Abstract
Free radical is a marker in various inflammatory diseases. The antioxidant effect protects us from this damage, which also plays an essential role in preventing inflammation. Inflammation protects the body from biological stimuli, and pro-inflammatory mediators are negatively affected in the immune system. Inflammation caused by LPS is an endotoxin found in the outer membrane of Gram-negative bacteria, which induces immune cells to produce inflammatory cytokines such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase. Based on this, the antioxidant and anti-inflammatory effects of plant extracts were investigated. First, the main phenolic compounds for the five peaks obtained from Stachys affinis extract (SAE) were identified. The antioxidant effect of each phenolic compound was confirmed through HPLC analysis before and after the competitive binding reaction between DPPH and the extract. Afterward, the anti-inflammatory effect of each phenolic compound was confirmed through competitive binding between COX2 and the extract in HPLC analysis. Lastly, the anti-inflammatory effect of SAE was confirmed through in vitro experiments and also confirmed in terms of structural binding through molecular docking. This study confirmed that phenolic compounds in SAE extract have potential antioxidant and anti-inflammatory effects, and may provide information for primary screening of medicinal plants.
Similar content being viewed by others
Introduction
Stachys affinis is a root plant of the Lamiaceae family, native to China and East Asia. It contains numerous phenolic compounds that activate brain function and prevent dementia1,2. It is also known as a preventive factor for fatty liver and has the effect of arteriosclerosis, and beneficial for colds, sore throat, bronchitis, and intestinal health3. Although research exists on the antioxidant and anti-inflammatory effects of Stachys affinis, antioxidant and anti-inflammatory research on individual compounds in the plant is lacking1. An accurate screening of plant components is required to conduct related research. High-Performance Liquid Chromatography (HPLC) can be used to efficiently screen complex plant extracts such as Stachys affinis and rapidly analyze the components within the extract4. Especially, Liquid Chromatography–Mass Spectrometry (LC–MS) technology provides an analytical method for high sample throughput and is well suited for the analysis of complex system components in plant extracts at low concentrations5. This analysis can identify various phenolic compounds in various plant extracts6.
Natural phenolic compounds, which are various secondary metabolites of plants, control oxidative stress and suppress inflammation7. Many scientists have tried to determine the antioxidant and anti-inflammatory effects of plant extracts rather than chemically synthesized drugs that have side effects. Most plant extracts are known to act as antioxidants, and they work to prevent this oxidation state by donating electrons and maintaining balance in the body by controlling free radicals8,9. Increased oxidative stress is closely related to diseases such as inflammation10. Inflammatory diseases are related to many chronic diseases, including neurological diseases, heart disease, alcoholic hepatitis, diabetes, malignant tumors, and geriatric diseases11.
In the inflammatory process, pro-inflammatory mediators such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) regulate inflammation12. COX is a protein that converts arachidonic acid into compounds such as thromboxane and prostaglandins13. The inflammatory response is mediated by COX-2, one of two cyclooxygenases14. iNOS is induced by inflammation, and when iNOS is activated, nitric oxide is released15. In particular, nitric oxide (NO) is an essential signaling molecule in the immune response and, as a retrograde neurotransmitter, is one of the prominent features of inflammation16.
Many studies, such as in vitro and biochemical analyses, are being conducted to confirm the antioxidant and anti-inflammatory effects of plant extracts. DPPH analysis is used to measure the antioxidant capacity of various ingredients. This analysis is performed using the stoichiometric method, which refers to the number of electrons or hydrogen atoms that can be donated to free radicals17. Additionally, DPPH analysis can measure the antioxidant capacity of bioactive compounds under various reaction conditions18. Some studies have been conducted to combine this DPPH analysis with HPLC to rapidly identify potential phenolic compounds affecting the antioxidant effect19. In addition, by using HPLC to identify phenol compounds that competitively react by binding COX2 to the extract, potential anti-inflammatory properties compounds can be identified19.
Lastly, it is known that molecular docking may anticipate functional locations on the surface of protein molecules. The form of protein–ligand binding sites and structure-based drug design makes it an extensively utilized technique20. This analytical bioinformatics modeling is also used to predict the binding affinity of specific compounds with target proteins21. Therefore, in order to validate the molecular docking outcome, the structural bonding must be visually verified22.
In this study, we screened potential phenolic compounds that can affect SAE's antioxidant and anti-inflammatory effects through the principle of binding specific compounds and enzymes. Afterward, the anti-inflammatory effect of the extract was confirmed in vitro. Through molecular docking, we identified the structural binding affinity of anti-inflammatory proteins with screened phenolic compounds.
Results
Separation and characterization of phenolic compound in SAE
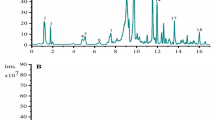
Qualitative analysis of compounds in Stachys affinis extract (SAE) was performed through HPLC–MS/MS. Five major peaks were obtained from UV–vis spectra and HPLC retention times (Fig. 1). The five phenolic compounds were identified 3-Indoleacrylic acid23, Chlorogenic acid24, Rutin25, Kaempferol26, and Astragalin27. In Table 1, the mass spectrometry of each compound was investigated using MS/MS data. It was based on fragmentation patterns generated from public sources for five compounds. The molecular ion peaks and mass patterns of the discovered phenols were compared with data already available in the literature23,24,25,26,27. Also these parameters of phenol were validated using published data28. The outcomes of predicting compound fragmentation using LC–MS/MS data are displayed in Fig. 2.
The HPLC chromatograms of the phenolic compounds in SAE. (A) The blue line is the original chromatogram of SAE phenolic compound, and the orange line is the chromatogram after the reaction with DPPH solution (B) Also the original chromatogram of SAE is blue, and the chromatogram following reaction with COX2 is orange peak. The detected compounds at the 284 nm wavelength are 3-Indoleacrylic acid (1), Chlorogenic acid (2), Rutin (3), Kaempferol (4), and Astragalin (5).
Screening for potential antioxidant phenolic compounds in SAE
The peak area values of the five selected compounds and their change rates before and after DPPH reaction are shown in Table 2. First, looking at the change in peak area value, the order was Rutin (214.27), 3-Indoleacrylic acid (211.67), Chlorogenic acid (199.67), Kaempferol (21.03), and Astragalin (7.67). However, the relative area change rate was highest for 3-indoleacrylic acid at 25.93%, followed by chlorogenic acid (23.25%) and rutin (6.87%). Therefore, by comprehensively examining the absolute area value difference and change rate before and after the DPPH reaction, 3-Indole acrylic acid and chlorogenic acid were screened as compounds that bind to DPPH and are significantly responsible for its antioxidant activity. From this result, the potential antioxidant ability of each phenolic compound contained in the SAE extract was evaluated.
Screening for potential anti-inflammatory phenolic compounds in SAE
The peak area values and rate of change of five selected compounds among the extracts bound to activated or deactivated COX2 are shown in Table 3. First, the peak area values are in the following order: Rutin (854), Chlorogenic acid (352), 3-Indoleacrylic acid (253), Astragalin (93.67), and Kaempferol (35). However, the relative peak area change rate is 3-Indoleacrylic acid (9.88), Chlorogenic acid (9.66%), and Rutin (6.52%). What is noteworthy is that the change value of Astragalin was 93.67, but the change rate was quite high at 4.31%. As a result, looking at the difference in peak area change value and change rate according to COX2 binding, it can be confirmed that 3-indole acrylic acid and chlorogenic acid among the phenolic components of the extract will play an important role in COX2-related anti-inflammatory action.
Anti-inflammatory effects of SAE
Phenolic compounds with potential antioxidant and anti-inflammatory effects were screened in SAE. This screening provides conditions under which the phenolic compounds in the extract may be effective in antioxidant and anti-inflammatory properties. Additionally, it is necessary to confirm the anti-inflammatory effects of the extract in vitro condition. Therefore, in vitro experiments were conducted by treating SAE.
Cytotoxicity of SAE on RAW264.7 Cells
The 3-(3,4-dimethyl-thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) assay was carried out in RAW264.7 cells to verify the cytotoxicity of the extract (Fig. 3A,B). With or without 1 µg/mL of LPS, the SAE treated RAW 264.7 cells at concentrations of (10, 25, 50, 75, 100, and 250 ng/mL) for 24 h. In Fig. 3A, the SAE concentration had no significant cytotoxicity through cell viability up to 75 ng/mL, and in Fig. 3B, the concentration (20 and 50 ng/mL) was set.
Cytotoxicity effect of SAE on RAW264.7 cells and the inhibition of inflammatory marker in LPS inflammation-induced RAW264.7 cells. RAW264.7 cells were pretreated without or with LPS (1 µg/mL) for 1 h at 37 °C. Following that, cells were treated with SAE (0, 10, 25, 50, 75, 100, 250 ng/mL) for 24 h at 37 °C. (A) Cytotoxicity effect of SAE on without LPS induced RAW264.7 cells. (B) SAE on LPS induced cell viability in RAW264.7 cells. The RAW264.7 cells were treated with SAE (25, and 50 ng/mL) at indicated concentration for 24 h. COX2 and iNOS levels were quantified. (C) The relative area of COX2 and (D) the relative area of iNOS. Results from three independent experiments were expressed as mean ± standard error of the mean (SEM) compared with control. ###p < 0.001 versus untreated group; *p < 0.05 **p < 0.01, ***p < 0.001 versus LPS treated group.
Inhibition of COX-2 and iNOS expression by SAE in LPS-induced RAW264.7 cells
The iNOS increases in NO production, and it has been suggested that iNOS inhibition may be a successful anti-inflammatory strategy. Additionally, numerous inflammatory cytokines are produced by COX2, which has been implicated in the development of chronic inflammatory diseases2. Therefore, the downregulation of iNOS and COX2 plays an important role in suppressing inflammation.
In RAW 264.7 cells inflammation induced by LPS, SAE treatment dose-dependently inhibited iNOS and COX2 (Fig. 3C,D). These results indicated that SAE suppresses LPS-mediated inflammation by downregulating inflammation-related marker COX2 and iNOS.
NO and PGE2 inhibition of SAE in LPS induced RAW264.7 cells
As a result of treating SAE in LPS-induced RAW264.7 cells, it can be confirmed that NO production is suppressed (Fig. 4A). These results showed that NO (Nitric Oxide) was significantly increased when RAW264.7 cells were treated with LPS, and NO was significantly decreased in a dose-dependent manner when treated with SAE. Therefore, SAE plays an important role in mediating inflammation by downregulating NO and suppresses the early inflammatory response. RAW264.7 cells were treated with LPS to induce an increase in PGE2 and then treated with SAE in a dose-dependent manner, resulting in a significant decrease in PGE2 (Fig. 4B). Figure 3C shows that SAE causes a significant decrease in COX2, predicting a decrease in PGE2. Accordingly, a decrease in PGE2, an inflammatory mediator, was also confirmed.
ROS production effect of SAE
ROS is a production of cellular metabolism, and upon LPS treatment, RAW264.7 cells generate ROS causing oxidative stress and cell damage29. Therefore, ROS was induced through LPS in this study, and the ROS inhibition effect of the extract was confirmed. RAW 264.7 cells were treated with LPS for 24 h to induce ROS production, and SAE was treated in a dose-dependent manner. Figure 5 shows that SAE treated at 50 ng/mL significantly reduced the level of ROS induced by LPS. In addition, RAW264.7 cells induced by LPS were treated with SAE in a concentration-dependent manner, and the intensity of ROS was confirmed through fluorescence cell imaging through staining (Fig. 5A). In Fig. 5B, it was confirmed that ROS increased in the LPS-treated group and that ROS decreased in a dose-dependent manner when treated with the extract.
ROS production of SAE on RAW264.7 cells. RAW264.7 cells were pretreated without or with LPS (1 µg/mL) for 1 h at 37 °C. Following that, cells were treated with SAE (0, 25, and 50 ng/mL) for 24 h at 37 °C. (A) ROS detection of SAE on LPS induced RAW264.7 cells. (B) Quantitative analysis of ROS production of SAE on LPS induced RAW264.7 cells. Results from three independent experiments were expressed as mean ± standard error of the mean (SEM) compared with control. ##p < 0.01 versus untreated group; **p < 0.01 versus LPS treated group.
Molecular docking of selected phenolic compounds with NF-κB
The structural affinity binding of selected phenolic compounds in SAE with NF-κB was analyzed through molecular docking (Fig. 6). Among the five phenolic compounds obtained from the identification of phenolic compounds from SAE, the two compounds (3-Indoleacrylic acid and Chlorogenic acid) that bind highly to DPPH and COX2 and one compound (Astragalin) that bind low were selected. Molecular docking of three compounds was performed through the UCSF Chimera program and AutoDock Vina. The binding active sites of 3-Indoleacrylic acid and NF-κB are (GLU184, ARG232, HIS183, LEU236, CYS149, and TYR227), and the docking energy score is − 6.5 kcal/mol. The binding sites for Chlorogenic acid and NF-κB are (TYR227, ARG237, PHE146, LEU236, ARG239, ASN240, and GLU233), and the docking energy score was the highest at − 6.8 kcal/mol. The binding score of astragalin was measured to be − 5.8 kcal/mol and the binding sites were (HIS187, ZN401, TYR227 and ASP194) (Table 4).
In the previous COX2 binding results (Tables 3), Astragalin was a compound with a relatively low binding reaction. However, Chlorogenic acid and 3-indoleacrylic acid are compounds that showed a high significantly competitive binding reaction with COX2. Therefore, it can be predicted that Chlorogenic acid and 3-Indoleacrylic acid will be higher than Astragalin in molecular docking scores. As expected, Table 4 shows that the scores of chlorogenic acid and 3-indole acrylic acid were relatively higher compared to astragalin. This result shows that there is a significant correlation between the molecular docking results and the competitive reactivity.
Molecular docking of selected phenolic compounds with COX2 and iNOS
COX-2 (Cyclooxygenase-2) and iNOS (Inducible Nitric Oxide Synthase), which play a mediating role in the inflammatory process, have been studied as direct indicators of inflammation, and research combining molecular docking is actively in progress30. Therefore, molecular docking of COX2 and the screened phenolic compounds was performed in this study (Fig. 7). In the molecular docking of 3-Indoleacrylic acid and COX2, the binding score was − 7.6 kcal/mol and the binding sites were LEU352, SER353, HIS90, VAL349, and TRP387. The binding score with chlorogenic acid and COX2 is the highest, − 8.6 kcal/mol, and has binding sites for ARG469, ASN34, CYS36, CYS41, GLU465, GLY45, and PRO153. Astragalin had the lowest binding score of − 6.9 kcal/mol, and its binding sites were THR212, HIS214, HIS386, HIS207, and VAL291 (Table 5).
Through additional docking analysis, we confirmed the structural binding of the three compounds to iNOS, another inflammatory marker (Fig. 8). As a result of docking with iNOS, the binding scores for Chlorogenic acid (ALA191, ARG193, GLU371, TYR483, MET349) were − 7.7 kcal/mol and 3-Indoleacrylic acid (ALA191, ARG193, PRO192, TRP188, TRP457, TYR485, LEU119, MET349) were − 7.4 kcal/mol. Astragalin (ASP376, GLU371, GLN257, TRP84, TRP457, MET114) showed a relatively low structure binding score of − 6.8 kcal/mol (Table 6).
In addition, it was confirmed that the screened phenolic compound was a component responsible for the anti-inflammatory effect in terms of structural binding of COX2 and iNOS docking. All three compounds screened for molecular docking contributed to anti-inflammatory effects but showed a significant correlation with previous peak area change results. In other words, it can be seen that the binding point energy of Chlorogenic acid and 3-indoleacrylic acid, which are the most reacted compounds in the COX2 binding HPLC results, is relatively high.
Discussion
The present study screened SAE, a Stachys affinis plant extract, for phenolic compounds with antioxidant and anti-inflammatory potential. Previous research evidence suggests that this Stachys affinis or Stachys affinis Bunge extract, obtained through various fractions, has potential antioxidant and anti-inflammatory effects1,31. However, the potential antioxidant and anti-inflammatory activities of each phenolic compound in the extract were confirmed through HPLC, and peaks with significantly high activity were identified in this study. The five major phenolic compounds identified in the study were 3-Indoleacrylic acid, Chlorogenic acid, Rutin, Kaempferol, and Astragalin. Each of these compounds is a phenolic compound with established antioxidant and anti-inflammatory properties. Although 3-Indoleacrylic acid has not been well studied for its antioxidant and anti-inflammatory properties, there is some evidence that microbially derived 3-Indoleacrylic acid may reduce inflammatory responses32. It is known that Chlorogenic acid has anti-inflammatory and antioxidant properties, largely due to its ability to activate the Nrf2 signaling pathway33. Rutin has also been shown to have antioxidant properties and exert anti-inflammatory effects by inhibiting pro-inflammatory factors such as tumor necrosis factor-α, interleukin (IL)-6, cyclooxygenase-2, and IL-1β, and by blocking nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) activation34. Additionally, kaempferol exerts anti-inflammatory effects by inhibiting phosphorylation of PI3K and AKT. Some studies have shown that it modulates the expression of heme oxygenase (HO)-1 and the mitogen-activated protein kinase (MAPK) pathways, thereby decreasing cellular oxidative stress and increasing antioxidant capacity35,36. Astragalin is a kaempferol-3-O-β-d-glucoside that exhibits antioxidant effects by modulating oxidative stress induced by endotoxin and anti-inflammatory effects by modulating pro-inflammatory factors such as IL- 1β and IL-637.
Radical scavenging activity assay using DPPH (2,2-Diphenyl-1-picrylhdrazyl) reagent is mainly used to investigate the antioxidant effect of natural products. 2,2-diphenyl-1-picrylhydrazyl (DPPH) is a reagent used to measure plant extracts or foods38. The DPPH analysis of the extract itself is a method that utilizes the scavenging ability of free radicals, so it is difficult to relate it to biological reactions. Furthermore, it has the disadvantage of requiring a constant calculation including the reaction rate and a process of normalization to the ascorbic acid concentration39. We performed the DPPH and HPLC combined analysis to screen the potential antioxidant capacity of five selected phenolic compounds from SAE. The antioxidant activity of each compound was evaluated through the peak area change value of the extract combined with DPPH using HPLC19.
Prostaglandins are hormone-like substances that are produced from arachidonic acid in response to injury or infection in the body and play an important role in the inflammatory response. The COX2 enzyme is involved in the conversion of arachidonic acid to prostaglandins during this process and many anti-inflammatory studies have targeted COX2@@40. In this study, HPLC analysis was performed by combining the extract with the COX2 enzyme to confirm the anti-inflammatory effect of the five phenolic compounds selected in SAE. The area values of the two HPLC peaks (COX2 activated vs. inactivated) were first analyzed to determine the difference in the reaction area values of the phenolic compounds in each peak.
Inflammation caused by LPS stimulation then binds to Toll-like receptors, leading to the expression of COX2 and iNOS, downstream through the NF-κB pathway41. An anti-inflammatory in vitro study was conducted using a model in which inflammation was induced by treating normal RAW264.7 cells with LPS, and in this regard, the NF-κB transcription factor acts as a key regulator41. Based on this, the NF-κB protein was selected as the target receptor for LPS-induced inflammation in RAW 264.7 cells. However, NF-κB controls a wide range of biological mechanisms beyond inflammation. Therefore, it is essential to confirm the molecular association of each screened phenolic compound by targeting COX2, iNOS and NF-κB. Molecular docking aims to predict the binding affinity of a ligand to a receptor protein42. Molecular docking is largely accomplished in two steps. First, the protein's active site and the ligand's predicted binding site are modeled with shape and chemical characteristics, and then binding affinity is indicated through two scoring functions: extended force-field-based scoring functions and knowledge-based scoring functions43.
Currently, various synthetically derived anti-inflammatory drugs are used, but side effects still exist compared to natural agents44. In particular, famous anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) exhibit anti-inflammatory effects by inhibiting COX action, but these also have side effects such as gastrointestinal bleeding45. This research has identified compounds in SAE extracts that are responsible for a range of anti-inflammatory effects, which can later be compared to a range of conventional chemical anti-inflammatory drugs to determine their relative efficacy.
Lastly, screening each compound identified in the extract through these results is significant because it can be used in various industrial fields. There is a limitation in that it is difficult to explain the exact action based on the effect of the entire natural extract. However, which characterization of each compound in the natural extracts through screening methods such as this study, can contribute to many food and pharmaceutical industries.
Material and method
Plant materials
Nationally designated research data Animal Bio Resources Bank (http://www.abrb.or.kr) provided Stachys affinis (Code number: 00286A) was used in the experiment. The plant material for this study was grown by a professional farm in Jirisan, Gyeongsangnam-do, and a code number is assigned after the Animal Bio Resources Bank professional identification manager completes the identification of the plant material. It was later stored in the herbarium for distribution and research purposes. Animal Bio Resources Bank is a nationally designated research data bank with optimal storage conditions for storing and distributing research data. The use of plant materials was approved after receiving permission from Bank President, Gon Sup Kim. Lastly, all experiment complied with relevant institutional, national, and international guidelines and legislation.
After receiving the plant material, and being rinsed with water, the plant was chopped and dried for 72 h in a 56 °C dry oven. It was then stored in sealed polyethylene bags containing silica gel at − 20 °C until use.
Reagents, chemicals and standards
The DPPH (2,2-Diphenyl-1-picrylhdrazyl) reagent and recombinant human COX-2(Cyclooxiganase2) were purchased Sigma-Aldrich Corp (St. Louis, MO, USA. cas no. 1898-66-4). A centrifugal ultrafiltration filter (YM-30) with a capacity of 30 kDa (Millipore Co., Ltd) was purchased. All other chemicals and solvents utilized were of analytical grade and purchased from Duksan Pure Chemical Co. Ltd. (Dongdaemun-gu, Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), and antibiotics penicillin/streptomycin (P/S) were purchased from Gibco (BRL Life Technologies, Grand Island, NY, USA). Antibodies to COX-2 (cat. no. 12282S), iNOS (cat. no. 13120S), and β-actin (cat. no. 3700S) were purchased from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies to antirabbit (cat. no. A120-101P) and antimouse (cat. no. A90-116P) were obtained from Bethyl Laboratories, Inc. (Montgomery, AL, USA).
Extraction and purification of SAE phenolic compounds
Stachys affinis was used to isolate phenolic compounds from plants in the following method46. Stachys affinis (162 g) was extracted with 4 L of 70% ethanol for 4 days and filtered using filter paper. (Whatman qualitative No. 6). The mixture was concentrated to 500 mL at reduced pressure and 45 °C using a rotary evaporator (N-1110, Eyela, Tokyo, Japan). The concentrate was washed three times with 500 mL of hexane to get rid of fatty particles. To separate the phenolic component from the remaining filtrate, it was extracted three times with 250 mL of ethyl acetate. The residue was first dehydrated with MgSO4. The residue was then eluted using silica gel solvent (40 cm \(\times\) 2.5 cm) and ethyl acetate. Highly polar components were eliminated. Finally, the solvent was concentrated at a lower pressure to produce a mixed phenolic powder and stored at − 70 °C (10.2 g, 6.3% of raw material dried plants).
HPLC and LC–MS/MS
As an HPLC sample, the extracted powder was diluted in 70% ethanol to a concentration of 1000 µg/mL. LC–MS/MS were carried out using 1260 series HPLC system (Agilent Technologies, Inc., California, USA) and Ultra Quadrupole Time of flight LC/MS/MS System (X500R) in positive ion mode. The DW and acetonitrile (ACN) with 0.1% formic acid were used as the solvents, and the gradient system was configured to flow at 0.5 mL/min. Prontosil C18 column (Phenomenex Co., Ltd. California, USA, length: 250 mm, inner diameter: 4.6 mm, particle size: 5 µm) was used. The analysis condition was a wavelength of 284 nm and temperature of 35 °C. The flow conditions in the mobile phase were acetonitrile concentrations of 0–10 min at 10–15%, 10–20 min at 20%, 30–40 min at 40%, 40–50 min at 70%, and 50–60 min at 95% At 95%, 60–70 min.
DPPH binding HPLC study to determine antioxidant activity
The potential antioxidant of phenolic compounds in SAE was investigated using a modified method19. The reaction of the extract (5000 µg/mL/70% ethanol) was mixed with 0.2 mg/mL DPPH reagent in a 1:1 (v:v) ratio and proceeded for 15 min at room temperature. Prior to HPLC analysis, the mixture was filtered through a 0.45 m filter, and methanol was employed as a control in place of the DPPH reagent. By contrasting the chromatographic peak region that underwent the DPPH reaction, the phenolic compounds that had reacted with the enzyme could be determined. This allows for the identification of primary antioxidant compounds in SAE.
Potential anti-inflammatory activity of phenolic compounds through UF-HPLC and COX2 reaction
The potential anti-inflammatory of phenolic compounds in SAE was investigated by a modified technique47. React 100 uL of extract and 20ul of COX2 (2U) in a water bath at 37 °C for 30 min. Inactivated COX2 was used in the control group, and activated COX2 was used in the experimental group. Centrifuge the combined solution at 10,000 rpm for 10 min at room temperature using 30 kd cutoff ultrafiltration (YM-30). Compounds that did not bind to COX2 were down through the filter and were removed. Afterwards, the filtrate remaining was washed three times with 200ul NE buffer (pH 7.9) for optimal enzyme activity. The remaining material was dissolved in 80% ACN for 10 min and then centrifuged 3 times. The components dissolved in ACN were recovered and analyzed through HPLC.
Measurement of anti-inflammatory effects
Cell culture and viability assay
The American Type Culture Collection (ATCC) provided the macrophage RAW264.7 cells. The cells were grown in full DMEM with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (P/S) and then incubated in a humid environment with 5% CO2 at 37 °C. In 96-well plates, RAW264.7 cells were seeded for 12 h at a density of 1 × 104 cells per well. Following this, the cells were exposed to SAE for 24 h at doses of 0, 10, 25, 50, 75, 100, and 250 ng/mL, either with or without 1 µg/mL of LPS (Sigma-Aldrich, Merck KGaA, Burlington, USA). The experiment was conducted in the LPS group as a positive control. SAE was dissolved in DMSO (negative control) and treated in a dose-dependent manner. The cells were grown for 4 h at 37 °C after each well treated 10 µL of MTT solution (5 mg/mL, dissolved in PBS). The insoluble crystals of formazan were dissolved using DMSO. Each sample was analyed in triplicate, and a microplate reader (BioTek, Winooski, VT, USA) was used to read the absorbance (OD) value of each well at 450 nm.
Nitric oxide (NO) assay
RAW264.7 cells were seeded at 1 × 104 cells, treated with or without LPS (1 μg/mL), and cultured at 37 degrees for 1 h. Subsequently, the cells were treated with SAE (0, 10, 25, 50, 75, 100, and 250 ng/mL) and cultured for 24 h. After that, 100 ul of supernatant was analyzed with a NO Plus detection kit (Intron Biotechnology, Gyeonggi, Republic of Korea); Cat. No. 21023). 50 ul of N1 and N2 buffers were added in accordance with the manufacturer's instructions, and a Multiskan FC microplate reader (Thermo Scientific, Rockford, IL, USA) was used to measure the results at 520 nm. Each group's NO concentration was measured using a standard, and standard sodium nitrite was utilized to create the curve.
Prostaglandin E2 (PGE2) enzyme-linked immunoassay (ELISA)
RAW264.7 cells were seeded at 5 × 104 cells in a 24-well plate, pre-treated with or without LPS (1 μg/mL), and cultured at 37 degrees for 1 h. After that, the cells were treated with SAE (25 and 50 ng/mL) and cultured for 24 h. To measure PGE2, a total of 100 μL of supernatant was used. PGE2 levels were measured using the PGE2 ELISA kit (Cat. No. ADI-900-001; Enzo Life Sciences, Inc., New York, NY, USA) according to the manufacturer's guidance. An immunoassay using a 4-parameter logistic curve fitting algorithm was used to handle the data.
Western blot analysis
RAW264.7 cells were seeded into 60 mm plates at a density of 1 × 106 cells per well and treated with LPS 1 μg/mL for 24 at 37 °C incubator for positive control. Next, treatment groups were treated with 25 and 50 ng/mL SAE. The incubated cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (iNtRON Biotechnology in Gyeonggi, Korea) which contains a protease inhibitor cocktail and a phosphatase inhibitor (Thermo Fisher Scientific in Waltham, Massachusetts, USA). By the protocol provided from the manufacturer, the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Waltham, MA, USA) was performed to determine the protein quantification of the sample. The equal amounts of protein (10 μg) were separated using 10–15% Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS PAGE). A semi-dry conveying system (JP/WSE-4040 HorizeBLOT 4 M-R WSE-4045, Atto Corp., Tokyo, Japan), was used to transfer the created polyacrylamide gels to polyvinylidene fluoride (PVDF) membranes. Then the PVDF were blocked by EzBlockChemi (ATTO Blotting System, Tokyo, Japan) for 2 h at room temperature. In a subsequent step, membranes were treated with a 1:1000 diluted primary antibody overnight at 4 °C. The membranes were washed 15 times for 2 h with Tween 20 (TBS-T, pH7.4), incubated with 1:5000 diluted anti-rabbit and anti-mouse for 3 h at room temperature. The membranes were then washed using TBS-T 10 times for 2 h. The proteins were detected by the ChemiDoc imaging system (Version 6.0, Bio-Rad Laboratories, Inc., California, USA) and processed using the Image Lab 4.1 (Bio-Rad) application. Detected using a buffer for enhanced chemiluminescence (ECL) (Bio-Rad, Hercules, CA, USA). The protein β-actin served as the loading control, blots were quantified by using Image J software from the National Institutes of Health.
Measurement of intracellular ROS production
RAW264.7 cells were seeded at 1 × 104 and 5 × 104 in a 96well black plate and 12-well plate, respectively, and treated with or without LPS 1 μg/mL for 24 at 37 ºC incubator to measure intracellular ROS production. Afterward, it was measured using the Total ROS detection kit (Enzo Life Sciences, NY) according to the manufacturer's instructions. ROS-generated cells were stained using the kit and measured using a fluorescence plate reader (Bio Tek, Epoch, VT, USA) with fluorescence filter settings (Ex/Em: 480 nm/530 nm), followed by cell imaging multi-board reader (Bio Tek, Cytation). 7, VT, USA) to confirmed fluorescence imaging.
Molecular docking analysis
The protein structure was obtained by searching the protein database (PDB) required for molecular docking (ID 4Q3J, NF-кB), (ID 6COX, COX2), and (ID 1R35, iNOS) (https://www.rcsb.org/, accessed on September 13, 2023). The 3D compound structures of 3-Indoleacrylic acid, (Compound CID: 5375048), Chlorogenic acid (Compound CID: 1794427), and Astragalin (Compound CID: 5282102) were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on September 13, 2023). UCSF Chimera and AutoDock Vina were used to analyze docking. Discovery Studio (DeLano, 2002) were used to show the docking structure. Using the total intermolecular energy and the expected free energy binding, the affinity of the binding is determined.
Statistical analysis
To express the data, the mean and ± SEM are utilized. Prism software (GraphPad Inc. version 9.3.1) was applied for data analysis. SPSS version 12.0 was used for the statistical examination (SPSS Inc., Chicago, Illinois, USA). It was analyzed using one-way factorial analysis of variance (ANOVA) to determine if the groups differed significantly from one another in any way. Dunnett’s multiple comparison test was used, and a statistically significant value of p < 0.05 was used. (#p < 0.05, ##p < 0.01, ###p < 0.001 vs. untreated group; and *p < 0.05, **p < 0.01, ***p < 0.001 vs. LPS-treated group).
Conclusion
In this study, five phenolic compounds were identified in SAE and screened for phenolic compounds with antioxidant activity potential in combination with DPPH. We also screened potential anti-inflammatory phenolic compounds through COX2 binding. The potential antioxidant properties of each compound were confirmed through a competitive binding reaction with DPPH and COX2 enzyme. Subsequently, the anti-inflammatory effect of the extract was confirmed through in vitro experiments (Fig. 9). Lastly, the molecular docking results showed that the molecular structure of the phenol compound contained in SAE is related to the anti-inflammatory activity of SAE. These results provide an effective method to determine the potential antioxidant and anti-inflammatory effects of phenolic compounds in the extract and suggest that Stachys affinis can be used as basic data in the industry of health foods and pharmaceuticals. In future research, we plan to conduct more in-depth antioxidant and anti-inflammatory research on SAE and, at the same time select compounds with high activity among the phenolic compounds present in the extract to conduct antioxidant and anti-inflammatory research.
Data availability
The data used to support the findings of this study are available upon request from the corresponding author.
References
Guo, H., Saravanakumar, K. & Wang, M.-H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal. Agric. Biotechnol. 15, 235–239 (2018).
Cheng, B.-H. et al. Anti-inflammatory action of YHQ by regulating 5-LOX/COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. J. Herbal Med. 17–18, 100269 (2019).
Guo, H.-F. & Wang, M.-H. Anti-inflammatory and anti-cancer effect of Stachys affinis tubers. Korean J. Plant Resour. 30(6), 679–685 (2017).
Kumar, B. R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 7(6), 349–364 (2017).
Liu, R. et al. Simultaneous determination of two galangin metabolites from Alpinia Officinarum Hance in rat plasma by UF LC-MS/MS and its application in pharmacokinetics study. PeerJ 9, e11041 (2021).
Uddin, R. et al. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 4(3), 273–281 (2014).
Li, Y. et al. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 17(8), 1258 (2016).
Saeed, N., Khan, M. R. & Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern. Med. 12, 221 (2012).
Baiano, A. & Del Nobile, M. A. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 56(12), 2053–2068 (2016).
Halliwell, B. Oxidative stress and cancer: Have we moved forward?. Biochem. J. 401(1), 1–11 (2007).
Yu, W. et al. HO-1 is essential for tetrahydroxystilbene glucoside mediated mitochondrial biogenesis and anti-inflammation process in LPS-treated RAW264.7 macrophages. Oxid. Med. Cell. Longev. 2017, 1–13 (2017).
Lee, H. J. et al. Membrane-free stem cell components inhibit interleukin-1α-stimulated inflammation and cartilage degradation in vitro and in vivo: A rat model of osteoarthritis. Int. J. Mol. Sci. 20(19), 4869 (2019).
Fang, Y., Yang, L. & He, J. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 143, 112104 (2021).
Cao, Y. et al. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients 11(11), 2794 (2019).
Kesavardhana, S., Malireddi, R. S. & Kanneganti, T.-D. Caspases in cell death, inflammation, and gasdermin-induced pyroptosis. Ann. Rev. Immunol. 38, 567 (2020).
Regehr, W. G., Carey, M. R. & Best, A. R. Activity-dependent regulation of synapses by retrograde messengers. Neuron 63(2), 154–170 (2009).
Yeo, J. & Shahidi, F. Critical re-evaluation of DPPH assay: Presence of pigments affects the results. J. Agric. Food Chem. 67(26), 7526–7529 (2019).
Dang, J. et al. Preparative isolation of highly polar free radical inhibitor from Floccularia luteovirens using hydrophilic interaction chromatography directed by on-line HPLC-DPPH assay. J. Chromatogr. B 1142, 122043 (2020).
Jeong, S. H. et al. Potential antioxidant and anti-inflammatory effects of Lonicera japonica and Citri Reticulatae Pericarpium polyphenolic extract (LCPE). Antioxidants (Basel) 12(8), 1582 (2023).
Crampon, K. et al. Machine-learning methods for ligand–protein molecular docking. Drug Discov. Today 27(1), 151–164 (2022).
Gunasekaran, M., Ravi, R. & Subramanian, K. Molecular docking analysis of lupeol with different cancer targets. Bioinformation 18(3), 134–140 (2022).
Stanzione, F., Giangreco, I. & Cole, J. C. Chapter four—use of molecular docking computational tools in drug discovery. In Progress in Medicinal Chemistry (eds Witty, D. R. & Cox, B.) 273–343 (Elsevier, 2021).
Zhang, J. et al. Phytochemistry and antioxidant activities of the rhizome and radix of Millettia speciosa based on UHPLC-Q-exactive orbitrap-MS. Molecules 27(21), 7398 (2022).
Willems, J. L. et al. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 933, 164–174 (2016).
Fu, Y. et al. Characterization and quantification of phenolic constituents in peach blossom by UPLC-LTQ-orbitrap-MS and UPLC-DAD. Nat. Prod. Commun. 15(1), 1934578X19884437 (2020).
Dong, Y. et al. Phytochemistry and comprehensive chemical profiling study of flavonoids and phenolic acids in the aerial parts of Allium mongolicum regel and their intestinal motility evaluation. Molecules 25(3), 577 (2020).
Xiao, X. et al. DPPH radical scavenging and postprandial hyperglycemia inhibition activities and flavonoid composition analysis of Hawk tea by UPLC-DAD and UPLC-Q/TOF MS(E). Molecules 22(10), 1622 (2017).
Al-Hakkani, M. F. et al. Cefoperazone rapidly and sensitive quantitative assessment via a validated RP-HPLC method for different dosage forms, in-use stability, and antimicrobial activities. BMC Chem. 17(1), 72 (2023).
Hwang, J. H. et al. Anti-inflammatory and antioxidant effects of MOK, a polyherbal extract, on lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 43(1), 26–36 (2019).
Kalita, A. et al. Molecular docking prediction and in vitro studies elucidate anti-inflammatory effect of Garcinia extract against inducible nitric oxide synthase and cyclooxygenase-2 targets. Beni Suef Univ. J. Basic Appl. Sci. 11(1), 32 (2022).
Venditti, A. et al. Polar constituents, protection against reactive oxygen species, and nutritional value of Chinese artichoke (Stachys affinis Bunge). Food Chem. 221, 473–481 (2017).
Wlodarska, M. et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22(1), 25-37.e6 (2017).
Huang, J. et al. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 14, 1218015 (2023).
Muvhulawa, N. et al. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 178, 106163 (2022).
Sharma, N. et al. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants (Basel) 10(9), 1419 (2021).
Wang, J. et al. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 13(5), e0197563 (2018).
Riaz, A. et al. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 9794625 (2018).
Baliyan, S. et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 27(4), 1326 (2022).
Schaich, K. M., Tian, X. & Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 14, 111–125 (2015).
Simon, L. S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 106(5B), 37S-42S (1999).
Ye, S. et al. Chlojaponilactone B attenuates lipopolysaccharide-induced inflammatory responses by suppressing TLR4-mediated ROS generation and NF-κB signaling pathway. Molecules 24(20), 3731 (2019).
Perez, S. & Tvaroska, I. Carbohydrate-protein interactions: Molecular modeling insights. Adv. Carbohydr. Chem. Biochem. 71, 9–136 (2014).
Meng, X. Y. et al. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 7(2), 146–157 (2011).
Piatczak, E. et al. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules 10(10), 1391 (2020).
Davis, A. & Robson, J. The dangers of NSAIDs: Look both ways. Br. J. Gen. Pract. 66(645), 172–173 (2016).
Kim, S. M. et al. Polyphenol mixture of a native Korean variety of Artemisia argyi H. (Seomae mugwort) and its anti-inflammatory effects. Int. J. Mol. Med. 44(5), 1741–1752 (2019).
Chen, G. L. et al. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 277, 706–712 (2019).
Acknowledgements
This study was supported by the National Research Foundation of Korea, funded by the Ministry of Science and ICT (grant nos. 2022R1A6A3A01086899 and RS-2023-0024337661382).
Author information
Authors and Affiliations
Contributions
Conceptualization, H.H.K; methodology, H.H.K, S.H.J; formal analysis, H.H.K, S.H.J, M.Y.P.; writing-original draft preparation, H.H.K, S.H.J.; writing-review and editing, A.A, P.B.B. H.H.K; investigation, S.J.L, J.K.S, D.I.K, H.W.K.; validation, J.D.H, A.A.; project administration, K.I.P G.S.K.; supervision, G.S.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, H.H., Jeong, S.H., Park, M.Y. et al. Binding affinity screening of polyphenolic compounds in Stachys affinis extract (SAE) for their potential antioxidant and anti-inflammatory effects. Sci Rep 14, 18095 (2024). https://doi.org/10.1038/s41598-024-68880-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68880-z
- Springer Nature Limited