Abstract
Observational studies have shown that non-alcoholic fatty liver disease (NAFLD) is strongly associated with metabolic dysfunction. However, there is a paucity of research on whether changes in indicators of serum metabolism contribute to the development of NAFLD. This study was conducted with 4084 participants who underwent healthy physical examinations at Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China, in 2022 and 2023. Baseline and follow-up measurements, including anthropometric data, abdominal ultrasound and blood samples were collected. The diagnosis of NAFLD was based on the 2010 Chinese Guidelines on Diagnosis and Treatment of NAFLD. Multiple logistic regression was utilized to analyze the odds ratios (ORs) for the 1-year risk of NAFLD in connection with both baseline metabolic indicators and changes in metabolic indicators observed over the course of 1 year. A total of 3425 study participants who were free of NAFLD at baseline, including 1146 men and 2279 women, were included in the final analysis. The mean age was 34.43 ± 7.20 years. Participants who developed NAFLD were older, male and had higher levels of body mass index (BMI), blood pressure, fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), free triiodothyronine (fT3), uric acid (UA), alanine aminotransferase (ALT) and aspartate aminotransferase (AST); and lower levels of high-density lipoprotein cholesterol (HDL-C) and free thyroxine (fT4) (all P values < 0.05). The multivariable model showed that baseline BMI, diastolic blood pressure (DBP), TG, TC, HDL-C, LDL-C, UA, fT4, fT3, ALT and changes in TG, HDL-C, and UA were associated with the 1-year risk of developing NAFLD. The risk of NAFLD increased by 56% [OR 1.56, 95% Confidence Interval (CI) 1.32–1.87] and 40% (OR 1.40, 95% CI 1.19–1.64) for each standard deviation (SD) increase in altered TG values (1.01 mmol/L) and altered UA values (55 µmol/L) respectively. Conversely, for each SD (0.27 mmol/L) increase in HDL-C change, the 1-year risk of incident NAFLD was reduced by 50% (OR 0.50, 95% CI 0.40–0.62). The present study suggested that increases in TG and UA, and decreases in HDL-C, significantly increase the risk of developing NAFLD. Therefore, more attention should be paid to these factors in the management and prevention of NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a prevalent form of chronic liver disease characterized by hepatic fat accumulation in individuals who abstain from excessive alcohol consumption1. Since the twenty-first century, NAFLD has gone from an obscure liver disorder to the most prominent chronic liver disease worldwide, with a prevalence of 25%2. NAFLD, which encompasses a range of liver abnormalities from nonalcoholic fatty liver to nonalcoholic steatohepatitis, can have a variable course but ultimately may progress to cirrhosis and hepatocellular carcinoma. Research by Byrne et al. indicated that numerous prospective and retrospective studies have established a significant association between NAFLD and cardiovascular disease, highlighting an elevated risk3. Furthermore, individuals with NAFLD experience heightened overall mortality rates compared to the general population1.
NAFLD, characterized by an accumulation of excessive fat in the liver, is a hepatic manifestation of metabolic syndrome4. Metabolic abnormalities, including high blood pressure, elevated glucose levels, dyslipidemia, high uric acid (UA) levels, are closely linked to the presence of excess fat in the liver5. These metabolic abnormalities are believed to play significant roles in both the cause and effect of NAFLD6. Dyslipidemia commonly coexists with NAFLD. A systematic review and meta-analysis of population-based cohort studies have confirmed that type 2 diabetes, obesity, hypertension, and lipid abnormalities [including low high-density lipoprotein cholesterol (HDL-C) and high triglycerides (TG)] are established risk factors for NAFLD7. Serum uric acid (UA) is the final product of purine metabolism in humans, and hyperuricemia stands as a prevalent metabolic disorder, exceeding a 20% prevalence rate in developed nations8. Recently, the relationship between serum UA and NAFLD is receiving increasing attention. The correlation between serum UA and NAFLD is gaining prominence. A substantial prospective cohort study in China involving 2832 participants identified elevated serum UA levels as an autonomous risk factor for NAFLD9. Similarly, a comprehensive population-based study in Western countries underscored a significant association between hyperuricemia and NAFLD10. The thyroid is an important endocrine organ responsible for hepatic fatty acid and cholesterol synthesis and metabolism11. Indeed, hypothyroidism has been associated with increased serum levels of TG and cholesterol as well as NAFLD12. Accumulating evidence has suggested that serum free thyroxine (fT4) and free triiodothyronine (fT3) levels were associated with the risk of NAFLD13,14,15.
Although numerous studies have been conducted on the correlation between metabolism-related indicators and NAFLD, previous studies have only considered the level of metabolism-related indicators at a single point in time. Consequently, the influence of their dynamic changes over time on NAFLD has been overlooked. The objective of the present study was to examine the associations between baseline metabolic-related markers, including BMI, plasma glucose, lipid profile, UA and thyroid hormone parameters, and changes in these markers over time with the risk of developing NAFLD in a Chinese population undergoing regular physical examinations.
Methods
Study design and population
This study was screened from participants who completed health check-ups in both 2022 and 2023 at Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China. During the course of these health examinations, participants were required to complete a standardized questionnaire pertaining to their lifestyle factors, medical history and disease. Additionally, professional nurses conducted anthropometric measurements and collected blood samples. Abdominal ultrasounds were performed by trained sonographers. Among the study population were individuals aged between 20 and 60 in 2022, who had complete 2-year health check-up data for both 2022 and 2023, including health questionnaires, physical examinations, laboratory tests, and abdominal ultrasound results. In total, 4084 participants were included in the study. Exclusion criteria for the study included individuals with a history of renal failure (n = 5), chronic liver failure (n = 2), or malignant tumors (n = 2) due to their potential impact on blood biochemical indicators. Moreover, participants diagnosed with NAFLD during the 2022 health assessment (n = 650) were excluded, resulting in a final cohort of 3425 eligible individuals. The study protocol was approved by the Institutional Review Board of Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University. All participants provided written informed consent.
Data collection
A standardized questionnaire was administered by trained physicians to obtain detailed information on age, sex, smoking status, alcohol consumption, medical history, and medication use. Smoking status was categorized as current (defined as smoking daily for more than 6 months) or non-current. Alcohol intake was classified as current (defined as drinking daily for more than 6 months) or non-drinker. Diabetes was defined as a previous diagnosis by a healthcare provider or current hypoglycemic therapy. Hypertension was defined as a previous diagnosis by a healthcare provider or current treatment with oral blood pressure medications. Body weight and height were measured with participants wearing lightweight clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured on the nondominant arm after at least a 10-min rest using an automated electronic device (Omron movable arm cylinder blood pressure monitor HEM-1000). Venous blood samples were collected from fasting subjects (fasting for a minimum of eight hours). Serum concentrations of fasting blood glucose (FBG), TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and UA were measured by an autoanalyzer (HITACHI 7600 automatic biochemical analyzer). Serum free triiodothyronine (fT3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4) were determined by chemiluminescent microparticle immunoassay using the Architect system (Beckman DXI 600, DXI 800). Laboratory test quality control was conducted in compliance with the "Medical Quality Control Indicators for Clinical Laboratory Specialties" (2015 edition) set forth by the National Health Commission of the People's Republic of China. Accuracy was evaluated through bias, while precision was assessed using the coefficient of variation (CV). Inter-laboratory quality control was overseen by the Clinical Laboratory Centre of the National Health Commission. Procedures for inter-laboratory quality control included conducting comparative tests on the same sample using different analytical methods or instruments of the same model, as well as comparing retained samples by multiple individuals or by the same operator. The specific values for the quality control of each laboratory indicator are presented in Table S1 of Supplementary Material. The change values of BMI, SBP, DBP, FBG, TG, TC, HDL-C, LDL-C, UA, fT3 and fT4 were calculated by subtracting the values in the 2022 physical examination from the values in the 2023 physical examination.
The hepatic ultrasonography examination was conducted by a skilled ultrasonographer using a high-resolution B-mode topographic ultrasound system with a 3.5-MHz probe. The diagnosis of non-alcoholic fatty liver disease (NAFLD) was based on the presence of at least two of the following three abnormal findings, as outlined in the 2010 Chinese Guidelines on Diagnosis and Treatment of NAFLD16: diffusely increased echogenicity of the liver compared to the kidney or spleen, ultrasound beam attenuation, and poor visualization of intrahepatic structures. Furthermore, all individuals met the following additional criteria: alcohol consumption of less than 30 g per day in men or 20 g per day in women; absence of hepatitis B virus (HBV) infection; no presence of chronic hepatitis or hepatic cirrhosis; and no use of medications associated with NAFLD within the past two weeks.
Statistical analysis
The statistical analysis was conducted using SAS software, version 9.4 (SAS Institute, Cary, NC). A two-sided P value of less than 0.05 was considered statistically significant. Continuous variables with a normal distribution were presented as the mean ± standard deviation (SD), and inter-group comparisons were made using a two independent samples t-test. Continuous data that did not follow a normal distribution were described as the median (interquartile range) [M (Q1, Q3)], and comparisons between groups were made using the Wilcoxon rank sum test. Categorical variables were presented as cases and percentages, and a comparison between the two groups was performed using the Chi-square test. A multiple logistic regression analysis was conducted to determine the odds ratios (ORs) for the 1-year risk of NAFLD based on baseline metabolism-related indices and 1-year changes in these indices.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China (No. 2024DZKY-015-01). All subjects provided consent for their data to be used in this study.
Informed consent
Written informed consent was obtained from all participants.
Results
Baseline characteristics of study population
Table 1 presented the baseline characteristics of participants according to their status of newly onset NAFLD at the 1-year follow-up. The mean age of the 3425 participants was 34.43 ± 7.20, with 33.5% (n = 1146) being male. During the 1-year follow-up, 255 participants developed NAFLD. The participants who developed NAFLD were older and exhibited higher levels of blood pressure, FBG, TG, TC, LDL-C, fT3, UA, ALT and AST, lower levels of HDL-C and fT4, and a higher proportion of males, current smokers, current drinkers, hypertension and diabetes (all P values < 0.05).
As illustrated in Fig. 1, the median of 1-year changes in BMI, FBG, TC, LDL-C, UA, and fT3 in patients with new-onset NAFLD were significantly higher than those in the non-NAFLD population, while the median of 1-year change in HDL-C was significantly lower than those in the non-NAFLD participants (all P values < 0.05).
Associations of baseline metabolic markers with 1-year risk of incident NAFLD
Table 2 presented the results of a logistic regression analysis investigating the association between age, sex, ALT, AST and baseline metabolism-related indexes with NAFLD in a logistic regression analysis. After adjustment for smoking status, drinking status, diabetes, hypertension, BMI, SBP, DBP, FBG, TG, TC, HDL-C, LDL-C, UA, ALT, AST, fT3 and fT4, the 1-year risk of incident NAFLD was found to increase by 5% for each additional year of age, with an OR of 1.05 [95% confidence interval (CI) 1.03–1.08, P < 0.0001]. For every SD increase in BMI (2.67 kg/m2), the risk of incident NAFLD increased by 157% (OR 2.57, 95% CI 2.13–3.10, P < 0.0001). For each SD increase in DBP (9 mmHg), the 1-year risk of incident NAFLD increased by 25% (OR 1.25, 95% CI 1.03–1.52, P = 0.02). The 1-year risk of incident NAFLD was found to increase by 11% (OR 1.11, 95% CI 1.01–1.22, P = 0.02) for each SD increase in FBG (0.75 mmol/L). The 1-year risk of incident NAFLD increased by 64% (OR 1.64, 95% CI 1.39–2.15, P < 0.0001) and 18% (OR 1.18, 95% CI 1.03–1.35, P = 0.02) for every SD increase in TG (0.86 mmol/L) and LDL-C (0.64 mmol/L), respectively. For every SD increase in UA (91 µmol/L), the 1-year risk of incident NAFLD increased by 53% (OR 1.53, 95% CI 1.28–1.84, P < 0.0001). For every SD increase in fT3 (1.10 pmol/L), the 1-year risk of incident NAFLD increased by 20% (OR 1.20, 95% CI 1.03–1.39, P = 0.02). For ALT, every SD increase (14 U/L) was associated with a 49% (OR 1.49, 95% CI 1.14–1.94, P = 0.003) increment in the 1-year risk of NAFLD. On the contrary, being female, having higher HDL-C levels, and higher fT4 levels were protective factors for NAFLD, with the 1-year risk of NAFLD being 52% (OR 0.48, 95% CI 0.31–0.74, P = 0.0009) lower in females than in males. For each SD increase in HDL-C (0.33 mmol/L) and fT4 (2.87 pmol/L), the 1-year risk of NAFLD was reduced by 29% (OR 0.71, 95% CI 0.59–0.85, P = 0.0003) and 21% (OR 0.79, 95% CI 0.63–0.99, P = 0.04), respectively. However, no association was found between SBP, TC and AST and the annual risk of NAFLD in the multifactorial adjusted model.
Associations between changes in metabolism-related indicators and the 1-year risk of incident NAFLD
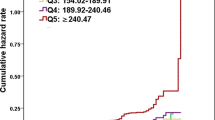
As illustrated in Fig. 2, after adjusting for age, sex, smoking and alcohol consumption, as well as the presence diabetes and hypertension, baseline metabolic measures, ALT and AST, changes in TG, HDL-C and UA were found to be significantly associated with the 1-year risk of NAFLD. The 1-year risk of NAFLD increased by 56% (OR 1.56, 95% CI 1.32–1.87, P < 0.0001) and 40% (OR 1.40, 95% CI 1.19–1.64, P < 0.0001) for each SD increase in altered TG values (1.01 mmol/L) and altered UA values (55 µmol/L), respectively. Conversely, for every SD (0.27 mmol/L) increase in HDL-C change, the 1-year risk of incident NAFLD was reduced by 50% (OR 0.50, 95% CI 0.40–0.62, P < 0.0001).
Subgroup analysis of baseline and changes in metabolism-related indicators and their association with the incidence of NAFLD
Tables S2 and S3 displays the results of subgroup analysis on baseline metabolic markers and the 1-year risk of incident NAFLD. Upon adjusting for potential confounders, significant associations were found between baseline BMI, TC, HDL-C, UA, and NAFLD events across different age groups and sexes. These results align with the overall population findings. Conversely, correlations between DBP, FBG, LDL-C, fT3, fT4, and ALT and NAFLD risk varied slightly among different population subgroups.
Tables S4 and S5 presents the subgroup analysis results of 1-year changes in metabolism-related indicators and the risk of incident NAFLD. Except for TG change, which showed no significant correlation with the 1-year risk of NAFLD incidence in females, the associations of 1-year changes in HDL-C and UA with NAFLD remained consistent across different population subgroups compared to the total population. Notably, in the subgroup of individuals aged under 40, significant correlations were observed between changes in BMI and fT3 levels and the risk of developing NAFLD. Specifically, for every standard deviation (SD) increase in BMI change (0.94 mmol/L) and fT3 change (0.74 pmol/L), the 1-year risk of incident NAFLD increased by 178% (OR 2.78, 95% CI 1.79–4.31, P < 0.0001) and 71% (OR 1.71, 95% CI 1.12–2.61, P = 0.01), respectively. Significant associations between altered FBG values and NAFLD risk were observed in individuals aged over 40 and in the male subgroup. For each SD increase in altered FBG values, the risk of NAFLD incidence increased by 40% (OR 1.40, 95% CI 1.08–1.82, P < 0.0001) in the over 40 age group and by 36% (OR 1.36, 95% CI 1.12–1.64, P < 0.0001) in the male subgroup.
Discussion
The present study has identified a number of baseline characteristics, including BMI, DBP, TG, TC, HDL-C, LDL-C, UA, fT3, fT4 and ALT, that are associated with the 1-year risk of developing NAFLD in a Chinese population undergoing health check-ups. Furthermore, we observed a significant correlation between the 1-year changes in TG, HDL-C, and UA and the 1-year incidence of NAFLD. The present study identified a significant correlation between elevated TG and UA levels, in conjunction with decreased HDL-C, and the 1-year risk of developing NAFLD. The novelty of this study is that in addition to prospectively examining the association between baseline metabolic markers and the risk of NAFLD, it also examined their dynamic changes over time on NAFLD, which could be more informative. Baseline metabolic markers such as high TG, low HDL-C and high UA increase the risk of developing NAFLD. Our findings suggest that changes in TG, HDL-C and UA are associated with NAFLD, indicating that active control of TG, HDL-C and UA may reduce the development of NAFLD.
Dyslipidemia, defined as elevated plasma TG, elevated LDL-C and reduced HDL-C, is a prevalent comorbidity in patients with NAFLD5. The liver is responsible for metabolizing fatty acids. However, in the presence of overnutrition and obesity, this process can be disrupted, leading to the accumulation of TG in liver cells and the development of NAFLD17. A previous physical examination population-based cohort study from China modelled the 2-year risk of NAFLD in a non-obese, lipid-neutral population. The study found that TG is one of the main aspects of the NAFLD risk factor score18. HDL-C, also known as "good cholesterol", has been associated with antioxidant, anti-inflammatory, anti-thrombotic, and other beneficial effects on the body. Recent studies have indicated that an imbalance in the function of HDL-C, particularly in its ability to remove cholesterol from cells, may be a major factor in the development of NAFLD19,20. Low levels of HDL-C are a common lipid disorder that is closely linked to the progression of NAFLD21. The findings of our study have confirmed the association between dyslipidemia and NAFLD and have also highlighted the role of altered lipid and lipoprotein metabolism in increasing the risk of NAFLD. Furthermore, promising therapies for NAFLD have also been shown to improve dyslipidemia in clinical trials22, suggesting that interventions targeting TG and HDL-C may be beneficial in preventing NAFLD in patients with high lipid levels.
UA is a byproduct of purine oxidation and is a well-known component of metabolic abnormalities, including abdominal obesity, glucose intolerance, insulin resistance, dyslipidemia, and hypertension23. Recently, the relationship between serum UA and NAFLD has been receiving increasing attention. Observational research and animal studies have provided strong evidence of the significant connection between high levels of UA and NAFLD24,25. Prospective studies have shown that a higher serum UA changing trajectory is a risk factor for NAFLD26. The findings of our study align with those of previous research in this field. Recent studies have also shown that low baseline serum UA levels and a decrease in serum UA levels over time are both associated with NAFLD resolution in young adults27. It is believed that hyperuricemia plays a role in the onset and progression of NAFLD. The underlying mechanisms can be summarized as follows: higher blood uric acid levels mediate insulin resistance28, induce endoplasmic reticulum stress29 leading to increased lipid synthesis, resulting in fat deposition in liver cells and NAFLD. Therefore, our study suggests that UA may play an important role in the pathogenesis of NAFLD and could potentially lead to new therapeutic strategies for treating hyperuricemia induced NAFLD.
It is notable that this study also identified a positive correlation between baseline fT3 and NAFLD, as well as a negative correlation between fT4 and NAFLD. These contradictory results have been corroborated in previous studies by Lai and Xu15,30. This suggests that the role of thyroxine in the development of NAFLD is complex, and that there may be a biologically plausible mechanism through which thyroid hormones exert significant effects on the development of NAFLD. The potential mechanism by which fT3 is positively correlated with the risk of NAFLD is that fT3 promotes long-term autophagy and damage in hepatocytes by translocating lipids to lysosomes via hepatic hippophagy and enhancing fatty acid oxidation31. The inverse relationship between fT4 and NAFLD may be attributed to the association between low serum fT4 levels and hypertriglyceridemia and obesity, which has been repeatedly confirmed in previous studies15,30. A previous study by Bilgin and Pirgon has proposed that increased conversion from fT4 to fT3 by increasing deiodinase activity may serve as a compensatory mechanism for excessive fat accumulation, with the aim of improving energy expenditure32. This may also provide a potential explanation for the observed inconsistency in the results for fT3 and fT4.
Previous study has shown that the prevalence of NAFLD is around five times greater in individuals with diabetes compared to those without diabetes33. In our study, in the older age group (> 40 years) and among males, a higher risk of NAFLD was observed with escalating FBG changes. FBG levels may serve as indicators of basal insulin secretion and function34. Glucose metabolism or insulin resistance (IR) appears to be implicated in the development of NAFLD, as indicated by previous research showing a strong connection between IR and NAFLD. Moreover, the research revealed a heightened susceptibility to NAFLD among individuals with elevated baseline BMI levels. In younger participants (under 40 years old), a notable rise in BMI change was observed concomitant with an increased likelihood of NAFLD onset. The established consensus underscores the association between obesity and the heightened prevalence and seriousness of NAFLD35. In light of the scarcity of approved pharmacological treatments for NAFLD, interventions targeting obesity present a feasible solution. Our findings support the proposition that reducing body weight, particularly in younger individuals, may contribute to the prevention of NAFLD.
Previous Mendelian randomization studies have established a causal link between hypertension, SBP, DBP, and NAFLD. While no significant association was found between baseline and altered SBP values and NAFLD, our study reveals that elevated DBP levels are correlated with an increased risk of NAFLD onset. The outcomes of our research diverge somewhat from prior investigations, potentially due to the relatively youthful age of the cohort, with 77% of participants being under 40 years old. Furthermore, a reciprocal relationship between NAFLD and hypertension has been suggested, wherein NAFLD may serve as both a consequence and a causal factor of hypertension36.
This retrospective cohort study utilized a large sample size from a health checkup population and adjusted for confounding factors such as gender, age, BMI, blood pressure, lipid levels, uric acid, aminotransferase and thyroid function to enhance the scientific reliability of the results. However, there are several limitations that need to be considered. Firstly, liver biopsies are the most accurate method for diagnosing NAFLD, whereas the use of ultrasound in this study may not be sensitive enough to detect mild steatosis. This could result in the oversight of early or mild NAFLD cases as potential diagnoses, potentially influencing the overall study findings. Secondly, the diagnosis of NAFLD necessitates the exclusion of specific liver diseases, including alcoholic liver disease, hepatitis C virus infection, autoimmune hepatitis, hepatomegaly, and other conditions that can lead to fatty liver. However, testing for HCV antibodies was not conducted in our study population, and therefore only those with exclusion of HBV infection and chronic liver failure and cirrhosis were considered in the diagnosis. Thirdly, the study was restricted to individuals undergoing healthy physical examinations, which may limit the generalizability of the findings to the entire population. Finally, the short 1-year follow-up time of the study only allows for an explanation of the short-term risk of NAFLD. Consequently, there is a need for a multicenter cohort study with increased sample sizes, refined diagnostic criteria, and extended follow-up to enhance the validation of our outcomes.
Despite these limitations, our study suggests a potential role in preventing and managing NAFLD by enhancing metabolic markers. We observed a correlation between baseline levels and short-term fluctuations in TG, HDL-C, and UA with the development of NAFLD. However, this association necessitates confirmation through more rigorous interventional randomized controlled clinical trials. Presently, there is a scarcity of data from randomized controlled trials involving patients with NAFLD and comorbid dyslipidemia or hyperuricemia.
Conclusions
In conclusion, the research indicates that an increase in TG, a decrease in HDL-C, and a rise in UA over the span of 1 year significantly heighten the risk of NAFLD. This implies that improving levels of TG, HDL-C, and UA could be of some important in managing and preventing NAFLD.
Data availability
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64(1), 73–84. https://doi.org/10.1002/hep.28431 (2016).
Ge, X., Zheng, L., Wang, M., Du, Y. & Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 10(8), e036663. https://doi.org/10.1136/bmjopen-2019-036663 (2020).
Byrne, C. D. & Targher, G. NAFLD: A multisystem disease. J. Hepatol. 62(1 Suppl), S47–S64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Marchesini, G. et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 37(4), 917–923. https://doi.org/10.1053/jhep.2003.50161 (2003).
Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2(11), 901–910. https://doi.org/10.1016/S2213-8587(14)70032-4 (2014).
Rich, N. E. et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16(2), 198-210.e2. https://doi.org/10.1016/j.cgh.2017.09.041 (2018).
Jarvis, H. et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 17(4), e1003100. https://doi.org/10.1371/journal.pmed.1003100 (2020).
Li, S. et al. Serum uric acid levels and nonalcoholic fatty liver disease: A 2-sample bidirectional mendelian randomization study. J. Clin. Endocrinol. Metab. 107(8), e3497–e3503. https://doi.org/10.1210/clinem/dgac190 (2022).
Wei, F. et al. Higher serum uric acid level predicts non-alcoholic fatty liver disease: A 4-year prospective cohort study. Front. Endocrinol. (Lausanne). 11, 179. https://doi.org/10.3389/fendo.2020.00179 (2020).
Sirota, J. C. et al. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: Liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism. 62(3), 392–399. https://doi.org/10.1016/j.metabol.2012.08.013 (2013).
Sinha, R. A., Singh, B. K. & Yen, P. M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 14(5), 259–269. https://doi.org/10.1038/nrendo.2018.10 (2018).
Tanase, D. M. et al. Hypothyroidism-induced nonalcoholic fatty liver disease (HIN): Mechanisms and emerging therapeutic options. Int. J. Mol. Sci. 21(16), 5927. https://doi.org/10.3390/ijms21165927 (2020).
Hu, Y. et al. The nonlinear relationship between thyroid function parameters and metabolic dysfunction-associated fatty liver disease. Front. Endocrinol. (Lausanne). 14, 1115354. https://doi.org/10.3389/fendo.2023.1115354 (2023).
Liu, Y., Wang, W., Yu, X. & Qi, X. Thyroid function and risk of non-alcoholic fatty liver disease in euthyroid subjects. Ann. Hepatol. 17(5), 779–788. https://doi.org/10.5604/01.3001.0012.3136 (2018).
Xu, C., Xu, L., Yu, C., Miao, M. & Li, Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin. Endocrinol. (Oxf). 75(2), 240–246. https://doi.org/10.1111/j.1365-2265.2011.04016.x (2011).
Fan, J. G. et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese-on-Chinese Journal of Hepatology 2010; 18:163–166). J. Dig. Dis. 12(1), 38–44. https://doi.org/10.1111/j.1751-2980.2010.00476.x (2011).
Alves-Bezerra, M. & Cohen, D. E. Triglyceride metabolism in the liver. Compr. Physiol. 8(1), 1–8. https://doi.org/10.1002/cphy.c170012 (2017).
Ji, L., Cai, X., Bai, Y. & Li, T. Application of a novel prediction model for predicting 2-year risk of non-alcoholic fatty liver disease in the non-obese population with normal blood lipid levels: A large prospective cohort study from China. Int. J. Gen. Med. 14, 2909–2922. https://doi.org/10.2147/IJGM.S319759 (2021).
März, W. et al. HDL cholesterol: Reappraisal of its clinical relevance. Clin. Res. Cardiol. 106(9), 663–675. https://doi.org/10.1007/s00392-017-1106-1 (2017).
Cohen, D. E. & Fisher, E. A. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin. Liver Dis. 33(4), 380–388. https://doi.org/10.1055/s-0033-1358519 (2013).
Verwer, B. J. et al. NAFLD is related to post-prandial triglyceride-enrichment of HDL particles in association with endothelial and HDL dysfunction. Liver Int. 40(10), 2439–2444. https://doi.org/10.1111/liv.14597 (2020).
Deprince, A., Haas, J. T. & Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 42, 101092. https://doi.org/10.1016/j.molmet.2020.101092 (2020).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359(17), 1811–1821. https://doi.org/10.1056/NEJMra0800885 (2008).
Petta, S., Cammà, C., Cabibi, D., Di Marco, V. & Craxì, A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 34(7), 757–766. https://doi.org/10.1111/j.1365-2036.2011.04788.x (2011).
Xie, D. et al. High uric acid induces liver fat accumulation via ROS/JNK/AP-1 signaling. Am. J. Physiol. Endocrinol. Metab. 320(6), E1032–E1043. https://doi.org/10.1152/ajpendo.00518.2020 (2021).
Ma, Z. et al. Changing trajectories of serum uric acid and risk of non-alcoholic fatty liver disease: A prospective cohort study. J. Transl. Med. 18(1), 133. https://doi.org/10.1186/s12967-020-02296-x (2020).
Cho, Y., Chang, Y., Ryu, S., Wild, S. H. & Byrne, C. D. Baseline and change in serum uric acid level over time and resolution of nonalcoholic fatty liver disease in young adults: The Kangbuk Samsung Health Study. Diabetes Obes. Metab. 26(5), 1644–1657. https://doi.org/10.1111/dom.15466 (2024).
Hu, Y. et al. High uric acid promotes dysfunction in pancreatic β cells by blocking IRS2/AKT signalling. Mol. Cell Endocrinol. 520, 111070. https://doi.org/10.1016/j.mce.2020.111070 (2021).
Choi, Y. J. et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 94(10), 1114–1125. https://doi.org/10.1038/labinvest.2014.98 (2014).
Lai, S., Li, J., Wang, Z., Wang, W. & Guan, H. Sensitivity to thyroid hormone indices are closely associated with NAFLD. Front. Endocrinol. (Lausanne). 12, 766419. https://doi.org/10.3389/fendo.2021.766419 (2021).
Sinha, R. A. & Yen, P. M. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell. Biosci. 6, 46. https://doi.org/10.1186/s13578-016-0113-7 (2016).
Bilgin, H. & Pirgon, Ö. Thyroid function in obese children with non-alcoholic fatty liver disease. J. Clin. Res. Pediatr. Endocrinol. 6(3), 152–157. https://doi.org/10.4274/Jcrpe.1488 (2014).
Khan, R. S., Bril, F., Cusi, K. & Newsome, P. N. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 70(2), 711–724. https://doi.org/10.1002/hep.30429 (2019).
Cai, X. et al. A prediction model based on noninvasive indicators to predict the 8-year incidence of type 2 diabetes in patients with nonalcoholic fatty liver disease: A population-based retrospective cohort study. Biomed. Res. Int. 2021, 5527460. https://doi.org/10.1155/2021/5527460 (2021).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 92, 82–97. https://doi.org/10.1016/j.metabol.2018.11.014 (2019).
Hu, J. et al. Relationship Between plasma aldosterone concentrations and non-alcoholic fatty liver disease diagnosis in patients with hypertension: A retrospective cohort study. Diabetes. Metab. Syndr. Obes. 16, 1625–1636. https://doi.org/10.2147/DMSO.S408722 (2023).
Acknowledgements
The present study would not have been possible without the participation of the participants.
Funding
Jiangsu Province elderly health research project (LKM2023021). National Postdoctoral Science Foundation (2018M633765).
Author information
Authors and Affiliations
Contributions
Y.H., T.J. and W.N. were responsible for the conceptualisation, statistical analysis, and manuscript writing and editing. Y.Z., R.Z., D.L. and Y.W. were involved in the collection of data and its subsequent statistical analysis. X.H. and Y.S. were responsible for data collection and laboratory tests. Y.Z. was responsible for the conceptualisation of the project, methodology, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Jin, T., Ni, W. et al. Baseline and change in serum lipid and uric acid level over time and incident of nonalcoholic fatty liver disease (NAFLD) in Chinese adults. Sci Rep 14, 18547 (2024). https://doi.org/10.1038/s41598-024-69411-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69411-6
- Springer Nature Limited