Abstract
A recent systematic review indicated that gut–microbiota–brain axis contributes to growth and rupture of intracranial aneurysms. However, gaps were detected in the role of intestinal microbiome in cerebral vasospasm (CVS) after aneurysmal subarachnoid hemorrhage (aSAH). This is the first pilot study aiming to test study feasibility and identify differences in gut microbiota between subjects with and without CVS following aSAH. A prospective nested case–control pilot study with 1:1 matching was conducted recruiting subjects with aSAH: cases with CVS; and controls without CVS based on the clinical picture and structured bedside transcranial Doppler (TCD). Fecal samples for microbiota analyses by means of 16S rRNA gene amplicon sequencing were collected within the first 96 h after ictus. Operational taxonomic unit tables were constructed, diversity metrics calculated, phylogenetic trees built, and differential abundance analysis (DAA) performed. At baseline, the groups did not differ significantly in basic demographic and aneurysm-related characteristics (p > 0.05). Alpha-diversity (richness and Shannon Index) was significantly reduced in cases of middle cerebral artery (MCA) vasospasm (p < 0.05). In DAA, relative abundance of genus Acidaminococcus was associated with MCA vasospasm (p = 0.00013). Two butyrate-producing genera, Intestinimonas and Butyricimonas, as well as [Clostridium] innocuum group had the strongest negative correlation with the mean blood flow velocity in anterior cerebral arteries (p < 0.01; rho = − 0.63; − 0.57, and − 0.57, respectively). In total, 16 gut microbial genera were identified to correlate with TCD parameters, and two intestinal genera correlated with outcome upon discharge. In this pilot study, we prove study feasibility and present the first preliminary evidence of gut microbiome signature associating with CVS as a significant cause of stroke in subjects with aSAH.
Similar content being viewed by others
Introduction
Cerebral vasospasm (CVS) is one of the dreadful sequelae of aneurysmal subarachnoid hemorrhage (aSAH). Clinically, it might lead to delayed cerebral ischemia (DCI) with delayed ischemic neurologic deficits (DIND). The latter one has been defined as a new focal deficit (hemiparesis, dysphasia) or decrease in Glasgow Coma Scale by two points not attributable to hydrocephalus, rebleeding, seizures, or hyponatremia. It accounts for death or disability of approximately 20% of the aSAH survivors1. Despite its significance, cerebral vasospasm fundamentals remain obscure and it lacks effective prophylaxis or treatment.
Gut–microbiota–brain axis (GMBA) is a bidirectional route of signaling between the digestive tract and central nervous system (CNS) that modulates health and disease and was shown to play a role in a plethora of neurological disorders such as ischemic stroke, multiple sclerosis, Alzheimer's disease, or others2,3,4. Moreover, our group recently published a systematic review with a narrative synthesis which highlighted the role of gut microbiota in growth and rupture of intracranial aneurysms5. However, as we indicated, currently there are no studies searching associations between intestinal microbiota and CVS following aSAH. Since CVS poses a significant risk of permanent neurological disability or death, it is of great concern to seek new diagnostic and therapeutic targets to prevent or counteract this phenotype.
Therefore, we aimed to investigate taxonomic components of gut microbiome as well as its ecological indices (alfa-diversity, beta-diversity) and their association with the presence of CVS and DIND after aSAH or with its surrogate markers in transcranial Doppler ultrasound examination as it is the only non-invasive and bedside modality to visualize in-vivo CVS. The primary objective of this pilot study was to assess feasibility at a small scale with recruitment rates, ultimate study completion rates, and troubleshooting as outcomes. The secondary objective was to search for preliminary clinical associations between microbiota and CVS utilizing the following outcomes: alfa-diversity, beta-diversity, differential abundance at a genus level, and correlation matrix of the microbial abundance.
Results
Patient characteristics
26 subjects with aSAH met the eligibility criteria and completed the entire study protocol. There were 18 women (69.2%) and eight men (30.8%), a proportion concordant with the female sex being a risk factor for aSAH (hazard ratio: 1.90 [95% CI 1.47–2.46] PMID 31521957). The most prevalent locations of aneurysms were anterior communicating artery (n = 6; 23.1%), middle cerebral artery (n = 6; 23.1%) and internal carotid artery (n = 6; 23.1%), followed by posterior communicating artery (n = 5; 19.2%). Posterior circulation aneurysms comprised 30.8% (n = 8). A group of 13 patients developed CVS with DIND and 13 patients did not. Upon discharge, subjects with CVS had worse clinical outcome defined as an unfavorable (3–6) modified Rankin scale (62% vs. 15%; p = 0.041, Fisher’s exact test). Tables 1 and 2 provide study group characteristics. All elements of the STROBE statement were addressed and can be viewed in Supplementary Material 1. Table 3 summarizes the measurements of transcranial Doppler ultrasound examination. Figure 1 presents an example of transcranial Doppler examination report.
An example of transcranial Doppler examination report demonstrating normal mean blood flow velocity in the left middle cerebral artery with Lindegaard ratio of 1.9 and increased mean blood flow velocity due to posthemorrhagic vasospasm in the right middle cerebral artery with Lindegaard ratio of 6.53. L left, R right, MCA middle cerebral artery, Int Carotid internal carotid artery, PI pulsatility index, RI resistance index.
Microbiota analyses
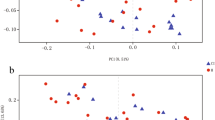
A significant decrease in alpha diversity was observed in subjects with MCA vasospasm (p = 0.031 for richness and p = 0.045 for Shannon index)—see Fig. 2. There was no difference in beta diversity for any group comparison (MCA vasospasm, ACA vasospasm, DIND)—see Supplementary Material 2.
In differential abundance analysis, Wilcoxon test with Total Sum Scaling (TSS) normalization indicated that genus Acidaminococcus was relatively abundant in cases of MCA vasospasm (p = 0.0001296). All subjects without MCA vasospasm had nonexistent abundance of genus Acidaminococcus whereas subjects with MCA vasospasm presented with significantly larger abundance (in MCA vasospasm group median = 0.000268, interquartile range = 0.0003195)—Fig. 3.
(A) Venn’s diagram illustrating the results of differential abundance analysis with four different normalization methods. Wilcoxon test with total sum scaling (WilcoxTSS) displayed that genus Acidaminococcus is more abundant in middle cerebral artery vasospasm. TSS is considered the best normalization method reducing experimental bias regarding statistical power and effect on false discovery rate (FDR) compared to other normalization schemes such as ALDEx2 or centered log ratio (CLR) (PMID: 36074761). (B) A box plot illustrating difference in abundance of genus Acidaminococcus between subjects without MCA vasospasm and with MCA vasospasm. All subjects without MCA vasospasm had nonexistent abundance of genus Acidaminococcus whereas subjects with MCA vasospasm presented with significantly larger abundance (in MCA vasospasm group median: 0.000268, interquartile range: 0.0003195).
The strongest negative linear correlation was found between butyrate-producing genus Intestinimonas and mean velocity in ACA (rho = − 0.63; p = 0.003; Q = 0.16), butyrate-producing genus Butyricimonas and ACA mean velocity (rho = − 0.57; p = 0.008; Q = 0.246), as well as genus [Clostridium]_innocuum_group and ACA mean velocity (rho = − 0.57; p = 0.004; Q = 0.246). See correlation matrix in Fig. 4. Positive correlations were weaker than the negative but initial significance threshold was observed for genus Sellimonas and ACA mean velocity, genus NK4A214_group and ACA mean velocity, genus Flavonifractor and basilar artery mean velocity (rho = 0.51; p = 0.025; Q = 0.508), genus Bifidobacterium and MCA mean velocity (rho = 0.42; p = 0.035; Q = 0.920)—see Fig. 4. In total, 16 gut microbial genera were identified to correlate with TCD parameters and two intestinal genera correlated with outcome upon discharge as determined by modified Rankin scale—negative correlation with genus Megasphaera (rho = − 0.45; p = 0.034; Q = 0.778) and positive correlation with genus Eisenbergiella (rho = 0.47; p = 0.021; Q = 0.781). Results that did not reach the significance threshold are displayed in Supplementary Material 2. Taxonomic data are available as Supplementary Material 3.
(A) Correlation matrix summarizing Spearman's correlation testing of the microbial abundance at the genus level with parameters of transcranial Doppler ultrasound examination in subjects with aneurysmal subarachnoid hemorrhage. The single asterisk (*) indicates correlations with p value in range 0.01–0.05; The double asterisk (**)—p value 0.001–0.01; The dot (.)—p value with tendency to significance 0.05–0.1. ACA mean velocity was obtained as a mean from both sides whereas ACA mean velocity 2 as a mean velocity from the side with higher velocity. Direction of correlation for both ACA velocities remains the same. (B–G) show graphs illustrating Spearman linear correlation testing. (B) Genus Intestinimonas and ACA mean velocity; (C) Genus Butyricimonas and ACA mean velocity 2; (D) Genus [Clostridium] innocuum group and ACA mean velocity 2; (E) Genus Flavonifractor and basilar artery mean velocity; (F) Genus Bifidobacterium and MCA mean velocity; (G) Genus Eisenbergiella and modified Rankin scale upon discharge. ACA anterior cerebral artery, BA basilar artery, MCA middle cerebral artery, mRankin modified Rankin scale upon discharge, Q False discovery rate-adjusted p value.
Feasibility assessment of the pilot study
Of 31 consecutive subjects with subarachnoid hemorrhage, 27 met the eligibility criteria, which produces a recruitment rate of 87.1%. Ineligibility was due to lack of acoustic bony window in two subjects, an aneurysm within the arteriovenous malformation in one, and Hunt–Hess grade V with early death in one. Of the 27 eligible participants, one did not complete the study because of the early transfer to another hospital in a different city upon request from the family (a completion rate of 96.3%). Stool sample collection, DNA isolation, library preparation, 16S rRNA gene amplicon sequencing, and bioinformatics were uneventful. The final outcome of the feasibility assessment for a future trial is: feasible with close monitoring.
Discussion
We conducted the very first study to find the link between the intestinal microbiome and CVS following aSAH. Thereby, we demonstrated that the methodology used to conduct this study was appropriate and can be used in the future to validate research hypotheses on the relationship between the microbiome and CVS after aSAH conducted in a larger group of patients. Here we present that alpha-diversity metrics were significantly reduced in cases of MCA vasospasm. Our study also showed that relative abundance of genus Acidaminococcus was associated with MCA vasospasm. Butyrate-producing genera, namely Intestinimonas and Butyricimonas, along with [Clostridium] innocuum group had the strongest negative correlation with the mean blood flow velocity in anterior cerebral arteries. At last, we identified 16 gut microbial genera correlating with TCD parameters and two with outcome upon discharge.
MCA vasospasm is linked to reduced microbial diversity
In our study, patients with MCA CVS had significantly lower alfa diversity of intestinal microbiome. It is accepted that composition of the microbiota fluctuates strongly and constantly with age, gender, genetic factors of the host, environmental conditions, and lifestyle6. Therefore, there is no single component that would be universally characteristic of healthy human gut microbiota. Rather, a broader term of high diversity was introduced and is considered desirable. Indeed, most of the human diseases of the westernized regions are associated with reduction in microbial diversity7. However, according to Shade and colleagues8 diversity is neither good nor bad, it simply “is”, and its deviations can be utilized in clinical settings. In terms of CVS, there is evidence that gut microbiome alterations including bacterial sub-population diversity might result in endothelial dysfunction, which in turn is strongly linked to vasospasm9. Also, bacterial diversity alters levels of plasma trimethylamine N-oxide (TMAO) of bacterial origin10. Interestingly, in ischemic stroke TMAO is usually elevated, whilst in aSAH it is reduced11,12,13,14. The question remains what levels of TMAO are observed in DCI after aSAH. Also, what needs further studies is whether modulation of microbial alfa diversity and TMAO levels could impact course of CVS after aSAH.
Butyrate-producing bacteria in cerebral ischemia
We presented that butyrate-producing genus Intestinimonas and genus Butyricimonas negatively correlate with mean flow velocity in anterior cerebral artery which is used as a diagnostic parameter of in-vivo cerebral vasospasm. Such vasospasm is a leading cause of stroke in subjects after aSAH. Moreover, it has been shown that abundance of butyrate-producing bacteria in gut could be a protective factor against the primary ischemic stroke15. Butyrate is a four-carbon short-chain fatty acid that readily absorbs from intestines into the portal circulation and modulates inflammation by exerting antioxidant and anti-inflammatory effects. It is also a potent neuroprotectant and modulates immune function as well as energy metabolism16. Importantly, this SCFA has been found to cross blood–brain barrier and its involvement in neural signaling has been proven17. There are data indicating that low levels of butyrate-producing bacteria are associated with a variety of cardiovascular disorders such as acute ischemic stroke18, hypertension19, or chronic kidney disease20. Moreover, an in vitro study elegantly demonstrated that mice orally supplemented with butyrate improved myocardial ischemia/reperfusion injury via a gut–brain neural circuit21.
However, we here provide the first preliminary evidence indicating negative correlation between two butyrate-producing genera, Intestinimonas and Butyricimonas, and mean flow velocity in anterior cerebral arteries, one of the surrogate markers of the in-vivo CVS following aneurysmal subarachnoid hemorrhage. Interestingly, in May 2023 Meng et al. found that a relative decrease in abundance of Intestinimonas could play a role in the pathomechanism of both large artery and small vessels ischemic strokes, conditions which share many similarities with the CVS and delayed cerebral ischemia22. Although the delayed cerebral ischemia and atherosclerotic ischemic stroke are of different etiologies, their end points are congruent—decreased cerebral blood flow leading to a cascade of responses including local hypoxia, excitotoxicity, release of free radicals, mitochondrial death pathways, protein misfolding, apoptosis, and necrosis23. Whether promoting the growth of butyrate-producing genera such as Intestinimonas or Butyricimonas and restoring levels of butyrate could be a protective factor or serve as a complementary therapy for subjects with ACA vasospasm deserves further studies.
Acidaminococcus and inflammatory fundamentals of CVS
In our cohort, subjects with vasospasm in middle cerebral artery had higher relative proportions of genus Acidaminococcus as detected in DAA approach using Wilcoxon test with Total Sum Scaling (TSS). TSS is considered the best normalization method reducing experimental bias regarding statistical power and effect on false discovery rate (FDR) compared to other normalization schemes such as ALDEx2 or CLR (centered log ratio)24. Thus, we consider this finding significant, however, it requires external validation with larger samples. CVS and Acidaminococcus could be linked via its role in modulation of inflammation. Even though the exact etiology and pathomechanism of CVS are unknown, one of the accepted theories is the antecedent inflammatory cascade of leukocytes, cytokines (interleukin-1 and interleukin-6), and cell adhesion molecules25,26. In fact, the abundance of Acidaminococcus has been observed in a number of inflammatory disorders such as ankylosing spondylitis, rosacea, oral mucositis after autologous hematopoietic stem cell transplantation, or primary biliary cholangitis27,28,29,30. Thus, we hypothesize that the so-called dysbiosis involving genus Acidaminococcus associates with CVS throughout the inflammatory mediators in the vessel wall and could corroborate the inflammatory theory of CVS. Indeed, Acidaminococcus was negatively correlated with plasma levels of omega-6-derived oxylipins31, and importantly, oxylipins such as 20-hydroxyeicosatetraenoic acid were reported to be involved in cerebral vasospasm following aSAH32—see Fig. 5. Moreover, only recently was the relative abundance of genus Acidaminococcus found to be also associated with lacunar cerebral infarcts33. Crucially, the lacunar pattern of infarct is seen in approximately 20% of MCA vasospasm-related cerebral ischemia34. Finally, this genus has been observed abundantly in obese subjects with increased visceral adipose tissue which could constitute a potential confounding factor35,36. However, the groups in our sample did not differ significantly in terms of Body Mass Index, so we do not consider it a confounder (27.97 kg/m2 in case group versus 25.43 kg/m2 in control group; p = 0.21). Overall, we provide preliminary evidence of association between Acidaminococcus and MCA vasospasm after aSAH, yet it remains unclear whether a true causal relationship exists, or it is merely an epiphenomenon.
Limitations and strengths
Although the sample size appears small, it is an inherent feature of pilot studies exploring no man’s land of research and, whatsoever, it was sufficient to detect significant differences and provide preliminary evidence of gut microbiome-based signature in CVS. Nonetheless, the generalizability of the study results is limited and it is supposed that this study will appeal to researchers to conduct external validation of the findings on larger cohorts. By the same token, only strong correlations were identified, whereas weaker but potentially significant correlations might remain undetected. A strong point of this investigation is the multidisciplinary expertise of the researchers involved in planning, microbiota analysis, and neurosurgical clinical evaluation of the subjects. The homogenous sample comes from the single center, whereas the expertise and assistance were obtained throughout multiple centers.
Another limitation refers to the radiographic measures of vessel narrowing. TCD was utilized because it is noninvasive, repeatable, and can be performed bedside, but it is prone to error stemming from the inter–examiner variability. The authors acknowledgle that digital subtraction angiography (DSA) could visualize the vasospasm more accurately and is considered a gold standard. However, DSA is more invasive, not repeatable at a bedside, and is not performed routinely at our institution in every single subject to determine CVS or lack thereof. Moreover, TCD allows for analysis of arterial flow velocity and development of correlation matrix, which would not be possible with binary evaluation of DSA.
Future perspectives and clinical implications
Considering strengths and limitations of the study, the results presented here, after external validation, have potential to impact further research and clinical practice in several ways. In future, prediction models might include microbial signature to aid in early diagnosis and prevention of DCI. Ideally, a test from the stool sample collected upon admission indicating a subject at a high risk of CVS could guide commencement of therapy, for instance permissive euvolemic hypertension, which is currently the mainstay of treatment37. Moreover, recognizing interventions that impact intestinal microbiome, prospective randomised placebo-controlled clinical trials with administration of probiotics promoting growth of butyrate-producing bacteria could be initiated to analyze whether the risk of CVS could be reduced by alteration of the gut microbiota. Thereby, more profound understanding of the CVS can introduce multidisciplinary approach to the individualized treatment.
Conclusions
In this pilot study, we confirmed that the used methodology was appropriate and we have presented the first preliminary evidence of gut microbiome-based signature associating with CVS as a significant cause of stroke in subjects with aneurysmal subarachnoid hemorrhage. It is the only attempt in the literature of sequencing 16S rRNA gene of the intestinal microbiota in subjects with CVS and DCI, which provides insights into its metagenomic landscape. Moreover, these findings could fuel further research on gut microbiome as a potential biomarker in diagnosis or a target for prevention and treatment of cerebral vasospasm.
Methods
Study design and eligibility
This is a prospective observational nested case–control pilot study evaluating the association between CVS after aSAH and gut microbiota structure. Recruitment started in June 2022 once Institutional Review Board of Pomeranian Medical University in Szczecin approval had been obtained (resolution no. KB-0012/61/2021-A-4897) and the pilot phase ended in July 2023. The authors confirm that all methods were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the participants. Inclusion criteria were as follows: (1) subarachnoid hemorrhage identified in non-enhanced computed tomography (CT), (2) culprit intracranial aneurysm detected on CT angiogram or digital subtraction angiography, (3) age > 18 years old, (4) successful management of the aneurysm. On the other hand, we dismissed subjects with (1) non-aneurysmal pretruncal subarachnoid hemorrhage, (2) antibiotics usage within the previous two months, (3) under the age of 18 years, (4) mycotic aneurysms or aneurysms within arteriovenous malformations, (5) admitted to hospital beyond postbleeding day 5, (6) remaining in Hunt Hess grades IV-V from admission up to postbleeding day 14 as this would preclude obtaining informed consent (consent from the next of kin was not attempted as it is not legally valid for observational studies in Poland), or (7) no acoustic bony window. Cases were eligible subjects with cerebral vasospasm as determined by transcranial Doppler (TCD) ultrasonography whereas controls were eligible patients who did not develop cerebral vasospasm (see section ‘Determination of cerebral vasospasm and delayed cerebral ischemia’). Matching was performed with 1:1 control-to-case ratio. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement was applied to maintain appropriate structure of the manuscript. Detailed gradings of aSAH severity can be viewed as Supplementary Material 4.
Stool sample collection
Between postbleeding days 1–4 (prior to cerebral vasospasm development), a single stool sample was collected with a swab into 5 ml Eppendorf tubes filled with 1 ml of ultraclean high-purity 96% ethyl alcohol (EMSURE). The sample was then transferred to the laboratory refrigerator and stored at a temperature of − 25 °C until analyses. Upon collection of the entire batch, DNA isolation took place.
DNA isolation
Auto-Pure Mini (Allsheng, Hangzhou) extraction machine was utilized for DNA isolation along with MagnifiQ (A&A Biotechnology) reagents and consumables kits. All samples were processed at the same time as a single batch in order to minimize the batch effect. Isolation was performed by trained and certified laboratory technicians in accordance with standard protocol of genomic DNA isolation provided by the manufacturer.
Quantification and quality assessment
Quantity of the isolated genomic DNA and purity of the samples were determined by means of spectrophotometry using DeNovix DS-11 machine by trained and certified laboratorians.
Library preparation
DNA libraries were prepared according to Illumina procedure with PCR amplification of V3–V4 fragment of 16S rRNA gene. Length of the fragment was 460 base pairs (bp). Next-generation sequencing of 16S rRNA gene amplicons was conducted with MiSeq system of Illumina generating fragments of 300 bp in pair-end reads (2 × 300 bp) obtaining approximately 100,000 reads per sample.
Determination of cerebral vasospasm and delayed cerebral ischemia
Radiological CVS was determined by transcranial Doppler ultrasound examination, which is the only non-invasive, safe, surrogate measure of the real in-vivo vasospasm. Moreover, TCD was proven to be a suitable intermediate measure for genetic association studies38. For research purpose, TCD is advantageous over more invasive DSA as it facilitates the quantitative assessment of blood flow velocity, resistence or pulsatility indices, and the creation of a correlation matrix, which would be unattainable with the binary evaluation of DSA. Measurements were performed by a neurosurgical resident (T.K.) and validated by a senior radiologist (M.S.) with over 20 years of experience. Primary hardware in use was Dolphin IQ Transcranial Doppler platform (Viasonix, Ra'anana, HaMerkaz, Israel) using 2 MHz linear probe whereas validation was conducted with Multi-Dop T digital (Compumedics DWL, Germany). Serial assessments were done up to three times on a structured basis on postbleeding days 3–5, 7–10, and 11–14, the schedule grounded upon the pathophysiology of cerebral vasospasm39. ALARA (as low as reasonably achievable) principle was applied to reduce ultrasound exposure. Care was taken to maintain the insonation angle below 30° to ensure measurement bias of the reflector speed below 15%. All measurements were conducted with a patient lying flat in bed. Middle cerebral arteries (MCAs) were found in transtemporal acoustic window at a depth between 40 and 62 mm with flow towards the probe—see Fig. 1. Extracranial segment of internal carotid artery (for calculation of the Lindegaard ratio and Sloan ratio) was found through the submandibular window in the carotid triangle of the neck. MCA vasospasm was declared if both conditions were met: Lindegaard ratio > 3 and MCA mean velocity > 120 cm/s. Anterior cerebral arteries (ACAs) could be found via the transtemporal acoustic window at a depth between 63 and 80 mm with flow away from the probe. ACA vasospasm was declared if both conditions were met: ACA mean velocity > 80 cm/s and Sloan ratio > 3. In case of hypoplastic ACA on one side, only the other side was insonated. Due to less sensitivity of TCD in ACA flow assessment, the measured ACA velocity was curated in two ways: ACA mean velocity from both sides and ACA mean velocity from the side with higher velocity. Basilar artery (BA) was found in suboccipital region at a depth 70–90 mm and its vasospasm was declared if BA mean flow velocity was > 70 cm/s and Sviri ratio > 2. Acknowledging the fact that in about 10% of the population no acoustic window can be identified40 those patients were excluded from the study. Clinically, delayed cerebral ischemia or delayed ischemic neurologic deficit was defined as a new neurological deficit and/or drop in Glasgow Coma Scale by 2 points not attributable to hydrocephalus, rebleeding, hyponatremia or other causes, lasting more than one hour, or the appearance of new infarct in the affected territory on CT or magnetic resonance imaging (MRI) scan.
Sequencing and microbiome bioinformatic analysis
The 16S sequence processing bioinformatics pipeline was initiated with Illumina paired-end sequencing, specifically targeting the V3–V4 region of the 16S rRNA gene. To ensure data quality, we conducted an initial rigorous assessment using FastQC (version 0.12.1) and MultiQC (version 1.12) tools. Subsequently, all data preprocessing procedures were executed using QIIME2 software41. The 16S rRNA gene sequences underwent processing through the DADA2 (Divisive Amplicon Denoising Algorithm 2) algorithm, as introduced by Callahan et al.42. DADA2 is primarily designed for identifying and correcting errors in high-throughput sequencing data. It employs a denoising approach to distinguish genuine biological sequence variants (amplicon sequence variants or ASVs) from sequencing errors. This is achieved by implementing a denoising algorithm that detects and removes errors inherent in sequencing reads. Before applying DADA2, primer trimming on the V3–V4 region was performed using the cutadapt plugin. Representative sequences resolved by DADA2 underwent filtering to exclude low-abundance features, specifically ASVs with a frequency below 10. This resulted in 1572 unique ASVs, each with abundance estimates within every sample. Metrics per sample included a minimum frequency of 21,393.0, a 1st quartile at 39,060.5, a median frequency of 43,536.00, a 3rd quartile at 50,829.0, a maximum frequency of 60,817.0, and an overall mean frequency of 43,477.6. Additionally, taxonomic profiling of microbial communities was conducted using the scikit-learn method in QIIME 2, employing a naive Bayes machine learning classifier43. The scikit-learn classifier was trained on the Silva 138–99 reference dataset of 16S rRNA gene sequences. Query sequences were associated with predicted taxonomic assignments at different taxonomic ranks (phylum, class, order, family, and genus). Following taxonomic profiling, ASVs assigned to categories such as Eukaryota, Archaea, mitochondria, and chloroplasts were excluded due to their likely representation as contaminants or non-bacterial sequences.
Statistical analysis
All analyses were performed in R (R Core Team, 2021), and, where necessary, False Discovery Rate (FDR) correction was applied to calculate Q value (FDR-adjusted p value). We predefined the following potential baseline confounders to be checked between the analyzed groups: age, sex, hypertension presence, BMI, aneurysm location and size, smoking cigarettes, alcohol abuse, multiple comorbidities, Hunt–Hess and WFNS grades. Fisher’s exact test was applied for categorical variables. For continuous variables we used unpaired two-sample Wilcoxon–Mann–Whitney test. Descriptive statistics of nonparametric data were presented as medians with interquartile ranges. Alpha diversity measures, including Shannon’s diversity, Pielou’s evenness, and richness, were calculated from rarefied samples to a minimum frequency depth of 21,393. The indices were compared between groups using a Wilcoxon test. Beta-diversity was calculated using Bray–Curtis distance at the genus level, and pairwise relations between samples were visualized through Principal Coordinate Analysis (PCA). Beta-diversity distances were compared between groups using Permutational Analysis of Variance (PERMANOVA). Differential abundance analysis was conducted using four different methods: ALDEx244, the Wilcoxon test on rarefied data (WilcoxRarefied), center-log-transformed data (WilcoxCLR), and data transformed using total sum scaling (WilcoxTSS). This approach, suggested by Nearing et al.45 served as a workaround for the lack of reproducibility and consistency among various differential abundance techniques. Correlations with numerical clinical variables and microbiome data were assessed using the ALDEx2 package. Missing data were handled by omission from the given analysis.
Data availability
The datasets generated and analysed during the current study are available in the BioProject repository, accession number: PRJNA1075509, accession hyperlink: www.ncbi.nlm.nih.gov/bioproject/PRJNA1075509. Taxonomic data are available as Supplementary Material 3. Detailed gradings of aSAH severity can be viewed as Supplementary Material 4.
References
Rosengart, A. J., Schultheiss, K. E., Tolentino, J. & Macdonald, R. L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 38(8), 2315–2321. https://doi.org/10.1161/STROKEAHA.107.484360 (2007).
Asghari, K. M. et al. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis: A randomized clinical trial. Sci. Rep. 13(1), 18577. https://doi.org/10.1038/s41598-023-46047-6 (2023).
Sciuto, M. & Catanzaro, R. Composition of gut microbiota and its correlations with neurological, intestinal, cardiovascular and metabolic diseases. Acta Microbiol. Immunol. Hung. https://doi.org/10.1556/030.2023.02134 (2023).
Zeng, H. et al. Unraveling the connection between gut microbiota and Alzheimer’s disease: A two-sample Mendelian randomization analysis. Front. Aging Neurosci. 15, 1273104. https://doi.org/10.3389/fnagi.2023.1273104 (2023).
Klepinowski, T., Skonieczna-Żydecka, K., Pala, B., Stachowska, E. & Sagan, L. Gut microbiome in intracranial aneurysm growth, subarachnoid hemorrhage, and cerebral vasospasm: A systematic review with a narrative synthesis. Front. Neurosci. 17, 1247151. https://doi.org/10.3389/fnins.2023.1247151 (2023).
Hou, K. et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 7(1), 135. https://doi.org/10.1038/s41392-022-00974-4 (2022).
Mosca, A., Leclerc, M. & Hugot, J. P. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem?. Front. Microbiol. 7, 455. https://doi.org/10.3389/fmicb.2016.00455 (2016).
Shade, A., Jacques, M. A. & Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 37, 15–22. https://doi.org/10.1016/j.mib.2017.03.010 (2017).
Rustia, A. J., Paterson, J. S., Best, G. & Sokoya, E. M. Microbial disruption in the gut promotes cerebral endothelial dysfunction. Physiol. Rep. 9(21), e15100. https://doi.org/10.14814/phy2.15100 (2021).
Zhen, J. et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. (Lausanne) 14, 1085041. https://doi.org/10.3389/fendo.2023.1085041 (2023).
Emonds, J. J. et al. Trimethylamine N-oxide (TMAO) in patients with subarachnoid hemorrhage: A prospective observational study. Acta Neurochir. (Wien) 165(5), 1277–1287. https://doi.org/10.1007/s00701-022-05485-3 (2023).
Nie, J. et al. Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 49(9), 2021–2028. https://doi.org/10.1161/STROKEAHA.118.021997 (2018).
Rexidamu, M., Li, H., Jin, H. & Huang, J. Serum levels of Trimethylamine-N-oxide in patients with ischemic stroke. Biosci. Rep. 39(6), BSR20190515. https://doi.org/10.1042/BSR20190515 (2019).
Schneider, C. et al. Trimethylamine-N-oxide is elevated in the acute phase after ischaemic stroke and decreases within the first days. Eur. J. Neurol. 27(8), 1596–1603. https://doi.org/10.1111/ene.14253 (2020).
Zeng, X. et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 9, 4. https://doi.org/10.3389/fcimb.2019.00004 (2019).
Stilling, R. M. et al. The neuropharmacology of butyrate: The bread and butter of the microbiota–gut–brain axis?. Neurochem. Int. 99, 110–132. https://doi.org/10.1016/j.neuint.2016.06.011 (2016).
Chen, T., Noto, D., Hoshino, Y., Mizuno, M. & Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J, Neuroinflamm. 16(1), 165. https://doi.org/10.1186/s12974-019-1552-y (2019).
Chang, Y. et al. Microbiota dysbiosis and functional outcome in acute ischemic stroke patients. Sci. Rep. 11, 10977. https://doi.org/10.1038/s41598-021-90463-5 (2021).
Calderón-Pérez, L. et al. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 10, 6436. https://doi.org/10.1038/s41598-020-63475-w (2020).
Gao, B. et al. Butyrate producing microbiota are reduced in chronic kidney diseases. Sci. Rep. 11, 23530. https://doi.org/10.1038/s41598-021-02865-0 (2021).
Yu, Z. et al. Oral supplementation with butyrate improves myocardial ischemia/reperfusion injury via a gut-brain neural circuit. Front. Cardiovasc. Med. 8, 718674. https://doi.org/10.3389/fcvm.2021.718674 (2021).
Meng, C. et al. Gut microbiome and risk of ischaemic stroke: A comprehensive Mendelian randomization study. Eur. J. Prev. Cardiol. 30(7), 613–620. https://doi.org/10.1093/eurjpc/zwad052 (2023).
Sekerdag, E., Solaroglu, I. & Gursoy-Ozdemir, Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr. Neuropharmacol. 16(9), 1396–1415. https://doi.org/10.2174/1570159X16666180302115544 (2018).
Cappellato, M., Baruzzo, G. & Di Camillo, B. Investigating differential abundance methods in microbiome data: A benchmark study. PLoS Comput. Biol. 18(9), e1010467. https://doi.org/10.1371/journal.pcbi.1010467 (2022).
Carr, K. R., Zuckerman, S. L. & Mocco, J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol. Res. Int. 2013, 506584. https://doi.org/10.1155/2013/506584 (2013).
Eisenhut, M. Vasospasm in cerebral inflammation. Int. J. Inflamm. 2014, 509707. https://doi.org/10.1155/2014/509707 (2014).
Joura, M. I., Brunner, A., Nemes-Nikodém, É., Sárdy, M. & Ostorházi, E. Interactions between immune system and the microbiome of skin, blood and gut in pathogenesis of rosacea. Acta Microbiol. Immunol. Hung. 68(1), 1–6. https://doi.org/10.1556/030.2021.01366 (2021).
Ponziani, F. R. et al. High prevalence of lower limb atherosclerosis is linked with the gut-liver axis in patients with primary biliary cholangitis. Liver Int. 43(2), 370–380. https://doi.org/10.1111/liv.15463 (2023).
Wong, S. P. et al. Oral and gut microbiota dysbiosis is associated with mucositis severity in autologous hematopoietic stem cell transplantation: Evidence from an Asian population. Transplant. Cell. Ther. 29(10), 633.e1-633.e13. https://doi.org/10.1016/j.jtct.2023.06.016 (2023).
Zhou, C. et al. Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J. Autoimmun. 107, 102360. https://doi.org/10.1016/j.jaut.2019.102360 (2020).
Xu, H. et al. Plasma levels of omega-3 and omega-6 derived oxylipins are associated with fecal microbiota composition in young adults. Nutrients 14(23), 4991. https://doi.org/10.3390/nu14234991 (2022).
Poloyac, S. M., Reynolds, R. B., Yonas, H. & Kerr, M. E. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J. Neurosci. Methods 144(2), 257–263. https://doi.org/10.1016/j.jneumeth.2004.11.015 (2005).
Ma, J. et al. The gut microbial signatures of patients with lacunar cerebral infarction. Nutr. Neurosci. 27, 1–17. https://doi.org/10.1080/1028415X.2023.2242121 (2023).
Weidauer, S. et al. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: A prospective MRI and DSA study. Stroke 38(6), 1831–1836. https://doi.org/10.1161/STROKEAHA.106.477976 (2007).
Freitas, R. G. B. O. N. et al. Gestational weight gain and visceral adiposity in adult offspring: Is there a link with the fecal abundance of Acidaminococcus genus?. Eur. J. Clin. Nutr. 76(12), 1705–1712. https://doi.org/10.1038/s41430-022-01182-7 (2022).
Pinart, M. et al. Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 14(1), 12. https://doi.org/10.3390/nu14010012 (2021).
Hoh, B. L. et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke 54(7), e314–e370. https://doi.org/10.1161/STR.0000000000000436 (2023).
Kim, H. et al. Cerebral vasospasm after sub-arachnoid hemorrhage as a clinical predictor and phenotype for genetic association study. Int. J. Stroke 8(8), 620–625. https://doi.org/10.1111/j.1747-4949.2012.00823.x (2013).
Macdonald, R. L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 10(1), 44–58. https://doi.org/10.1038/nrneurol.2013.246 (2014).
Purkayastha, S. & Sorond, F. Transcranial Doppler ultrasound: Technique and application. Semin. Neurol. 32(4), 411–420. https://doi.org/10.1055/s-0032-1331812 (2012).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37(8), 852–857. https://doi.org/10.1038/s41587-019-0209-9 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13(7), 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6(1), 90. https://doi.org/10.1186/s40168-018-0470-z (2018).
Fernandes, A. D. et al. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2, 15. https://doi.org/10.1186/2049-2618-2-15 (2014).
Nearing, J. T. et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 13(1), 342. https://doi.org/10.1038/s41467-022-28034-z (2022).
Funding
This study is partly funded by National Science Center, Poland. Grant number: 2021/41/N/NZ2/00844 to TK as a principal investigator and LS as a supervisor and internal funds of Pomeranian Medical University in Szczecin.
Author information
Authors and Affiliations
Contributions
T.K., K.S.Ż and I.Ł. wrote the main manuscript. T.K. and K.S.Ż. prepared Figs. 1 and 5. M.K. prepared Figs. 2, 3 and 4. M.S., W.P., N.A. and C.O. provided expertise on cerebral vasospasm diagnosis. L.S. provided neurosurgical expertise. T.K. collected stool samples. T.K. performed transcranial Doppler examination. M.S. validated transcranial Doppler examination. A.W.W., I.Ł., K.S.Ż. and M.K. provided resources for 16S rRNA sequencing. J.P. assisted with sample collection and handling. M.K. performed biostatistical analysis. T.K. prepared all tables. K.S.Ż., M.K., S.P. and D.T. provided expertise on metagenomics. T.K. and L.S. acquired national funding. E.S. and L.S. supervised the entire project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Klepinowski, T., Skonieczna-Żydecka, K., Łoniewski, I. et al. A prospective pilot study of gut microbiome in cerebral vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Sci Rep 14, 17617 (2024). https://doi.org/10.1038/s41598-024-68722-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68722-y
- Springer Nature Limited