Abstract
We aimed to evaluate the correlation between endoscopic papillary abnormalities (PA) and high renal papilla Hounsfield density (PHD) on CT scan in patients who underwent flexible ureteroscopic treatment (fURS) for renal stones. We retrospectively assessed patients from a prospectively collected database who were treated with fURS for renal stones between May 2016 and October 2020. PHD was measured on preoperative CT-scan by a radiologist blinded from the intraoperative aspect of the papillae. Correlation was examined between high PHD (≥ 43 HU) and PA described in fURS, stone composition, metabolic abnormalities, … Out of 159 consecutive cases, 131 were eligible for analysis with available preoperative CT-scan. Median age was 55 years (IQR 43–67) and median PHD was 40 (IQR 36–45). Eighty patients (61%) had PHD < 43, and 51 patients (39%) had PHD ≥ 43. In univariate and multivariate analysis, only young age (p-value = 0.017) and insufficient diuresis (p-value = 0.008) were correlated with high PHD. No significant correlation was found with PA described during endoscopy, including the intensity of Randall's plaques. In this study, high PHD appears to be only a sign of insufficient diuresis, with no significant correlation with potential PA.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
There is a significant difference in renal papilla Hounsfield density (PHD) measured by computer tomography (CT) between stone formers and the others1.
In the 1930s, Randall described Papillary calculi that resulted from subepithelial lesions2. Since then, it has also been shown that stone formation on Randall plaques (RP) was not the only way to heterogeneous crystallization. Indeed, plugs of carbapatite (CA) in the Bellini ducts have been described, with a high risk of interstitial fibrosis surrounding these ducts3. It is now accepted that crystallization on Randall's plaques should most often suggest a dietary origin with insufficient diuresis4,5,6,7 and intratubular crystallization (“plugs”), related to calcium phosphate stones, with hypercalciuria and hypocitraturia, is often associated with a disease at high risk of stone formation (e.g. distal renal tubular acidosis)3,8,9.
The aim of our study was to evaluate the correlation between PHD and endoscopic description of papillary abnormalities during flexible ureteroscopies. The secondary objective was to evaluate the correlation between PHD and stone composition or biochemical evaluation.
Methods
A retrospective analysis of a prospective dataset of consecutive flexible ureterorenoscopies in stone formers from May 2016 to October 2020 was performed. A declaration MR-4 has been made to the CNIL for the research under the number 2234942 with approval from the ethics committee IRB00010835 under the reference COS-RGDS-2024-07-001-ALMERAS-C. All methods were carried out in accordance with relevant guidelines and regulations and informed consent was obtained from all subjects. We included only patients treated with flexible digital ureterorenoscopes. Patients without preoperative CT scan were excluded.
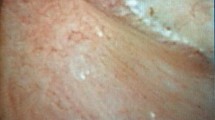
During the surgery, a systematic endoscopic exploration of all the calyces and their papillae was performed before the stone treatment. A scoring system was applied for each patient (one score per kidney and patient) by the operator (who was blinded to all patients characteristics regarding his stone history) using the Sx nPx Rx classification published in 2016 and validated in 20219,10 (Fig. 1). It has been then demonstrated its interest to determine the severity of the lesions, the potential risk of recurrence and to distinguish the type of crystallization (on RP or on intraductal plugs), and therefore this is why endoscopic description of the papillary abnormalities appeared in some international guidelines11.
The stones were assessed by microscopy and infrared spectrometry (Perlin Elmer) and classified accordingly12. Patients systematically underwent biochemical investigations (serum analysis, 24 h urine analysis) at least one month after the surgical procedure.
Every preoperative CT-scan were retrospectively reviewed by an uro-radiologist. The radiologist was blinded to the patient’s clinical characteristics. The PHD was measured according to the technique described by Eisner in 20081. Coronal reformatted images of 2.5 mm thickness were obtained in all patients. Renal papilla Hounsfield density was measured by placing ROIs (mean size 0.1–0.2cm2) in the region of renal papilla and the attenuation measurements were recorded (HU values). The images were magnified to 5× to prevent contamination of the ROI with the fat in the renal sinus. The densities of the one upper, middle and lower pole renal papillae were measured separately in both kidneys including the papillae in the region of calculi (Fig. 2).
Statistical analysis
Descriptive statistics were delineated for the available variables. Quantitative variables were reported in median, interquartile range [IQR] and analyzed by Mann–Whitney test. Categorical variables were described as numbers and percentages and were analyzed by chi-square test. Variables with p < 0.20 who were associated with PHD > 43 HU in univariate analysis were included in a multivariate logistic regression model. The cut-off of PHD > 43 HU has been chosen to describe high risk stone formers according to the results of previous works13,14. To detect multicollinearity, we calculated the Variance Inflation Factors (VIF) of each variable included in the model. Factors with VIF > 3 were excluded to avoid the effects of dominance or confusion in the analysis. The area under the receiver operating characteristic (ROC) curve was used to evaluate the model discrimination. Statistical analyses were performed using R Version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). For all tests, a two-side p < 0.05 was considered statistically significant.
Results
Out of 159 consecutive cases, 131 were eligible for analysis with available preoperative CT scan. For 31 patients, the biochemical investigations were missing. The median age of patients at the time of treatment was 55 years.
The median PHD was 40 (IQR 36–45). Eighty patients (61%) had PHD < 43, and 51 patients (39%) had PHD ≥ 43.
In univariate analysis, only young age (p-value = 0.017) and insufficient diuresis (p-value = 0.008) were significantly associated with PHD > 43 HU (Tables 1 and 2).
Multivariate stepwise logistic regression analysis revealed that low fluid intake (OR 3.54; 95% CI, 1.43 to 9.28; p = 0.008) was the only factor associated with PHD > 43 (Fig. 3). All VIF were < 3. The evaluation of the discrimination accuracy of our model using the area under ROC curve was 0.770, indicating good discrimination power.
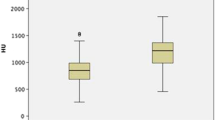
Even there was a trend for intraductal crystallization, extrophic papillae and severe amount of RP to have higher PHD, no significant correlation was found with papillary abnormalities described during endoscopy (Fig. 4).
Distribution of PHDs according to endoscopic papillary abnormalities (Sx nPx Rx Classification9). S0: no papillary stone; Sa: papillary anchored stone; Sc: intraductal crystallization; P0: no papillary abnormality; Pa: erosion; Pb: extrophic papilla; R0-a : mild amount of RP; Rb-c: high amount of RP.
Discussion
High PHD may be often observed on CT images, but not always described by radiologists (Fig. 5). This high density of papillae could also appear with medullary hyper echogenicity on renal ultrasound with false stone diagnosis. A highly statistically significant difference in PHD measured by computer tomography (CT) scan has been described between patients with nephrolithiasis and control patients1, with average HU values ranged from 37 to 54 for stone formers and from 21 to 37 for controls. High PHD values might correlate with first incident stones15 and with an increased risk of recurrence13,14,15,16,17,18. A cut-off of PHD > 43 HU has been specified for high risk of recurrence and PHD > 36.8 HU for a risk × 4013,14,15. A logic theorical correlation between PHD and the amount of Randall plaques (RP) has logically raised19,20, but remained hypothetical.
The fact that papillary calculi resulted from subepithelial lesions is well known since Randall in the 1930s2, but stone formation on RP is not the only cause of crystallization21. If crystallization on a Randall's plaques should most often suggest a dietary origin with insufficient diuresis4,5,6,7, intratubular crystallization (“plugs”), often linked to calcium phosphate stones with hypercalciuria and hypocitraturia9,22, is often associated with an underlying pathology at high risk of stone formation8. It has been demonstrated that the type of crystallization(on RP or intraductal) can be specified endoscopically during retrograde flexible ureteroscopies9,23,24.
Our study is the first at this time aiming to compare papillary abnormalities described by digital flexible ureteroscopy in stone formers with PHD measured on CT scans. We have chosen the technique of PHD measurement described by Eisner in 20081, that averages the densities of the upper, middle and lower papillae of both kidney in a same patient. In contrast to a papilla-by-papilla measurement, this technique has proven effective and limits the risk of biases due to mismatch between the HU measurement and the described papilla. Furthermore, this technique, more reproducible in routine practice, seems to be more correlated with lithogenesis that follows a global crystallization pathway21 in a same patient.
In univariate analysis, only young age (p-value = 0.017) and insufficient diuresis (p-value = 0.008) were correlated with high PHD. In multivariate analysis low fluid intake (OR 3.54; 95% CI, 1.43 to 9.28; p = 0.008) was the only factor associated with PHD > 43. Our results suggest that high PHD is not a discriminatory factor to predict one type of crystallization nor the amount of RP.
According to the results of previous studies, we did not find any correlation between PHD and stone composition25 nor hypercalciuria26 but correlation between low fluid intake and high PHD16,17. Ferraro has also demonstrated a decrease of PHD (from 43.2 + / − 6.6 to 35.4 + / − 4.2 (− 7.8UH) p < 0.001) with the consumption of 3 additional glasses of water per day for 1 year16. High PHD appears to be a prognostic factor of recurrence in connection with mild diuresis and low fluid intake, but without any correlation with the endoscopic description of type of crystallization, nor stone composition and the results of metabolic investigations.
Insufficient diuresis as risk factor for stone formers is obviously not a new data. It turns out that stone formers do not systematically have high PHD on CT scans, and that high PHD without urinary stone is also sometimes observed in patients who have CT scan indicated for acute transient abdominal pain. High PHD, when it is observed and which has a strong correlation with low diuresis, can give a strong information concerning this lithogenic risk factor, and could become a radiological semiological sign of insufficient diuresis.
Our study has some limitations. First, this is a monocentric study, and second, based on two difficult evaluations with complex reproducibility: the endoscopic recognition of the papillary abnormalities and the PHD measure on CT scan. To limit the bias related to the learning curve, we excluded the endoscopic data that were collected before evaluating the classification of the papillary abnormalities9. The PHD has been retrospectively measured on archive CT scan storage images that may have an enlarged thickness to reduce the size of the files to be stored. Therefore, a loss of information and accuracy could occur in comparison to measurements made at the time of acquisition. In addition, the technique of PHD measurement used in this study has been described 15 years ago, and could be improved nowadays in connection with the evolution of modern CT scans.
However, if there is a trend on the results to find high PHD in case of intraductal crystallization, extrophic papillae or high amount of RP (Fig. 4), this study has shown that nowadays high PHD on CT scan cannot predict one pathway of crystallization21, but the lack of fluid intake, insufficient diuresis and also the risk of first stone incident or recurrence.
Conclusion
The identification of high PHD on CT scan appears to be a prognostic factor of recurrence and is mainly correlated to mild diuresis and low fluid intake. Nevertheless, there was no correlation with the endoscopic description of type of crystallization, nor stone composition and the results of metabolic investigations. However, assigned to its correlation with low diuresis, PHD could be used in addition to EPR, stone composition analysis and biochemical investigations to follow stone formers and prevent recurrences.
Data availability
Data can be shared if needed.
References
Eisner, B. H. et al. Differences in computed tomography density of the renal papillae of stone formers and non-stone-formers: a pilot study. J. Endourol. 22, 2207–2210 (2008).
Randall, A. The origin and growth of renal calculi. Ann. Surg. 105, 1009–1027 (1937).
Evan, A. P. et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 71, 795–801 (2007).
Kuo, R. L. et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 64, 2150–2154 (2003).
Coe, F. L., Worcester, E. M. & Evan, A. P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 12, 519–533 (2016).
Bouderlique, E. et al. Vitamin D and calcium supplementation accelerates randall’s plaque formation in a murine model. Am. J. Pathol. 189, 2171–2180 (2019).
Siener, R. & Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur. J. Clin. Nutr. 57(Suppl 2), S47-51 (2003).
Dessombz, A. et al. Calcium phosphate stone morphology can reliably predict distal renal tubular acidosis. J. Urol. 193, 1564–1569 (2015).
Almeras, C. et al. Classification of the renal papillary abnormalities by flexible ureteroscopy: Evaluation of the 2016 version and update. World J. Urol. 39, 177–185 (2021).
Almeras, C. et al. Endoscopic description of renal papillary abnormalities in stone disease by flexible ureteroscopy: A proposed classification of severity and type. World J. Urol. 34, 1575–1582 (2016).
Almeras, C. et al. 2022 Recommendations of the AFU Lithiasis Committee: Endoscopic description of renal papillae and stones. Prog. En Urol. J. Assoc. Francaise Urol. Soc. Francaise Urol. 33, 766–781 (2023).
Daudon, M., Bader, C. A. & Jungers, P. Urinary calculi: Review of classification methods and correlations with etiology. Scan. Microsc. 7, 1081–1104 (1993) (discussion 1104–1106).
Ciudin, A. et al. Abdominal computed tomography—A new tool for predicting recurrent stone disease. J. Endourol. 27, 965–969 (2013).
Arrabal-Polo, M. A. et al. Usefulness of measuring renal papillae in Hounsfield units in stone—Forming patients. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 42, 973–976 (2016).
Ciudin, A. et al. The evolution of CT diagnosed papillae tip microcalcifications: Can we predict the development of stones?. J. Endourol. 28, 1016–1021 (2014).
Ferraro, P. M. et al. Changes in renal papillary density after hydration therapy in calcium stone formers. BMC Urol. 18, 101 (2018).
Arda, E. et al. Comparison of Turkish primary, recurrent, and non stone-forming patients using hounsfield unit measurements: How useful is it?. Curr. Urol. 12, 158–163 (2019).
Cakiroglu, B. et al. Renal papillary attenuation differences between primary and recurrent idiopathic calcium stone disease patients. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 66, 107–112 (2014).
Bhuskute, N. M., Yap, W. W. & Wah, T. M. A retrospective evaluation of Randall’s plaque theory of nephrolithiasis with CT attenuation values. Eur. J. Radiol. 72, 470–472 (2009).
Ciudin, A. et al. Validation of Randall’s plaque theory using unenhanced abdominal computed tomography. Urology 81, 246–249 (2013).
Coe, F. L. et al. Three pathways for human kidney stone formation. Urol. Res. 38, 147–160 (2010).
Williams, J. C. et al. Papillary ductal plugging is a mechanism for early stone retention in brushite stone disease. J. Urol. 199, 186–192 (2018).
Almeras, C. et al. Endoscopic papillary abnormalities and stone recognition (EPSR) during flexible ureteroscopy: A comprehensive review. J. Clin. Med. 10, 2888 (2021).
Borofsky, M. S. et al. A proposed grading system to standardize the description of renal papillary appearance at the time of endoscopy in patients with nephrolithiasis. J. Endourol. 30, 122–127 (2016).
Deshmukh, S. et al. Hounsfield density of renal papillae in stone formers: Analysis based on stone composition. J. Urol. 193, 1560–1563 (2015).
Shavit, L. et al. Increased renal papillary density in kidney stone formers detectable by CT scan is a potential marker of stone risk, but is unrelated to underlying hypercalciuria. Urolithiasis 44, 471–475 (2016).
Author information
Authors and Affiliations
Contributions
C.A.: design of the study, collect of the data and wrote the manuscript, B.P.: reviewed the manuscript, J.A: measured the PHD, M.B.: statistics, A.T.: reviewed the data, and designed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Almeras, C., Assoun, J., Baboudjian, M. et al. Correlation analysis between renal papillae Hounsfield density (PHD) and endoscopic papillary description in stone formers. Sci Rep 14, 18582 (2024). https://doi.org/10.1038/s41598-024-69390-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69390-8
- Springer Nature Limited