 167–173 (2018).
Sundrarajan, M. et al. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol B Biol. 171, 117–124 (2017).
Ishak, N. M., Kamarudin, S. K. & Timmiati, S. N. Green synthesis of metal and metal oxide nanoparticles via plant extracts: An overview. Mater. Res. Express 6, 112004 (2019).
Gomaa, I., Abdel-Salam, A. I., Khalid, A. & Soliman, T. S. Fabrication, structural, morphological, and optical features of Mn2O3 polyhedron nano-rods and Mn2O3/reduced graphene oxide hybrid nanocomposites. Opt. Laser Technol. 161, 109126 (2023).
He, Q., Liao, X., Li, G., He, Y. & Shen, J. Synthesis of cubic Mn2O3 and its catalytic performance in activating peroxymonosulfate for degradation of MB. Chem. Phys. 571, 111912 (2023).
Shao, D. et al. Synthesis of porous Mn2O3 architecture for supercapacitor electrode application. Colloids Surf. A Physicochem. Eng. Asp. 658, 130532 (2023).
Al-Salihi, S., Bayati, M., Jasim, A. M., Fidalgo, M. M. & Xing, Y. Magnetic mesoporous TiO2/Fe3O4 nanocomposite adsorbent for removal of sulfamethazine from water. Environ. Adv. 9, 100283 (2022).
Xu, L. et al. Pore structure and fractal characteristics of different shale lithofacies in the Dalong formation in the western area of the lower yangtze platform. Minerals 10, 72 (2020).
Cheung, O. et al. Nanostructure and pore size control of template-free synthesised mesoporous magnesium carbonate. RSC Adv. 6, 74241–77429 (2016).
Kim, M. G. et al. Effects of calcination temperature on the phase composition, photocatalytic degradation, and virucidal activities of TiO2 nanoparticles. ACS Omega 6, 10668–10678 (2021).
Nassar, M. Y., Amin, A. S., Ahmed, I. S. & Abdallah, S. Sphere-like Mn2O3 nanoparticles: facile hydrothermal synthesis and adsorption properties. J. Taiwan Inst. Chem. Eng. 64, 79–88 (2016).
Shao, Y. et al. Dual-porosity Mn2O3 cubes for highly efficient dye adsorption. J. Hazard. Mater. 333, 222–231 (2017).
Qi, G. et al. Synthesis of mono-dispersed mesoporous Mn2O3 powders with micro-nanostructure for removing Congo red dye from aqueous solution. Adv. Powder Technol. 30, 930–939 (2019).
Rout, D. R., Chaurasia, S. & Jena, H. M. Enhanced photocatalytic degradation of malachite green using manganese oxide doped graphene oxide/zinc oxide (GO-ZnO/Mn2O3) ternary composite under sunlight irradiation. J. Environ. Manage. 318, 115449 (2022).
Elmoubarki, R. et al. Adsorption of textile dyes on raw and decanted Moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resour. Ind. 9, 16–29 (2015).
Mirzaei, K., Mohammadi, A., Jafarpour, E., Shojaei, A. & Moghaddam, A. L. Improved adsorption performance of ZIF-8 towards methylene blue dye by hybridization with nanodiamond. J. Water Proc. Engineering. 50, 103254 (2022).
Jyoti Bora, H., Paul, C., Sen Sarma, N. & Kalita, A. Facile synthesis of regenerative framework adsorbent for organic dyes: Experimental and artificial neural modelling studies. ChemistrySelect. 45, 03766 (2022).
Ho, Y. S. & McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 34, 451–465 (1999).
Hu, Q. & Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 277, 646–648 (2019).
Kaur, S., Rani, S., Mahajan, R. K., Asif, M. & Gupta, V. K. Synthesis and adsorption properties of mesoporous material for the removal of dye safranin: Kinetics, equilibrium, and thermodynamics. J. Ind. Eng. Chem. 22, 19–27 (2015).
Zhou, Y. et al. Adsorption optimization of uranium (VI) onto polydopamine and sodium titanate co-functionalized MWCNTs using response surface methodology and a modeling approach. Colloids Surf. A Physicochem. Eng. Asp. 627, 127145 (2021).
Hu, Y. et al. Dye adsorption by resins: effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 228, 392–397 (2013).
Mohamed, N. A., El-Ghany, N. A. & Fahmy, M. M. Novel antimicrobial superporous cross-linked chitosan/pyromellitimide benzoyl thiourea hydrogels. Int. J. Biol. Macromol. 82, 589–598 (2016).
Elsayed, N. H., Monier, M. & Youssef, I. Fabrication of photo-active trans-3-(4-pyridyl) acrylic acid modified chitosan. Carbohydr. Polym. 172, 1–10 (2017).
Spiridon, I., Apostol, I., Anghel, N. C. & Zaltariov, M. F. Equilibrium, kinetic, and thermodynamic studies of new materials based on xanthan gum and cobalt ferrite for dye adsorption. Appl. Organomet. Chem. 36, 6670 (2022).
Thuy Luong Thi, T., Ta, H. S. & Le Van, K. Activated carbons from coffee husk: Preparation, characterization, and reactive red 195 adsorption. J. Chem. Res. 45(5–6), 380–394 (2021).
Çelebi, H., Gök, G. & Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Sci. Rep. 10(1), 17570 (2020).
Liu, H., Zhang, F. & Peng, Z. Adsorption mechanism of Cr (VI) onto GO/PAMAMs composites. Sci. Rep. 9(1), 3663 (2019).
Acknowledgements
The authors are thankful to Dr. Mohsen Sanikhani for identification of the plant species, Department of Hortticultural Science, University of Zanjan.
Funding
This research did not receive any specific grant.
Author information
Authors and Affiliations
Contributions
S.H.M.: Performed the experiments and collecting data; M.Y.: Conceiving and designing the experiments; Supervision; Analyzed and interpreted the data; Prepared reagents; Data validation; Writing (review and editing). F.A.: Reviewing and editing; Contributed reagent, and data validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mousavi, S.H., Yaghoobi, M. & Asjadi, F. Highly efficient adsorption of congo red and methyl orange dyes using mesoporous α-Mn2O3 nanoparticles synthesized with Pyracantha angustofolia fruit extract. Sci Rep 14, 18505 (2024). https://doi.org/10.1038/s41598-024-69432-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69432-1
- Springer Nature Limited