Abstract
Members of the Metal Tolerance Protein (MTP) family are critical in mediating the transport and tolerance of divalent metal cations. Despite their significance, the understanding of MTP genes in mustard (Brassica juncea) remains limited, especially regarding their response to heavy metal (HM) stress. In our study, we identified MTP gene sets in Brassica rapa (17 genes), Brassica nigra (18 genes), and B. juncea (33 genes) using the HMMER (Cation_efflux; PF01545) and BLAST analysis. For the 33 BjMTPs, a comprehensive bioinformatics analysis covering the physicochemical properties, phylogenetic relationships, conserved motifs, protein structures, collinearity, spatiotemporal RNA-seq expression, GO enrichment, and expression profiling under six HM stresses (Mn2+, Fe2+, Zn2+, Cd2+, Sb3+, and Pb2+) were carried out. According to the findings of physicochemical characteristics, phylogenetic tree, and collinearity, the allopolyploid B. juncea’s MTP genes were inherited from its progenitors, B. rapa and B. nigra, with minimal gene loss during polyploidization. Members of the BjMTP family exhibited conserved motifs, promoter elements, and expression patterns across subgroups, consistent with the seven evolutionary branches (G1, G4–G9, and G12) of the MTPs. Further, spatiotemporal expression profiling under HM stresses successfully identified specific genes and crucial cis-regulatory elements associated with the response of BjMTPs to HM stresses. These findings may contribute to the genetic improvement of B. juncea for enhanced HM tolerance, facilitating the remediation of HM-contaminated areas.
Similar content being viewed by others
Introduction
Rising levels of heavy metal (HM) contamination in the environment, resulting from industrial activities such as mining, exhaust emissions, waste management, artificial fertilizer use in agriculture, and urban wastewater treatment, have become a growing concern. This contamination poses a serious threat to agricultural production, highlighting the urgent need for effective mitigation measures1,2. Trace levels of essential HMs, such as manganese (Mn), iron (Fe), copper (Cu), and zinc (Zn), are present in plants to regulate numerous physiological processes3. However, high concentrations of these essential HMs, as well as low concentrations of non-essential HMs such as cadmium (Cd), lead (Pb), mercury (Hg), and antimony (Sb), can trigger phytotoxicity. Such toxicities were characterized by growth restriction, leaf chlorosis, root discoloration, and even plant death2,4,5,6.
Plants have evolved various defense mechanisms against HMs, such as detoxification, sequestration, cell membrane transport processes, and increased concentrations of compatible solutes2,7. Transport proteins in these systems maintain cellular homeostasis by recognizing metals and facilitating their movement to particular locations within plant tissues2,8. Studies have shown that families of transport proteins, such as Zn-regulated iron-regulated transporter-like proteins (ZIPs), ATP-binding cassettes (ABCs), natural resistance-associated macrophage protein (NRAMPs), and cation diffusion facilitators (CDFs), sequester HMs into vacuoles and chelate them to reduce their toxicity7,9. Metal tolerance proteins (MTPs), also known as the cation transporter family (CDF), were first discovered in bacteria and are involved in the tolerance and transport of divalent metal cations, including Mn2+, Fe2+, Zn2+, and Cd2+7,10,11. Structurally, MTPs were characterized by transmembrane domains (TMDs), metal ion recognition motifs (HXXXD or DXXXD), and cation transport domains12. Based on substrate specificity, these proteins were categorized into three major subgroups: Zn-CDF, Zn/Fe-CDF, and Mn-CDF, facilitating the transport of ions like Zn2+, Fe2+, Mn2+, Co2+, Cd2+, and Ni2+ 13. In Arabidopsis, the 12 MTP proteins were further divided into seven branches: G1, G5, G6, G7, G8, G9, and G12, highlighting their complexity and specificity11. Previous studies have demonstrated the diverse functions of MTP proteins. For instance, AtMTP3 acted as a vacuolar zinc transporter, essential for regulating intracellular Zn2+ levels and maintaining homeostasis14. AtMTP8 was crucial for Mn2+ transport during seed germination and development and also regulated Fe2+ transport15. Additionally, a combination of AtMTP5 and AtMTP12 proteins enhanced Zn2+ transport into the Golgi apparatus16.
Brassica juncea L. is an important oilseed, vegetables, and spice crop globally. Known for its rapid vegetative growth and substantial aboveground biomass, it has been recognized as an effective accumulator of various HMs, such as Pb, Cd, selenium (Se), Zn, chromium (Cr), Cu, and nickel (Ni). Consequently, this plant is widely used in studies on soil decontamination and remediation17,18,19. Up to now, only a few cation-efflux family genes, particularly BjCET1-4, had been identified in B. juncea. Heterologous expression of these genes in yeast had showed their ability to confer tolerance against Zn2+ and Cd2+ 20,21,22. However, the comprehensive identification and functional characterization of other cation-efflux family members in B. juncea in response to HMs remain underexplored.
Here, we investigated the BjMTP proteins using bioinformatics techniques, with a focus on the expression dynamics of the BjMTP genes under various HM stresses. This research lays a foundation for further investigation into the molecular mechanisms of HM homeostasis in B. juncea and provides valuable insights for the bioremediation of HM-contaminated soils.
Results
Identification and physicochemical characterization of MTP proteins
A total of 68 MTP proteins from three species, including B. rapa (17), B. nigra (18), and B. juncea (33), were identified by rigorous validation methods comprising analysis of the Cation_efflux (PF01545) domain characteristic, PFAM, SMART, and BLAST. Given their similarity and phylogenetic relationship with AtMTPs in Arabidopsis, all MTPs were named BxMTP1-BxMTP12 (x = r, n, and j). By analyzing the gene counts and physicochemical characteristics of MTPs, it was shown that the majority of MTPs in the allopolyploid B. juncea (AABB) were formed by merging MTPs from its diploid ancestors, B. rapa (AA) and B. nigra (BB). However, BnB06.MTP4b and BnB01.MTP12 homologs were not found in B. juncea, and orthologs of AtMTP2 and AtMTP3 were missing among the identified BjMTPs. Among the 33 BjMTPs, the number of orthologs ranged from 2 to 4, with distribution scores varying a minimum of 1 (BjMTP12) and a maximum of 6 (BjMTP8) (Table S1).
The length of BjMTP proteins varied from 163 to 1106 amino acids, with molecular weights ranging from 18.34 kDa (BjB06.MTP4) to 122.28 kDa (BjA04.MTP1). Generally characterized as acidic, the BjMTPs had an average isoelectric point (pI) < 7, except for BjB06.MTP4, which had a pI of 10.46. Subcellular localization analysis indicated that most BjMTPs were present in the vacuoles, whereas others localized to the cell membrane. Further investigation revealed that most BjMTPs possessed 4–8 TMDs, with BjA04.MTP12 carrying 14 TMDs in particular. In contrast, BjA03.MTP6 and BjB08.MTP6 lacked discernible TMDs (Table S1).
Phylogenetic analysis and chromosomal localization of BjMTPs
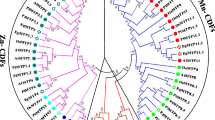
Based on the phylogenetic tree and structural characteristics of 80 MTP proteins from A. thaliana, B. rapa, B. nigra, and B. juncea, the MTPs could be categorized into three main subgroups: Mn-CDF, Zn-CDF, and Fe/Zn-CDF. These subgroups were further subdivided into seven branches: G1, G5, G6, G7, G8, G9, and G12 (Fig. 1a), where BjMTP proteins displayed a close evolutionary relationship with their equivalent orthologs in BrMTPs and BnMTPs. In addition, the branches G12 and G9 contained the fewest and largest BjMTP genes, with 1 and 10 genes, respectively. Chromosomal localization analysis indicated that the A and B subgenomes of B. juncea had 17 and 16 BjMTP genes, respectively, dispersed unevenly across 11 chromosomes. Notably, chromosome B06 showed the maximum number of genes (8), while chromosomes B03 and B04 each had just a single BjMTP. No BjMTP genes were discovered on chromosomes A01, A02, A08, A10, B02, B05, and B07 (Fig. 1b).
Phylogenetic analysis and chromosomal localization. (a) Phylogenetic analysis of 80 MTP proteins in A. thaliana, B. rapa, B. nigra, and B. juncea. The blue star, green circle, orange square, and red triangle represent MTPs from A. thaliana, B. rapa, B. nigra, and B. juncea, respectively. (b) Chromosomal localization of BjMTP genes. Fonts in various colors indicate distinct branches of the BjMTP genes.
Structural analysis of BjMTP proteins
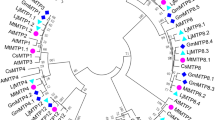
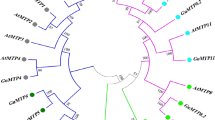
By evaluating 15 conserved motifs among the BjMTP proteins, subgroup-specific patterns were revealed. This research identified distribution patterns unique to each branch, with specific motifs characterizing them. Specifically, G1 was distinguished by motifs 6 and 7; G5 by motif 6; G6 by motif 14; G7 by motifs 4, 5, 8, 13, and 14; G8 by motifs 1, 2, 3, 4, 5, 8, 9, 11, and 12; and G9 by motifs 1, 2, 4, 8, and 11 (Fig. 2a,b). Further protein domain analysis using the SMART database revealed that the BjMTP family members featured a Cation_efflux domain. Members from branches G6, G8, and G9 also had a Zinc Transporter dimerization (ZT_dimmer) domain (Fig. 2c), suggesting that motifs 2, 4, and 8, and motifs 1 and 11 correspond to the Cation_efflux and ZT_dimmer domains, respectively. Additionally, BjA04.MTP1, BjB01.MTP1, and BjB06.MTP1 contained a single helix-loop-helix (HLH) domain, although its function remains unknown. Investigation of 3D structures for 10 representative BjMTP proteins using the AlphaFold database indicated that the cation efflux domain typically comprised 4–6 transmembrane helices, arranged into a compact cluster structure (Fig. S1). In contrast, Zn-MTP and Fe/Zn-MTP subgroups displayed either 1–2 HXXXD or DXXXD residues (Fig. S1a,b), while the Mn-CDF subgroup consistently had two DXXXD residues (Fig. S1c). Prior studies had shown a substantial link between these conserved residues and metal ion selectivity23,24. Consequently, it can be inferred that these residues in BjMTPs may also be closely related to metal ion transport.
Phylogenetic relationship, conserved motif, and protein domain analyses of BjMTP proteins. (a) Phylogenetic tree of BjMTP proteins. The phylogenetic tree was constructed with the ML method of MEGA 7 (1000 bootstrap replicates). (b) The analysis of conserved motifs in BjMTP proteins. In total, 15 different motifs are represented by squares with different colors. (c) Analysis of protein domains in BjMTP proteins. Various symbols represent different protein structural domains.
The analysis of conserved domains and the 3D structure of BjMTPs revealed that all BjMTP proteins contain the Cation_efflux domain and HXXXD or DXXXD residues, which are essential for metal ion binding. Additionally, significant structural differences were observed among various CDF subfamilies or branches, further explaining the functional diversity of BjMTP genes.
Analysis of promoters in BjMTP genes
Using PlantCARE software, the cis-regulatory elements (CREs) of the BjMTP promoters were examined to clarify their roles in tissue-specific expression and stress responses. After that, these elements were categorized and analyzed statistically. Among the 806 CREs identified across the promoters of 33 BjMTP genes, apart from the common CREs, three distinct categories25 were recognized: development and growth (298 elements), hormone response (335 elements), and stress responsiveness (173 elements) (Table S2). Development-related elements included light response (ACE, ATC-motif, Box-4, and G-box), meristem development (CAT-box), flowering (CCAAT-box), and circadian rhythm (Circadian), with light response elements accounting for 65.4% of this category (Fig. 3a). Numerous hormone-related elements were also identified, including those responsive auxin (AuxRR-core, TGA-element, TGA-box), MeJA (CGTCA-motif, TGACG-motif), gibberellin (GARE-motif, TATC-box, P-box), abscisic acid (ABRE), and salicylic acid (TCA-element). Of these, abscisic acid response elements were the most prevalent, accounting for 33.43% (112/335), whereas salicylic acid response elements were the least abundant at 5.07% (Fig. 3a). Stress-related elements included those responsive to anaerobic induction (ARE), anoxic induction (GC-motif), cold (LTR), drought (MBS), and general stress (TC-rich repeats). ARE elements constituted 54.33% of this category, suggesting a significant role for BjMTPs in anaerobic stress adaptation (Fig. 3a). Except for BjA04.MTP4 and BjB01.MTP5, the number of ARE elements varied among BjMTP genes, ranging from one (BjA04.MTP8) to nine (BjA03.MTP1) (Fig. 3b).
Once again, significant patterns across evolutionary branches and unique distributions of each gene were identified through the study of CREs in BjMTP gene promoters. In particular, genes in the G1 branch generally shared highly conserved MeJA-responsive elements, despite variations in the number of promoter elements. Promoters of homologous genes in the G5, G6, G7, and G8 branches displayed comparable CREs. Interestingly, the G9 branch, comprising 10 genes, was characterized by an abundance of G-box, ABRE, and ARE elements, along with substantially conserved MeJA-responsive elements (Fig. 3b). As a result, the presence of a wide variety of CREs in BjMTP promoters support the comprehensive regulation of development, hormone responses, and stress mechanisms. These conserved characteristics in promoters likely influence the functional diversity of BjMTP genes throughout evolutionary branches.
Synteny analysis of MTP genes
Whole genome duplication, segmental duplication, and tandem duplication are crucial mechanisms driving gene family evolution26. To further explore the collinear relationships of MTP gene families between B. juncea and its diploid ancestors, we performed a collinear analysis among B. juncea & B. rapa and B. juncea & B. nigra. A total of 107 orthologous gene pairs were identified (Fig. S2). We then successfully calculated the nonsynonymous (Ka) substitutions and synonymous (Ks) substitutions values for 107 pairs across three combinations: 49 pairs of between B. juncea & B. rapa and 67 pairs of between B .juncea & B.nigra (Table S3). It is generally believed that Ka/Ks < 1 shows negative (purify) selection, while Ka/Ks > 1 or = 1 means positive or neutral selection, respectively. As shown in Table S3, 107 duplicated gene pairs exhibited purifying selection pressures. Also, syntenic analysis identified 44 duplicated gene pairs among the 32 BjMTP genes in B. juncea, excluding BjA04.MTP12, all related to segmental duplication (Fig. 4; Table S3). Among them, all gene pairs had Ka/Ks ratios < 1, indicating purifying selection. Overall, all 151 pairs of duplicated MTP genes have experienced purifying selection, suggesting that these MTP gene pairs in Brassica species are highly conserved and have not acquired new functional differentiation. These findings indicate that these duplicated gene pairs, whether from the same or closely related species, likely perform similar functions in response to HM stresses. Specifically, they may facilitate the transport of similar metal ions, thereby providing a basis for further functional predictions of MTP genes.
Expression patterns and GO enrichment analysis
Utilizing RNA-seq data from the NCBI database, the expression patterns of BjMTP genes in the different tissues of B. juncea were clarified. A total of 33 BjMTP genes were thoroughly analyzed to evaluate their expression levels across various tissues, including roots, stems, leaves, buds, siliques at 7 and 15 days after flowering (DAF), pods at 20 DAF, seeds, and seed coats. The distribution of CREs within the promoters of BjMTPs was shown to correlate with their expression. Homologous BjMTP genes generally exhibited similar expression patterns across tissues, with few exceptions. For instance, BjMTP4 was barely expressed in stems, leaves, seed coats, and seeds, whereas BjMTP11 exhibited higher expression levels. Moreover, unique expression features were shown by the G9 branch genes BjMTP9, BjMTP10, and BjMTP11, with higher expression in leaves and buds and lower expression in seeds (Fig. 5a, Table S4). This implies a correlation between the evolutionary branches of BjMTPs and tissue-specific expression, potentially linked to the CREs in their promoters. Additionally, several BjMTPs showed patterns of tissue-specific or ubiquitous expression. For instance, BjA04.MTP1 had high expression levels across various tissues, while BjB06.MTP9, BjA07.MTP9, and BjB01.MTP11 showed considerable increases in leaf tissues, indicating that those BjMTPs had specialized functions in specific tissues.
GO enrichment analysis further clarified the molecular functions of the BjMTP genes. The top 20 GO terms encompassed biological activities such as efflux, cation, and ion transmembrane transporter activity (Fig. 5b). Notably, the predicted biological functions of BjMTP genes were linked to essential processes like zinc ion homeostasis maintenance and vacuolar membrane transport facilitation. These results align with previous findings on protein structure and subcellular localization7,11, underscoring the significant role of BjMTP genes in metal cation transport.
Expression profiles of BjMTPs under six HM stresses
Phenotypic alterations are critical indicators of a plant’s reaction to biotic or abiotic stress. In the early stages of HM treatment (12 and 24 h), B. juncea plants in our study did not display any discernible phenotypic alterations. However, after 48 h, the effects of the other metals were minimal except for 250 mg/L Mn2+, 250 mg/L Fe2+, and 10 mg/L Sb3+, which noticeably influenced plant morphology (Fig. 6a–c). Mn2+ exposure caused in uneven white spots on the leaves. Fe2+ stress was associated with visible dehydration signs, and low Sb3+ concentration resulted in root yellowing. Thus, in B. juncea, both non-essential (Sb3+) and essential (Mn2+ and Fe2+) metals might adversely affect B. juncea health.
The phenotypic characteristics of plants after 48 h of exposure to various HMs. (a–c) The leaf and root phenotypes of plants treated with 250 mg/L Mn2+ (a), 250 mg/L Fe2+ (b), and 10 mg/L Sb3+ (c). (d, e) Expression heatmap of BjMTP genes in the root (d) and leaf (e) tissues under various HMs treatments. The red or blue bar represents positive and negative regulatory expression, respectively.
To thoroughly evaluate the impact of various HMs on BjMTP genes expression, 10 representative genes from the expression profiles of 33 BjMTPs (Fig. 5a) were selected for qRT-PCR analysis under different HM conditions. These genes included BjA04.MTP1, BjA04.MTP4, BjB01.MTP5, BjB08.MTP6, BjA06.MTP7, BjA09.MTP8, BjB06.MTP9, BjA09.MTP10, BjA05.MTP11, and BjA04.MTP12. They demonstrated spatiotemporal and tissue-specific expression patterns across six HM conditions, with roots displaying a stronger response than leaves (Fig. S3, Fig. 6d,e). BjA04.MTP4 and BjB01.MTP5 were markedly elevated in roots during the first 12 and 24 h of Fe2+ and Sb3+ exposure. After 48 h of HM treatments, the majority of genes exhibited substantial changes in expression, aligning with the observed phenotypic alterations in B. juncea. Within the Mn-CDF subgroup, only BjA09.MTP10 reacted to Mn2+ stress in roots after 48 h. All HM treatments activated MTP8, which is known to modulate Mn2+ transport and tolerance15,27, in leaf tissues. Notably, BjA04.MTP4, BjA09.MTP10, and BjB01.MTP5 exhibited the greatest expression levels under Sb3+ and Pb2+ exposures, with increases of 997.1-, 370.5-, and 2137.3-fold compared to the control, respectively (Fig. S3; Table S5). Additionally, the consistently downregulated expression of BjB06.MTP9 under various HM stresses revealed its potential negative regulatory role in HM stress responses. Overall, comparable gene expression patterns were observed in root tissues exposed to HMs, with genes such as BjA04.MTP4, BjB01.MTP5, BjB08.MTP6, BjA06.MTP7, BjB06.MTP9, BjA09.MTP10, and BjA05.MTP11 responding positively to six types of HM stress. In the leaf tissues, BjA04.MTP4, BjB01.MTP5, and BjA09.MTP8 were discovered as potentially co-regulating Zn2+ transport, indicating a coordinated response to maintain metal homeostasis across diverse plant tissues.
Correlation analysis between BjMTPs expression under different HM treatments and CREs in BjMTP promoters
CREs in promoters are essential for controlling transcription in response to abiotic stress28. However, it remains unclear to what extent the CREs of BjMTP genes can explain the expression changes under stress responses. In this study, we investigated the correlations between the expression patterns of 10 BjMTP genes under different metal treatments over various time periods and their CREs in promoters (Fig. S4; Table 1). The findings revealed significant differences in the correlation between BjMTP expression and BjMTP promoter CREs under different heavy metal treatments in root and leaf tissues. Most CREs, such as ACE, CCAAT-box, GCN4_motif, LTR, and TC-rich repeats, exhibited positive correlations with various HMs in root tissues (Fig. S4a). Among these, ACE, CCAAT-box, and TC-rich repeats elements had consistent positive correlations with multiple tested metals. Notably, during a 48 h period in response to Sb and Pb treatments, the ACE and CCAAT-box elements exhibited the highest correlation coefficients of 0.941 and 0.975, respectively (Table 1). The findings indicate that the ACE and CCAAT-box elements in BjMTP promoters may play a role in recognizing and binding relevant proteins, which in turn affects the expression of genes during Sb and Pb stresses. Within 48 h of treatments, the Box 4 (light-responsive) element in leaf tissues exhibited positive correlations with Fe, Cd, and Sb (Fig. S4b). Conversely, several CREs including ABRE, AuxRR-core, and ARE, exhibited negative correlations with Zn, Pb, and Sb treatments. Specifically, the auxin-responsive element AuxRR-core displayed a strong negative correlation (correlation coefficient of − 0.866) with BjMTP gene expression during Pb tolerance in both root and leaf tissues. This finding implies that auxin signaling may negatively regulate Pb tolerance in B. juncea.
Discussion
MTPs are a class of membrane proteins crucial for regulating the cellular efflux of metal ions and maintaining metal homeostasis13. MTP proteins have been identified in various plant species, including A. thaliana (12 genes), Solanum lycopersicum (11 genes), Medicago truncatula (12 genes), Glycine max (20 genes)29, Solanum tuberosum (21 genes)30, Arachis hypogaea L. (22 genes)31, and B. napus (33 genes)32. However, the identification of MTP family members in B. juncea and their responses to HM stresses have not been reported. In this study, we compared the characteristics of MTP gene family members in B. juncea with those in its progenitor species and analyzed BjMTP responses to various HMs. These studies will enhance our understanding of the genetic and evolutionary characteristics of the MTP gene family and provide insights into the potential molecular basis for stress responses in B. juncea.
Inheritance characteristics of MTP gene family in allopolyploid Brassica juncea
B. juncea (AjAjBjBj, 2n = 4x = 36), a natural allotetraploid, originated from the hybridization and genome doubling of diploid progenitors B. rapa (ArAr, 2n = 20) and B. nigra (BnBn, 2n = 16) approximately 8000–14,000 years ago33. Theoretically, the number of MTP members in B. juncea should be equal to the sum of the MTPs in the diploid B. rapa and B. nigra. Here in this study, we found that the total number of MTPs in B. juncea was a little fewer than the sum of its two diploid progenitors (33 < 17 + 18). This suggests that, except for BnB06.MTP4b and BnB01.MTP12 in B. nigra, where no corresponding BjMTP homologs were identified, the majority of MTP genes in B. juncea inherited genetic materials from their ancestral species during allopolyploidization. The aforementioned finding was highly supported by the comparison of physicochemical characteristics and collinearity studies of MTP gene family members across various Brassica species. Moreover, all duplicate gene pairs in B. juncea & B. rapa, B. juncea & B. nigra, and B. juncea & B. juncea have experienced purifying selection, indicating that these MTP genes have not evolved new functions.
Previous studies had shown that Brassica species and A. thaliana originated from a common ancestor within the Brassicaceae family, sharing a close evolutionary relationship. Following their divergence from Arabidopsis, Brassica species underwent a unique whole genome triplication (WGT) event34,35. Consequently, corresponding homologous genes are expected between the genomes of Arabidopsis and Brassica species. However, in this study, we did not identify direct orthologs of AtMTP2 and AtMTP3 in any of the three examined Brassica species, providing concrete evidence that these two genes were lost during the WGT events in Brassica species.
The seven-branch evolutionary model may elucidate BjMTP functional diversity
Phylogenetic tree analysis is a powerful tool for elucidating evolutionary relationships and histories among different species and genes. It plays a crucial role in understanding population origins and uncovering the phylogenetic relationships between species. Our phylogenetic analysis revealed that the BjMTP gene family, similar to those in A. thaliana11, B. napus32, and G. max29, can be categorized into three subgroups (Zn-CDF, Mn-CDF, and Fe/Zn-CDF) and seven major branches (G1, G5, G6, G7, G8, G9, and G12). Previous research divided BjMTPs into three subgroups based on their substrate-binding selectivity for Zn2+, Mn2+, and Fe2+ 13. However, phylogenomic investigations of plants and algae genomes have revealed seven branches within the CDF family, which are believed to date back to the origin of land plants11. Our analysis of conserved motifs, promoter elements, and RNA-seq expression patterns suggested that most BjMTP genes aligned more closely with the seven-branch classification. Genomic studies of the model plant A. thaliana indicate that it has undergone a characteristic WGT event within the Brassicaceae tribe34,35,36, this gives a clear theoretical basis for the increase from 12 AtMTPs to 33 BjMTPs (even though gene loss occurs following WGT). Also, these BjMTP genes consistently inherit all seven ancient branches, with gene counts per branch varying from one to six (Table S2). By establishing a relationship between the conserved Cation_efflux domain's metal transport properties and the variations observed among the seven branches of the BjMTP family, it can be inferred that the functional variations in metal ion transport exhibited by BjMTPs may be dependent on the specific branches.
Specific CREs in BjMTP promoters may facilitate the response of BjMTPs to various metal stresses
Related studies have shown that transcriptional regulation driven by CREs plays a crucial role in responding to abiotic stresses28. To elucidate the specific functions of CREs in responding to HM stresses, a correlation analysis was performed between the CREs on BjMTP gene promoters and the expression levels of BjMTP genes across different metals, treatment durations, and tissue types. It was surprising to discover that light-responsive elements not only play a role in plant growth and development37 but also participate in responses to HM stresses. Specifically, ACE and Box 4 positively regulate BjMTP gene responses to HMs in root and leaf tissues, respectively. In contrast, hormone-responsive elements such as ABRE, AuxRR-core, and P-box exhibited a strong negative correlation. Nuclear Factor-Y (NF-Y) transcription factors could specifically recognize the CCAAT-box and were involved in regulating responses to various abiotic stresses, including drought, salinity, nutrient deficiency, osmotic stress, and temperature extremes38. Our research confirmed that the CCAAT-box element likely plays a significant function in the response of BjMTP genes in root tissues to HM stresses such as Fe, Sb, and Pb. TC-rich repeats were well-known stress-responsive CRE38. Correlation studies demonstrated their role in the response of BjMTP genes to six types of HM stresses. In conclusion, our analysis revealed numerous CREs that may be related to the stress response of MTPs in B. juncea. In other words, these tightly correlated CREs may be used to identify candidate genes that respond to HM stresses, hence facilitating future functional gene research. However, this correlation analysis method has its limitations, and the conclusions should be validated using additional gene expression data or direct molecular biology experiments. As a result, these findings can serve as a valuable reference for future CRE research.
BjMTP genes exhibit a broad-spectrum response to heavy metal stress
Polyploid plants undergo a series of genetic and epigenetic changes during polyploidization, facilitating new phenotypes and adaptation to diverse environments39,40. Allotetraploid B. juncea, a globally important oilseed and vegetable crop, was known for its high tolerance to various abiotic stresses, including salt, drought, and HMs18,41,42. Prior research has clarified the function of MTP genes in the detoxification of divalent metals, such as Zn2+, Mn2+, Fe2+, Cu2+, Pb2+, Hg2+, and Se2+, both essential and non-essential29,32. In this study, six HM ions (Mn2+, Fe2+, Zn2+, Cd2+, Sb3+, and Pb2+) were introduced to B. juncea seedlings to clarify the role of BjMTP genes in response to HM stresses. After 48 h of treatment, Mn2+, Fe2+, and Sb3+ exhibited varying effects on different tissues of B. juncea seedlings, likely related to the metals' absorption, translocation, and storage within the plant, while the lack of phenotypic changes at certain concentrations indicates the seedlings' high tolerance to these metals.
In A. thaliana, the cation efflux transporter AtMTP1 was involved in Zn detoxification43. Numerous metals, including Zn, Fe, Co, and Ni, translocate in part due to MTP144,45. In this study, Zn2+ and Fe2+ stresses elevated BjA04.MTP1 expression in B. juncea roots. However, compared to BjA04.MTP4 (of the same G1 branch), which helps cucumbers maintain Zn2+ homeostasis and sequester Cd45, its expression was less noticeable. Notably, root tissues showed substantial upregulation of BjA04.MTP4, BjB01.MTP5, and BjA09.MTP10 in response to six HMs, suggesting these genes’ potential for comprehensive metal tolerance and transport. MTP8 proteins regulate Mn2+ transport and provide Mn2+ tolerance15,27. For improved Mn2+ resistance, OsMTP8 sequesters Mn in the vacuoles of rice panicle cells27. Surprisingly, our findings showed that BjA09.MTP8 overexpression was stimulated only by Fe2+ in roots, while BjA09.MTP8 responded to almost all HMs in leaves, possibly explaining the speckled leaves observed after Mn2+ exposure. Related to the Mn-CDF subfamily, BjB06.MTP9 showed opposing regulatory effects on HMs tolerance and stress responses in root and leaf tissues. These findings highlight the interspecific functional variety and spatial expression of MTP genes in HM stress responses. Furthermore, this is the first study to incorporate Sb3+ into stress response tests on BjMTPs. Our results indicated that most BjMTPs were triggered by low concentrations of Sb3+, indicating that MTP proteins may be involved in the uptake and transport of multivalent metals. This study revealed specific B. juncea MTP genes implicated in the response to HM stresses, laying the groundwork for future research into the molecular mechanisms regulating HM stresses by BjMTPs.
Conclusion
The MTP family plays a crucial role in the transport and tolerance of divalent metal cations in plants. Here, we identified 33 MTP members in B. juncea for the first time using a combination of the Cation_efflux domain (PF01545) and BLAST analyses. Comprehensive analysis of physicochemical properties, conserved motifs, promoter elements, and RNA-seq expression data revealed that the MTP genes in B. juncea originated from its two diploid progenitor species, elucidating the functional diversification of the seven evolutionary branches of the MTP gene family. Spatiotemporal expression profiling under HM stresses successfully identified specific genes and potential crucial CREs associated with the response of BjMTPs to HM stresses. These findings laid a solid foundation for further investigation into the functions of BjMTP genes and provided valuable insights for the bioremediation of HM-contaminated soils.
Materials and methods
Identification of MTPs
The genome and protein sequences of B. rapa (Chiifu v3.5), B. nigra (NI100 v2), and B. juncea (Sichuan Yellow v1.2) were obtained from the Brassicaceae database (http://brassicadb.cn/#/) and the Molecular Breeding of Oilseed website (http://www.oilseedhunan.net/), respectively. In addition, 12 Arabidopsis MTP proteins were downloaded from the NCBI (http://www.ncbi.nlm.nih.gov/), and the HMM file of the MTP conserved domain (Cation_efflux, PF01545) was acquired from the PFAM database (http://pfam.xfam.org/). To identify MTP proteins in B. rapa, B. nigra, and B. juncea, the HMMER (v3.2.1) program under TBtools-II (v2.028)46 was used to screen the protein files for the cation efflux domain, setting the E-value cutoff at 1E-07. Subsequently, the identified MTP proteins were aligned with AtMTPs, retaining only those with an identity greater than 75% for further analysis. The ExPasy tool (https://web.expasy.org/compute_pi/) was utilized to predict the isoelectric point (pI), molecular weight (MW), and physicochemical properties of BjMTP proteins. For subcellular localization and transmembrane domain (TMD) analysis, we used the Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) and TMHMM-2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) programs, respectively, to perform our predictions.
Phylogenetic, chromosomal location, conserved motif, and protein structure analyses
The maximum likelihood (ML) method, implemented in MEGA7.047 with 1000 bootstrap replicates, was utilized to construct a phylogenetic tree of MTP protein families in A. thaliana, B. rapa, B. nigra, and B. juncea. For enhanced visualization, the phylogenetic tree was refined and displayed using EvolView (https://www.evolgenius.info/evolview/). The chromosomal location of BjMTP genes were obtained from the "Sichuan Yellow" genome GFF3 file33. Subsequently, to identify conserved motifs in the BjMTP proteins, we used the MEME Suite 5.5.3 (https://meme-suite.org/meme/tools/meme), specifying a maximum of 15 motifs. The conserved domains of BjMTP proteins were then analyzed using the SMART database (http://smart.embl.de/), which provided the corresponding annotations. The evolutionary tree, chromosomal locations, conserved motifs, and structural domains of the BjMTP proteins were visualized using TBtools-II46 software. Additionally, a comprehensive 3D structural prediction of the BjMTP proteins was conducted using the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/) to gain further insights into their structure and function.
Analysis of the promoter elements and collinearity of BjMTPs
The 2-kb upstream sequences preceding the start codon of the BjMTPs were analyzed for promoter elements using PlantCare (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The results were carefully evaluated, statistically analyzed, and categorized. TBtools-II was then used to generate visual representations based on the processed data. To gain a deeper understanding of the evolutionary patterns and expansion within the BjMTP family members, MCScanX and Ka/Ka calculator programs (both integrated within TBtools-II) were employed to analyze collinearity and calculate selection pressure among the MTPs in different species.
Expression pattern and GO enrichment analysis
To elucidate the expression patterns of MTPs in various tissues of B. juncea, RNA-seq datasets for "Sichuan Yellow" were retrieved from the NCBI database. These datasets included RNA-seq data for roots (SRR11787772), stems (SRR11787777), leaves (SRR11787776), buds (SRR11787782), siliques at 7 (SRR11787779) and 15 (SRR11787783) DAF, pods at 20 DAF (SRR11787780), seeds (SRR11787781), and seed coats (SRR807368). Data analysis followed the methodology described by Kang et al.33, using fragments per kilobase of transcript per million fragments mapped (FPKM) values to quantify gene expression levels. A heatmap was subsequently generated to visualize the expression patterns of the BjMTP genes by plotting the Log2 (FPKM + 1) values.
To gain insights into the biological functions of BjMTP genes, the entire gene family was selected as the target set for enrichment analysis using the Gene Ontology database (https://geneontology.org/). Significant enrichment results were then visualized using the Omicsmart platform (https://www.omicsmart.com/RNAseq/home.html).
Plant materials, growth conditions, and treatments
The "Sichuan yellow"seeds used in this study were provided by the Rapeseed Molecular Breeding Laboratory of Hunan Agricultural University. The seeds were disinfected with a 50% sodium hypochlorite (v/v) solution, followed by thorough rinsing with distilled water to remove any residual disinfectant. The seeds were then planted on germination beds soaked in Hoagland's solution. After one week of germination, the seedlings were carefully transplanted into opaque black containers filled with Hoagland's solution and exposed to a 16 h light/8 h dark photoperiod. Each container lid was equipped with six 1 cm-diameter holes, each accommodating one seedling. In the phytotron, environmental conditions suitable for plant growth were precisely regulated: a light/dark cycle of 16 h/8 h, relative humidity of 50–60%, and a temperature of 24 ± 2 °C.
After four weeks of growth, upon reaching the 4–5 leaf stage, the plants were subjected to stress research using known concentrations of four metals (Mn, Fe, Zn, and Cd) that induce chlorosis in wheat leaves48, as well as concentrations of two other metals (Sb and Pb) identified to have a notable influence on the phenotype of B. juncea. Fresh Hoagland solution were supplemented with the following metal concentrations: 250 mg Mn2+ (MnSO4·H2O), 250 mg Fe2+ (FeSO4·7H2O), 200 mg Zn2+ (ZnSO4·7H2O), 100 mg Cd2+ (CdCl2·2.5H2O), 10 mg Sb3+ (KSbC4H4O7·0.5H2O), and 250 mg Pb2+ (PbCl2). Each treatment was replicated across three containers, with six seedlings per container. Subsequently, root and leaf samples were collected at 12, 24, and 48 h after HM stress for RNA isolation using the Trizol Up plus RNA kit (TransGen Biotech, Beijing, China). The isolated RNA was reverse transcribed using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) and analyzed by qRT-PCR to assess gene expression levels, employing the AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China). β-actin49 was used as an internal control. Relative expression levels of target genes were calculated using the 2−ΔΔct method. Each experiment included three biological and three technical replicates per treatment. All primers were synthesized by TSINKE Biotech (Table S6). To investigate the function of gene promoter CREs in response to HM stress, we used the Omicsmart platform (https://www.omicshare.com/tools/Home/Soft/ica2) to examine the relationship between the promoter elements (Table S2) in representative genes and their expression patterns in roots and leaves (Table S5) under various HM stresses. Cytoscape v.3.7.2 was utilized to construct the correlation network diagrams using the obtained Pearson correlation coefficients.
Data availability
The RNA-seq data of "Sichuan Yellow" in this study were retrieved from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/). These datasets included RNA-seq data for various tissues such as roots (SRR11787772), stems (SRR11787777), leaves (SRR11787776), buds (SRR11787782), siliques at 7 (SRR11787779) and 15 (SRR11787783) days after flowering (DAF), pods at 20 DAF (SRR11787780), seeds (SRR11787781), and seed coats (SRR807368).
References
Tiwari, S. & Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 9, 336111 (2018).
Riyazuddin, R. et al. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 12(1), 43 (2021).
Williams, L. E. & Mills, R. F. P(1B)-ATPases—An ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 10(10), 491–502 (2005).
Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11), 1707–1719 (2006).
Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A. & Hakeem, K.R. Plants, pollutants and remediation. Berlin/Heidelberg, Germany: Springer Netherlands, pp. 251–268 (2015).
Zhang, Y., O’Loughlin, E. J. & Kwon, M. J. Antimony redox processes in the environment: A critical review of associated oxidants and reductants. J. Hazard. Mater. 431, 128607 (2022).
Vishwakarma, K. et al. Avenues of the membrane transport system in adaptation of plants to abiotic stresses. Crit. Rev. Biotechnol. 39(7), 861–883 (2019).
Yadav, B. et al. Plant mineral transport systems and the potential for crop improvement. Planta 253(45), 1–30 (2021).
Hall, J. L. & Williams, L. E. Transition metal transporters in plants. J. Exp. Bot. 54(393), 2601–2613 (2003).
Nies, D. H. & Silver, S. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14, 186–199 (1995).
Gustin, J. L., Zanis, M. J. & Salt, D. E. Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol. Biol. 11(76), 1–13 (2011).
Kolaj-Robin, O., Russell, D., Hayes, K. A., Pembroke, J. T. & Soulimane, T. Cation diffusion facilitator family: Structure and function. FEBS Lett. 589(12), 1283–1295 (2015).
Montanini, B., Blaudez, D., Jeandroz, S., Sanders, D. & Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 8(107), 1–16 (2007).
Arrivault, S., Senger, T. & Krämer, U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 46(5), 861–879 (2006).
Eroglu, S. et al. Metal Tolerance Protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiol. 174(3), 1633–1647 (2017).
Fujiwara, T. et al. A high molecular mass zinc transporter MTP12 forms a functional heteromeric complex with MTP5 in the Golgi in Arabidopsis thaliana. FEBS J. 282(10), 1965–1979 (2015).
Mourato, M. P. et al. Effect of heavy metals in plants of the genus Brassica. Int. J. Mol. Sci. 16(8), 17975–17998 (2015).
Małecka, A. et al. Activation of antioxidative and detoxificative systems in Brassica juncea L. plants against the toxicity of heavy metals. Sci. Rep. 11(1), 22345 (2021).
Yang, Z. et al. Metabolic profiles in the xylem sap of Brassica juncea exposed to cadmium. Physiol. Plant. 175(2), e13886 (2023).
Xu, J., Chai, T., Zhang, Y., Lang, M. & Han, L. The cation-efflux transporter BjCET2 mediates zinc and cadmium accumulation in Brassica juncea L. leaves. Plant Cell Rep. 28, 1235–1242 (2009).
Lang, M. et al. Functional characterization of BjCET3 and BjCET4, two new cation-efflux transporters from Brassica juncea L. J. Exp. Bot. 62(13), 4467–4480 (2011).
Han, L. et al. Identification and functional analysis of cation-efflux transporter 1 from Brassica juncea L. BMC Plant Biol. 22(1), 174 (2022).
Lu, M. & Fu, D. Structure of the zinc transporter YiiP. Science. 317(5845), 1746–1748 (2007).
Jogawat, A., Yadav, B. & Narayan, O. P. Metal transporters in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiol. Plant. 173(1), 259–275 (2021).
Wang, Q. et al. Identification, classification, and expression analysis of the Triacylglycerol Lipase (TGL) Gene family related to abiotic stresses in Tomato. Int. J. Mol. Sci. 22(3), 1387 (2021).
Xu, G., Guo, C., Shan, H. & Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 109(4), 1187–1192 (2012).
Chen, Z. et al. Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J. Exp. Bot. 64(14), 4375–4387 (2013).
Zou, C. et al. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl. Acad. Sci. 108(36), 14992–14997 (2011).
El-Sappah, A. H. et al. Genome-wide identification and expression analysis of metal tolerance protein (MTP) gene family in soybean (Glycine max) under heavy metal stress. Mol. Biol. Rep. 50(4), 2975–2990 (2023).
Wu, D. et al. OPT gene family analysis of potato (Solanum tuberosum) responding to heavy metal stress: Comparative omics and co-expression networks revealed the underlying core templates and specific response patterns. Int. J. Biol. Macromol. 188, 892–903 (2021).
Wang, X., Wang, C., Zhang, Z. & Shi, G. Genome-wide identification of metal tolerance protein genes in Peanut: differential expression in the root of two contrasting cultivars under metal stresses. Front. Plant Sci. 13, 791200 (2022).
Xie, T. et al. Genome-wide identification and expressional profiling of the metal tolerance protein gene family in Brassica napus. Genes (Basel) 13(5), 761 (2022).
Kang, L. et al. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 53(9), 1392–1402 (2021).
Vision, T. J., Brown, D. G. & Tanksley, S. D. The origins of genomic duplications in Arabidopsis. Science 290(5499), 2114–2117 (2000).
Schranz, M. E., Lysak, M. A. & Mitchell-Olds, T. The ABC’s of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci. 11(11), 535–542 (2006).
Laha, S. D., Dutta, S., Schäffner, A. R. & Malay, D. Gene duplication and stress genomics in Brassicas: Current understanding and future prospects. J. Plant Physiol. 255, 153293 (2020).
Shariatipour, N. & Heidari, B. Meta-analysis of expression of the stress tolerance associated genes and uncover their-regulatory elements in rice (L.). Open Bio. J. 13(1), 39–49 (2020).
Zhang, H. et al. Crucial abiotic stress regulatory network of NF-Y transcription factor in plants. Int. J. Mol. Sci. 24(5), 4426 (2023).
Soltis, D. E., Visger, C. J., Marchant, D. B. & Soltis, P. S. Polyploidy: Pitfalls and paths to a paradigm. Amer. J. Bot. 103(7), 1146–1166 (2016).
Segraves, K. A. The effects of genome duplications in a community context. New Phytol. 215(1), 57–69 (2017).
John, R., Ahmad, P., Gadgil, K. & Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 3, 65–76 (2009).
Shekhawat, K., Rathore, S. S., Premi, O. P., Kandpal, B. K. & Chauhan, J. S. Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj. Cosson): An overview. Int. J. Agric. 2012, 1–14 (2012).
Desbrosses-Fonrouge, A. G. et al. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett. 579(19), 4165–4174 (2005).
Menguer, P. K. et al. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 64(10), 2871–2883 (2013).
Migocka, M. et al. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 66(3), 1001–1015 (2015).
Chen, C. et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 16(11), 1733–1742 (2023).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1870–1874 (2016).
Yang, L. et al. Bioinformatic analysis of wheat metal tolerance protein (MTP) gene family and its role under stress. Acta Bot. Boreal. Occident. Sin. 40(7), 1123–1134 (2020) (In Chinese).
Xiao, L. et al. Mutations in the CDS and promoter of BjuA07. CLV1 cause a multilocular trait in Brassica juncea. Sci. Rep. 8(1), 5339 (2018).
Acknowledgements
This research was supported by Research Foundation of Education Bureau of Hunan Province, China (23B0809, 22B0844), Hunan Provincial Natural Science Foundation of China( 2023JJ50083) and the National Natural Science Foundation of China (32371589).
Author information
Authors and Affiliations
Contributions
YL, CY, and LXJ provided the research idea and wrote the manuscript. YL, JLS, JGX,CH, YYH, GS, YML, HJH, XGH, and DRY contributed reagents, materials, and analysis tools. YL, JLS, JGX and CH performed the experiments. YL, LXJ and DRY revised the manuscript and provided fund support. All authors participated in writing and reviewing the manuscript.
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, L., Sheng, J., Jiang, G. et al. Molecular characterization and expression patterns of MTP genes under heavy metal stress in mustard (Brassica juncea L.). Sci Rep 14, 17857 (2024). https://doi.org/10.1038/s41598-024-68877-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68877-8
- Springer Nature Limited