Abstract
The relationship of adipose tissue insulin resistance (AT-IR, a product of fasting insulin and free fatty acids) and homeostasis-model assessment-insulin resistance (HOMA-IR) to β-cell function was studied cross-sectionally in the setting of subtle glucose dysregulation. Associations of AT-IR and HOMA-IR with fasting and post-glucose glycemia and β-cell function inferred from serum insulin kinetics during a 75 g oral glucose tolerance test were studied in 168 young female Japanese students. β-cell function was evaluated by disposition index calculated as a product of the insulinogenic index (IGI) and Matsuda index. AT-IR, not HOMA-IR, showed positive associations with post-glucose glycemia and area under the glucose response curve although both indices were associated with fasting glycemia. HOMA-IR, not AT-IR, was associated positively with log IGI whereas both indices were inversely associated with Matsuda index. AT-IR, not HOMA-IR, showed inverse associations with log disposition index. Associations of adipose tissue insulin resistance with β-cell function (inverse) and glucose excursion in young Japanese women may suggest that lipotoxicity to pancreatic β-cells for decades may be associated with β cell dysfunction found in Japanese patients with type 2 diabetes. Positive association of HOMA-IR with insulinogenic index may be associated with compensatory increased insulin secretion.
Similar content being viewed by others
Introduction
Insulin resistance and impaired insulin secretion are major hallmarks of type 2 diabetes. In the course of the development of the disease, insulin resistance may cause elevation of blood glucose, which may increase insulin secretion to maintain glucose homeostasis. However, chronic insulin resistance may lead to β cell exhaustion and eventually to β cell dysfunction and/or cell death to cause overt diabetes. It is widely recognized that type 2 diabetes is characterized primarily by β cell dysfunction in East Asians whereas insulin resistance predominates in Caucasians1.
In subjects with normal β-cell function, a relationship between insulin sensitivity and insulin responses is hyperbolic2. Therefore, the product of these two variables, referred to as the disposition index (DI), can evaluate the ability of the β-cell to compensate for insulin resistance. As prospective studies showed that DI predicted the development of future diabetes3, low DI is an early marker of inadequate β-cell compensation.
Chronically elevated circulating concentrations of glucose and lipid in the form of free fatty acids (FFA) have toxic effects, referred to as glucotoxicity and lipotoxicity, respectively, both of which emerged as established mechanisms participating in the loss of β-cell function4,5. Homeostasis-model assessment- insulin resistance (HOMA-IR) is an index that focuses on glucose metabolism, which encompasses hepatic and muscle tissue, and is calculated as a product of fasting insulin and glucose6. HOMA-IR is a proxy of hepatic and muscle insulin resistance and elevated HOMA-IR may be associated with elevations in blood glucose and eventually glucotoxicity. As the adipose tissue insulin resistance (AT-IR) index is calculated as a product of fasting insulin and FFA7,8, elevated AT-IR may be associated with elevations in circulating FFA and eventually lipotoxicity. We showed that AT-IR may be a simple and useful surrogate index of adipose tissue insulin resistance even in young and middle-aged Japanese women without diabetes and obesity9. We further suggested that the adipose insulin-resistant but normal weight phenotype may be associated with increased sympathetic nervous system and low-grade systemic inflammation10. We recently reported that AT-IR was associated with reduced leg fat, a subtle partial lipodystrophy-like phenotype associated with reduced adipose tissue expandability in young Japanese women11.
Studies on associations of AT-IR with pancreatic β-cell function were scarce and limited in the setting of obesity as described later. Further, little is known in non-obese populations. We, therefore, tested, in non-obese populations, whether AT-IR may be associated with pancreatic β-cell function, whether the relationship to β-cell function may be different between AT-IR and HOMA-IR, and whether the relationship may differ concerning age, which has a substantial influence on glucose metabolism. To do this, we investigated the relationship of AT-IR and HOMA-IR to glucose-stimulated insulin secretion and β-cell function (glucose-stimulated insulin secretion adjusted for insulin resistance/insulin sensitivity) not only in young Japanese women but also in middle-aged Japanese women, who were nondiabetic and nonobese.
Methods
We cross-sectionally studied 168 Japanese female students (50 collegiate athletes and 118 non-athletes) of the University, and 68 biological mothers of the students, who underwent a standard 75 g oral glucose tolerance test (OGTT) as previously reported11,12,13,14. Athletes were students of the Department of Health and Sports Sciences and nonathletes were students of the Department of Food Sciences and Nutrition. We excluded women with clinically diagnosed acute or chronic diseases, those on hormonal contraception and those on a diet to lose weight from the study. Nobody reported to receiving any medications or having regular supplements. The study was in accordance with the Helsinki declaration. All subjects were recruited as volunteers and gave written consent after the experimental procedure had been explained. Participant recruitment and data collections were described in detail previously12.
After a 12-h overnight fast, height and weight were measured and participants underwent a standard 75 g OGTT with glucose and insulin measurements at 0 (fasting), 30 min, 1 h, and 2 h. Glucose was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (inter-assay coefficient of variation (CV) < 2%). Serum insulin was measured by an ELISA method with a narrow specificity excluding des-31, des-32, and intact proinsulin (interassay CV < 6%). FFA was measured using enzymatic colorimetric methods (Wako, Tokyo, Japan). Incremental 30 min glucose and insulin concentrations were calculated in each participant (ΔGlucose30 and ΔInsulin30, respectively). Area under the response curve of glucose (AUCg) and insulin (AUCi) were calculated by the trapezoidal method. HOMA-IR, the Matsuda index, and the insulinogenic index (IGI)), a measure of glucose-stimulated insulin secretion, were calculated as previously reported6,15,16. People with borderline-type OGTT and IGI < 0.4 have been reported to have a high risk of progressing to type 2 diabetes17. AT-IR was calculated as a product of fasting FFA and insulin7,8. To evaluate β-cell function, DI (the insulin secretion × sensitivity index) was calculated as the product of IGI and Matsuda index.
Data were presented as mean ± SD. Due to deviation from normal distribution, IGI and DI were logarithmically transformed for analyses. Bivariate correlations of AT-IR and HMA-IR with other parameters were evaluated by Pearson correlation analysis. Differences between the two groups were analyzed with a t-test and χ2 test when appropriate. A two-tailed value of p < 0.05 was considered significant. Statistics were performed with SPSS system 23.0 (SPSS Inc, Chicago, IL).
Ethical approval
The study was approved by the Ethics Committees of the Mukogawa Women’s University (No. 07-28 on 19/02/2008).
Results
Young and middle-aged women on average were normoglycemic (Table 1). Normal glucose tolerance was found in 161 young (95.8%) and 51 middle-aged women (78.5%) as previously reported13,14. Glucose levels at 4 time points, AUCg, and HbA1c were higher in middle-aged mothers. They had higher ΔGlucose30, lower ΔInsulin30, and hence lower IGI and disposition index. However, their IGI averaged 1.1, which is much higher than 0.417, suggesting preserved glucose-stimulated insulin secretion in mothers. Extremely high mean IGI and ODI in young women were due to extremely low ΔGlucose30 (5 mg/dL and lower) in 13 students. Extremely low ΔGlucose30 was found in two mothers as well. There was no difference in 1-h and 2-h insulin, AUCi, HOMA-IR, AT-IR, and Matsuda index probably because middle-aged women were non-obese rather lean although BMI was higher in middle-aged compared with young women.
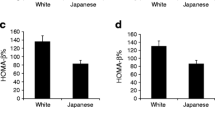
In young women (Table 2 and Fig. 1), AT-IR, but not HOMA-IR, showed positive associations with post-glucose glycemia including 2-h glucose and area under the glucose response curve although both indices were associated with fasting glycemia. Both indices were inversely associated with the Matsuda index. HOMA-IR was associated positively with log IGI but not with log DI. In contrast, AT-IR showed no association with log IGI but an inverse association with log DI (Table 2 and Fig. 2).
We divided young women into two groups according to a median AT-IR. Women with elevated AT-IR had lower log DI and higher AUCg (Fig. 2).
In middle-aged women (Table 3), both AT-IR and HOMA-IR showed an inverse association with the Matsuda index, no association with log IGI, and an inverse association with log DI and hence a positive association with AUCg.
Discussion
The present study has shown that elevated adipose tissue insulin resistance (measured by AT-IR) was associated with impaired β-cell function (glucose-stimulated insulin secretion adjusted for insulin sensitivity evaluated by DI) and hence with higher glucose excursion (evaluated by AUCg) in women in their early twenties, in whom more than 95% had normal glucose tolerance and BMI averaged 20.8 kg/m2. In contrast, HOMA-IR showed a positive association with glucose-stimulated insulin secretion (measured by IGI) and no association with log DI in young women. In middle-aged women, both AT-IR and HOMA-IR were associated with β-cell dysfunction and higher glucose excursion.
Associations of AT-IR with pancreatic β-cell function were reported. in obese adolescents, obese youth with impaired glucose tolerance, and obese middle-aged people including lean controls whose BMI averaged 25 kg/m218,19,20. However, little is known in non-obese populations. The present study has shown that an inverse association of AT-IR with DI was found even in young Japanese women whose BMI averaged 20.8 kg/m2 and HbA1c 5.2 %. The positive association of HOMA-IR with IGI (glucose-stimulated insulin secretion) in young women may be in line with the hyperbolic relationship between insulin resistance and insulin secretion2. Despite the inverse association with the Matsuda index, there was no association of HOMA-IR with β-cell function (DI).
Lipotoxicity to pancreatic β-cells5 may be a likely explanation for an inverse association of AT-IR with DI as AT-IR is calculated as a product of fasting insulin and FFA7,8. Studies reported that higher FFA levels were associated with lower insulin secretion21,22,23,24,25,26. For example, in children and adults with normal glucose tolerance. Higher fasting FFA levels were cross-sectionally associated with lower insulin secretion and prospectively associated with a greater risk of subsequent type 2 diabetes21. We found an inverse association of fasting FFA concentrations with log DI in young and middle-aged Japanese women (r = − 0.21, p = 0.007 and r = − 0.28, p = 0.027, respectively) (paper in preparation). We also reported that in another set of young Japanese female students, higher fasting and postprandial FFA levels were associated with lower meal-induced insulin response22.
Subcutaneous gluteofemoral AT is the largest and safe place to store excess fat to protect against cardiometabolic diseases27,28. As previously reviewed27,29, a major determinant of metabolic health is the ability of subcutaneous gluteofemoral (leg) fat to expand. The impaired ability of leg fat to store excess lipids leads to hypertrophic, dysfunctional, and insulin-resistant adipose cells29. Asian populations including Japanese may have a reduced ability to expand gluteofemoral fat30. It is proposed that increased FFA overflow from dysfunctional AT associated with impaired expansion of subcutaneous AT may lead to ectopic lipid accumulation on the one hand and may be toxic to β-cell function on the other hand in non-obese Asian people. Taken together, the present study suggests that AT-IR associated with impaired adipose tissue expandability11 may be at play through lipotoxicity in the very early stage of the development of impaired β-cell function in Japanese, in whom type 2 diabetes is characterized primarily by β cell dysfunction1. We reported in young Japanese female university students that low gluteofemoral fat was associated with a positive family history of type 2 diabetes and low birthweight31,32, both of which are well-known risk factors for type 2 diabetes33,34.
Glucotoxicity to pancreatic β cell function4 may be associated with an inverse association of HOMA-IR with DI in middle-aged women, in whom fasting serum triglycerides averaged 81 mg/dL9.
The strength of the present study includes a homogeneous study population with few confounding factors35. Several limitations of this study warrant consideration. The cross-sectional design complicates the drawing of causal inferences, and a single measurement of biochemical variables may be susceptible to short-term variation, which would bias the results toward the null. We used crude measures of insulin sensitivity/IR and insulin secretion, which may be less accurate. Statistical power including the sample size calculation was not estimated. As we studied young Japanese women only, results may not be generalized to other genders, age populations, races, or ethnicities.
In conclusion, an inverse association of AT-IR with DI, an early marker of inadequate β-cell compensation and a reliable predictor of type 2 diabetes3, in young Japanese female university students may suggest that lipotoxicity to pancreatic β-cells5 may be associated with impaired β cell function found in Japanese patients with type 2 diabetes1. Nearly half of all adults with type 2 diabetes mellitus live in Asia, mainly in India and China36. A systematic review suggested that East Asian people including Japanese had high sensitivity to insulin but a severely limited innate capacity to secrete insulin37. This limited capacity may be associated with the inverse association of AT-IR with DI observed in young and middle-aged Japanese people in the present study.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Yabe, D., Seino, Y., Fukushima, M. & Seino, S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr. Diab. Rep. 15(6), 602 (2015).
Kahn, S. E. et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: Evidence for a hyperbolic function. Diabetes 42, 1663–1672 (1993).
Utzschneider, K. M. et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32, 335–341 (2009).
Weir, G. C. Glucolipotoxicity, β-cells, and diabetes: The emperor has no clothes. Diabetes 69, 273–278 (2020).
Ye, R., Onodera, T. & Scherer, P. E. Lipotoxicity and β cell maintenance in obesity and type 2 diabetes. J. Endocr. Soc. 3, 617–631 (2019).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Søndergaard, E. & Jensen, M. D. Quantification of adipose tissue insulin sensitivity. J. Investig. Med. 64, 989–991 (2016).
Søndergaard, E. et al. How to measure adipose tissue insulin sensitivity. J. Clin. Endocrinol. Metab. 102, 1193–1199 (2017).
Kitaoka, K. et al. Determinants and correlates of adipose tissue insulin resistance index in Japanese women without diabetes and obesity. BMJ Open Diabetes Res. Care 8(1), e001686 (2020).
Minato-Inokawa, S. et al. Higher fasting glucose, triglycerides, resting pulse rate and high-sensitivity C reactive protein in adipose insulin-resistant but normal weight young Japanese women. BMJ Open Diabetes Res. Care 10(6), e003013 (2022).
Minato-Inokawa, S. et al. Associations of adipose insulin resistance index with leg (gluteofemoral) fat (inverse) and serum alanine aminotransferase (positive) in young Japanese women. Metab. Open 22, 100289 (2024).
Takeuchi, M. et al. Weight trajectory since birth, current body composition, dietary intake, and glucose tolerance in young underweight Japanese women. Womens Health Rep. (New Rochelle) 3, 215–221 (2022).
Tsuboi, A. et al. Association of serum orosomucoid with 30-min plasma glucose and glucose excursion during oral glucose tolerance tests in normal weight young Japanese women. BMJ Open Diabetes Res. Care 6(1), e000508 (2018).
Tsuboi, A. et al. Higher circulating orosomucoid, an acute-phase protein, and reduced glucose-induced insulin secretion in middle-aged Japanese people with prediabetes. BMJ Open Diabetes Res. Care 8(2), e001392 (2020).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Stumvoll, M. et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23, 295–301 (2000).
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 19, 212–28 (2010).
Hershkop, K. et al. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J. Clin. Endocrinol. Metab. 101, 2423–2431 (2016).
Kim, J. Y. et al. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes 66, 3085–3090 (2017).
Gastaldelli, A., Gaggini, M. & DeFronzo, R. A. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: Results from the San Antonio Metabolism Study. Diabetes 66, 815–822 (2017).
Salgin, B. et al. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J. Clin. Endocrinol. Metab. 97, 3302–3309 (2012).
Takeuchi, M. et al. Higher fasting and postprandial free fatty acid levels are associated with higher muscle insulin resistance and lower insulin secretion in young non-obese women. J. Clin. Med. Res. 10, 822–829 (2018).
Shitole, S. G. et al. Fasting and postload nonesterified fatty acids and glucose dysregulation in older adults. Am. J. Epidemiol. 191, 1235–1247 (2022).
Johnston, L. W. et al. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia 61, 821–830 (2018).
Il’yasova, D., Wang, F., D’Agostino, R. B. Jr., Hanley, A. & Wagenknecht, L. E. Prospective association between fasting NEFA and type 2 diabetes: Impact of post-load glucose. Diabetologia 53, 866–874 (2010).
Stefan, N., Stumvoll, M., Bogardus, C. & Tataranni, P. A. Elevated plasma nonesterified fatty acids are associated with deterioration of acute insulin response in IGT but not NGT. Am. J. Physiol. Endocrinol. Metab. 284, E1156–E1161 (2003).
Manolopoulos, K. N., Karpe, F. & Frayn, K. N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. (Lond.) 34, 949–959 (2010).
Lotta, L. A. et al. Association of genetic variants related to gluteofemoral vs. abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA 320, 2553–2563 (2018).
Smith, U. & Kahn, B. B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 280, 465–475 (2016).
Yaghootkar, H., Whitcher, B., Bell, J. D. & Thomas, E. L. Ethnic differences in adiposity and diabetes risk—Insights from genetic studies. J. Intern. Med. 288, 271–283 (2020).
Honda, M. et al. Reduced gluteofemoral (subcutaneous) fat mass in young Japanese women with family history of type 2 diabetes: An exploratory analysis. Sci. Rep. 12(1), 12579 (2022).
Honda, M. et al. Birth weight was associated positively with gluteofemoral fat mass and inversely with 2-h postglucose insulin concentrations, a marker of insulin resistance, in young normal-weight Japanese women. Diabetol. Int. 13, 375–380 (2021).
Carlsson, S. et al. Low birth weight, family history of diabetes, and glucose intolerance in Swedish middle-aged men. Diabetes Care 22, 1043–7 (1999).
Grill, V. et al. Family history of diabetes in middle-aged Swedish men is a gender unrelated factor which associates with insulinopenia in newly diagnosed diabetic subjects. Diabetologia 42, 15–23 (1999).
Tanaka, M. et al. FTO, abdominal adiposity, fasting hyperglycemia associated with elevated HbA1c in Japanese middle-aged women. J. Atheroscler. Thromb. 19, 633–642 (2012).
Ke, C., Narayan, K. M. V., Chan, J. C. N., Jha, P. & Shah, B. R. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat. Rev. Endocrinol. 18, 413–432 (2022).
Kodama, K. et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 36, 1789–1796 (2013).
Acknowledgements
We thank all participants for their dedicated and conscientious collaboration.
Author information
Authors and Affiliations
Contributions
S.MI., M.H., A.TK. and M.T. collected data and prepared tables. K.K., M.K. and B.W. analyzed data and prepared figures. T.K. wrote the manuscript, and K.F. reviewed and edited it. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Minato-Inokawa, S., Honda, M., Tsuboi-Kaji, A. et al. Associations of adipose insulin resistance index with pancreatic β cell function (inverse) and glucose excursion (positive) in young Japanese women. Sci Rep 14, 18590 (2024). https://doi.org/10.1038/s41598-024-69181-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69181-1
- Springer Nature Limited