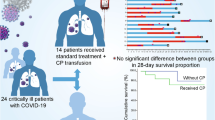
Abstract
The evidence supporting additional hemoperfusion (HP) with cytokine adsorbents for improving clinical outcomes in severe to critical coronavirus disease 2019 (COVID-19) patients remains limited. We compared severe to critical COVID-19 patients who received additional HP with a cytokine adsorbent to matched cases receiving standard medical treatment (SMT). The primary outcome was hospital mortality. In our study, we matched 45 patients who received additional HP 1:1 with the SMT group based on key clinical parameters. The hospital mortality rates did not differ between the groups (33% vs 38%, p = 0.83). The HP group had a significantly shorter ICU stay (22 vs 32 days; p = 0.017) and reduced mechanical ventilation duration (15 vs 35 days; p < 0.001). Additionally, the incidence of pulmonary complications (20% vs 42%; p = 0.04), sepsis (38% vs 64%; p = 0.02), and disseminated intravascular coagulopathy (DIC) (13% vs 33%; p = 0.046) were significantly lower in the HP group. In conclusion, among severe to critical COVID-19 patients, additional HP with a cytokine adsorbent did not improve hospital mortality. However, it reduced ICU length of stay, mechanical ventilator days, and incidences of lung complications, sepsis, and DIC.
Trial registration: TCTR20231002006. Registered 02 October 2023 (retrospectively registered).
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) not only predominantly causes respiratory tract infections, but also induces a wide range of clinical manifestations, in which the systemic inflammatory response plays an important role in the pathophysiology of the severity of the disease1,2. Additionally, an exaggerated inflammatory response identified by high levels of pro-inflammatory cytokines (i.e., interleukin (IL)-2, IL-7, and tumor necrosis factor alpha) called 'cytokine storm' was correlated with worse outcomes3,4,5,6.
The use of corticosteroids concomitantly with immunomodulatory agents, such as IL-6 receptor antagonists (e.g., tocilizumab) has shown an improvement in outcomes for patients with severe to critical COVID-197. However, immunomodulatory drugs are relatively contraindicated in pregnancy and in patients with active infection8,9,10. Moreover, such treatments are relatively expensive and were in short supply during the pandemic. According to numerous signaling factors involved in the inflammatory response, the results of treatment with a specific cytokine targeted became inconclusive8,11,12. However, hemoperfusion (HP) with a cytokine adsorbent is an extracorporeal blood purification modality that has the ability to remove various cytokines13,14. This treatment was approved by the United States Food and Drug Administration (US FDA) to treat patients with COVID-19 admitted to the intensive care unit (ICU) with confirmed or imminent respiratory failure15. Although studies in patients with severe COVID-19 reported that HP was associated with a decrease in cytokine levels, a decrease in severity of acute respiratory distress syndrome (ARDS), a lower rate of intubation, an improvement in oxygen saturation, and an increase in hospital discharge rates16,17, the published data and safety outcomes were still limited and unclear.
Consequently, we conducted the study to examine the efficacy of additional HP with a cytokine adsorbent alongside standard medical treatment (SMT) in patients with severe to critical COVID-19.
Material and methods
Study design
This is a retrospective observational study conducted in a single tertiary center to compare the efficacy of add-on HP with a cytokine adsorbent with SMT in cases with COVID-19 infection admitted to the ICU of Siriraj Hospital, Bangkok, Thailand, between January 1, 2020, and December 31, 2021. The study was approved by the Siriraj Hospital Medical Research Ethics Committee, Mahidol University, Thailand (approval number Si-355/2020).
Patients
Adult patients > 18 years of age who had confirmed SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction (PCR) tests and were classified as having severe to critical COVID-19 disease according to the World Health Organization (WHO) classification18, were enrolled in the study. HP with cytokine adsorbent was authorized in the local treatment guideline of COVID-19 at Siriraj Hospital19. This strategy was recommended to use in critical cases defined by worsening of oxygenation or chest radiograph findings, elevated blood levels of IL-6 or C-reactive protein (CRP), contraindications or unresponsiveness to immunomodulatory drugs, or when immunomodulatory drugs were not available, as shown in Fig. 1. Patients were excluded from the study if they had (a) comorbidities associated with a life expectancy of less than 6 months, (b) uncontrolled bleeding, or (c) moribund conditions with an expected imminent death within 24 h.
Study procedure
Patient data was obtained from an electronic medical record. Patient data during hospital admission including baseline demographic data, comorbidities, history of COVID-19 vaccination, day of onset of symptoms, severity score including Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, laboratory investigations and chest radiographs (CXR). Medical treatments (e.g., antiviral medication, type and dose of corticosteroids, IL-6 inhibitors), and the need for organ support devices such as invasive mechanical ventilation (IMV), renal replacement therapy (RRT), and extracorporeal membrane oxygenation therapy (ECMO) were reviewed. In our hospital, we considered an immunomodulatory drug (e.g., Tocilizumab 4–8 mg/kg/dose (maximum of 400 mg)) or performed 3-h session of HP with a cytokine adsorbent (HA330 cartridge, Jafron Biomedical Co., China) as an adjunctive therapy in patients with severe to critical COVID-1919. Hemoperfusion with a cytokine adsorptive cartridge was performed alone and the blood flow rate was set at 150–200 mL/min. All methods were carried out in accordance with relevant guidelines and regulations.
Outcomes
The primary outcome of the study was hospital mortality. Secondary outcomes included 14-day, 28-day, and 90-day mortality; ICU mortality; ICU and hospital length of stay; and ventilator days. We also reported complications, including sepsis and hospital-acquired infections, disseminated intravascular coagulation (DIC), and pulmonary complications such as pneumomediastinum, pneumothorax, and pleural effusion.
Statistical analysis
Categorical variables were presented as numbers and percentages and continuous variables were presented with mean ± standard deviation (SD) or median and Interquartile range (IQR) as appropriate. Student's t test and Mann–Whitney U test were used to compare non-normal continuous variables. The chi square test was used to compare the frequency of categorical variables. We considered potential confounders of the variables of the baseline characteristics variables, including the SOFA score, ARDS, and the need for IMV, which were relevant to the intervention and outcomes in the propensity score model. Next, the matching algorithm using the 'nearest neighbor matching' with the R software package 'matchIt' was used with a 1:1 ratio to match the SMT group to the intervention group. A standard mean difference (SMD) less than 0.1 was considered balanced in these covariates20. A p-value < 0.05 was will be considered as evidence of statistical significance. Statistical analyzes were performed using R software version 4.0.2 (R Foundation, Vienna, Austria) and a p-value of 0.05 was considered statistically significan.
Ethics approval and consent to participate
The study was approved by the Siriraj Hospital Medical Research Ethics Committee, Mahidol University, Thailand (approval number Si-355/2020). Written informed consent was obtained from the patient for publication of the details of their medical case (this consent may be obtained retrospectively).
Results
Patients
From January 2020 to December 2021, a total of 500 patients were screened for eligibility, and 272 (54%) were identified as severe to critical COVID-19 disease. Among all recruited patients (n = 272), 45 patients received HP in addition to a cytokine adsorbent and 227 patients received SMT treatment. Subsequently, propensity score matching was applied, matching 45 patients from the SMT group with a 1:1 ratio to the control group, as shown in Fig. 2. The baseline characteristics and demographic data of the overall cohort are shown in Table 1. In the overall cohort, there were no differences in age, sex, weight, body mass index (BMI), vasopressor dose, and treatment between the two groups. The HP group had a higher SOFA score at baseline (3 vs. 2; p = 0.035), a higher percentage of immunosuppressive drugs used (16% vs. 4%; p = 0.013), a higher number of ARDS (80% vs. 48%; p < 0.001), a higher IMV requirement (69% vs. 51%; p = 0.038) and a higher level of CRP (97 mg/L vs. 52 mg/L; p < 0.001) compared to the SMT group. Four patients (9%) in the HP group and six patients (3%) in the SMT group were pregnant. After propensity score matching, 45 patients from the SMT group were matched with a 1:1 ratio to the control group using the SOFA score, ARDS, and the need for IMV and all variables were balanced after matching with the mean difference between the groups was < 0.1 as shown in Fig. 3. Of the 45 patients in the HP group, 49% received three HP sessions.
Standard Mean Difference before and after matching. The figure showed the matching strategy which hollow circles represented all patients while black circles represented the matched patients using covariates including sequential organ failure assessment score (SOFA), acute respiratory distress syndrome (ARDS) and use invasive mechanical ventilation (IMV). After balancing with those variables, the mean standard difference value was less than 0.1.
Outcomes
Primary outcome
Hospital mortality was not different between HP addition with a cytokine adsorbent group and controls matched treated with SMT (33% vs 38%, respectively; p = 0.826) (Table 2).
Secondary outcomes
There was no difference in mortality from the ICU, mortality on days 14, 28, and 90 between the two groups (Table 2). Compared to the SMT group, the HP group was significantly associated with a shorter hospital LOS (22 days vs 32 days; p = 0.017). Additionally, among survivors, the HP group still had a shorter hospital LOS [22 days vs. 30 days; Mean Difference (MD), -16.96; 95% confidence interval (CI), -33.06 to -0.86]. The duration of IMV was also shorter in the HP group compared to the SMT group (15 vs. 35 days; p < 0.001), and these analyzes similarly found the shorter duration of IMV in patients who survived to hospital discharge in the HP group compared to the matched SMT group (10 vs 42 days; MD, -33.92; 95% CI, -52.39 to -15.44).
After completion of HP with a cytokine adsorbent, there was no significant change in the SOFA score and the PF ratio. However, the change in CRP was significantly higher in the HP group (80.4 mg/L vs. 36.18 mg/L; p = 0.047) (Table 2).
Complications
Pulmonary complications were significantly lower in the HP group compared to the matched SMT group (20% vs. 42%; p = 0.04). Furthermore, HP with a cytokine adsorbent was associated with a significantly lower risk of sepsis (38% in HP vs. 64%; p = 0.02) and disseminated intravascular coagulation (DIC) (13% vs. 33%; p = 0.046) (Table 2).
Discussion
Key findings
In this single-center cohort study of patients with severe to critical COVID-19 who underwent HP addition with a cytokine adsorbent found that there were no statistically different hospital mortality nor ICU mortality, mortality on day 14, 28 and 90 between the intervention group and the matched control group. However, this study indicated that additional HP was independently associated with a lower risk of hospital LOS and the duration of IMV. Furthermore, the study showed that HP has a significantly lower risk of lung complications, sepsis, and DIC.
Relationship with previous studies
Extracorporeal blood purification in COVID-19 is adjunctive treatment in severe to critical patients who developed multiple organ failures according to the hyperinflammatory response14,21,22. Several cytokines have played an important role in this pathogenesis and could be removed with the adsorptive technique23,24. Cytokine Adsorption in Patients with Severe COVID-19 Pneumonia Requiring Extracorporeal Membrane Oxygenation (CYCOV) study showed that HP use with a cytokine adsorbent (Cytosorp) in severe COVID-19 cases requiring veno-venous extracorporeal membrane oxygenation (VV-ECMO) was associated with an increase in mortality at 30 days compared to the control group (76% vs. 18%, p = 0.0075)25. On the other hand, Surasit et al.26 reported that early initiation of HP was associated with improved hospital mortality (13% vs 93%; p < 0.001). These findings suggested that delay in HP initiation when patients had already developed severe ARDS and multiorgan failure (MOF) and required ECMO as rescue therapy was associated with a worse outcome. Thus, the appropriate time for HP initiation is a critical question. In the present study, the time from DOS to the first HP and the level of CRP were comparable to the previous study which indicated that our patients were in the hyperinflammatory state26,27. Our study did not show a relationship between adjunctive HP and an improvement in mortality as previous studies, which may be due to underpower of study to detect the difference26,27,28. Furthermore, patients in our study had a higher severity of the disease identified by a lower PF ratio and a higher rate of hospital mortality in the control group compared to previous studies26,27.
However, our study showed that the median length of hospital stays and the duration of IMV were significantly shorter in the HP group. These findings were in line with those previously reported29,30 and is explained by the substantial reduction in cytokine levels in the intervention group31,32. Progressive lung injury in COVID-19 is caused not only by hyperinflammation but also by ventilator-induced lung injury (VILI). Moreover, excessive respiratory effort creates large transpulmonary pressure swings, known as patient self-inflicted lung injury (P-SILI), which exacerbate lung injury in COVID-19 patients29,30. We hypothesize that hemoperfusion decreases pulmonary complications by reducing inflammation, as evidenced by the greater decrease in CRP levels (Table 2), which could reduce the respiratory drive of the patients and VILI. Additionally, clinicians likely adjusted ventilator settings to minimize VILI and weaned patients off mechanical ventilation faster than the standard medical treatment group. This relationship may be elucidated that the persistent inflammation in SMT group is compatible with “secondary hit” model in sepsis leading to multiple organs failure, adverse outcomes and hospital complications31. Then this intervention led to a reduction in the risk of pulmonary complication, sepsis, and DIC. A multicenter retrospective cohort study using large-scale registry data from Japan reported that the incidence of DIC was 1.1% on admission, increasing to 10.9% by day 15. Therefore, we hypothesize that the higher proportion of DIC in the SMT group is likely a complication of sepsis32. The potential benefits of hemoperfusion, as mentioned above, are particularly important during the pandemic, especially the overwhelming number of cases of COVID-19, when ICU beds, mechanical ventilator capacity, and the ability of healthcare workers are limited. However, these benefits should be outweighed by the drawbacks of this intervention, including catheter-related complications, drug adsorption, and nutrient loss33,34,35.
In this cohort, vaccination did not influence the outcome. There are several possible explanations for this. First, the characteristics of our patients were more severe COVID-19 compared to previous studies26,27. Because the risk of COVID-19 hospitalization and death depend on severity and specific underlying health conditions36. Second, the total number of patients receiving vaccination was relatively low (Table 1) (4% vs. 20% in the HP and SMT groups, respectively) and third, during this period, the available vaccine was Sinovac, an inactivated form of the COVID-19 virus. Although the Sinovac vaccine was validated by the World Health Organization (WHO) for emergency use and has shown efficacy in reducing hospitalization and ICU admission when fully vaccinated37, its efficacy compared to mRNA vaccines indicated that patients receiving Sinovac were more likely to be infected and to develop severe COVID-19 disease38.
Clinical implications
Our findings implied that additional use of hemoperfusion with HA330 cartridge in patients with severe to critical COVID-19 with hyperinflammatory state could improve patient outcomes, which is particularly relevant in the resource limitation period during the COVID-19 pandemic (e.g. A shorter hospital stay and mechanical ventilation). Furthermore, this study implied that this intervention is relatively safe and could potentially decrease complications related to severe to critical cases of COVID-19. Finally, it also implied that this technique could be considered in other diseases associated with cytokine release syndrome.
Strengths and limitations
The present study was performed in a large university-affiliated referral hospital. Since January 2020, we have been actively involved in the management of critically ill COVID-19 patients39. Therefore, we had a relatively large sample size of patients receiving hemoperfusion26,27,28,40. We also reported a well-defined matching process based on baseline characteristics and disease severity.
We acknowledge several limitations for this study. First, this is a single-center study conducted in a tertiary hospital and had a relatively large sample size. However, the findings have limited the generalizability to other ICUs and were not able to detect the primary outcome. Second, this is a retrospective observational study. Therefore, it inevitably has some residual unmeasured confounders. However, propensity score matching was used to reduce unbalanced variables between groups. Third, we cannot accurately compare the severity of the patient at the time of intervention due to the nature of the study and the incidence of sepsis or bacterial co-infection on the day of ICU admission, which likely acts as a significant confounder for both primary and secondary outcomes. However, we reported the time from DOS to HP and CRP level, which were similar to the time from DOS to immunomodulatory drugs in the landmark trial. These implied that the patients were in a hyperinflammatory state41 and hemoperfusion was performed in this phase of COVID-19. Furthermore, in Table 1, the time from DOS to ICU admission was 8 days in the HP group and 9 days in the SMT group. ARDS developed in 80% of both groups upon ICU admission. The HP group started hemoperfusion only 2 days after ICU admission. Thus, we believe that the severity scores from ICU admission to the day of starting hemoperfusion were not significantly different. Fourth, we did not report the COVID-19 genotype. Because disease severity in COVID-19 genotypes has different clinical outcomes. However, the most common genotype in that period was the Delta variant42. Fifth, we performed hemoperfusion using only a cytokine adsorbent (HA330 cartridge, Jafron Biomedical Co., China) and performed approximately 3 sessions per patient. Thus, these results probably have not been referred to other hemoperfusion cartridges or other techniques of blood purification on COVID-19. However, the recommendations for extracorporeal blood purification in patients with COVID-19 are still unclear43. Sixth, the disadvantages of this intervention were not reported, including catheter-related complications, drug adsorption, and nutrients. However, we showed the overall complications related to COVID-19 management in the ICU.
Conclusion
Among severe to critical COVID-19 patients, the use of add-on HP with a cytokine adsorbent did not reduce hospital mortality. However, this intervention was associated with a lower risk of prolonged hospital stay, mechanical ventilation duration, pulmonary complications, sepsis, and DIC.
Data availability
All the data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- APACHE II:
-
Acute physiology and chronic health evaluation II
- ARDS:
-
Acute respiratory distress syndrome
- BID:
-
Bis in die
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CAPA:
-
COVID-19 associated pulmonary aspergillosis
- CI:
-
Confidence Interval
- CKD:
-
Chronic kidney disease
- CRBSI:
-
Catheter-related bloodstream infections
- CXR:
-
Chest X-rays
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- DIC:
-
Disseminated intravascular coagulation
- DNR:
-
Do-not-resuscitate
- DOS:
-
Day onset of symptom
- ECMO:
-
Extracorporeal membrane oxygenation therapy
- H:
-
Hour
- HAP:
-
Hospital acquired pneumonia
- HFNC:
-
High-flow nasal cannula
- HP:
-
Hemoperfusion
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- IMV:
-
Invasive mechanical ventilation
- IQR:
-
Interquartile
- IV:
-
Intravenous
- LOS:
-
Length of stay
- MD:
-
Mean Difference
- MOF:
-
Multiorgan failure
- NE:
-
Norepinephrine
- OD:
-
Once daily
- PCR:
-
Polymerase chain reaction
- PF:
-
PaO2/FiO2 ratio
- PO:
-
Per os
- RA :
-
Room air
- RRT:
-
Renal replacement therapy
- SMT:
-
Standard medical treatment
- SOFA:
-
Sequential organ failure assessment
- SpO2:
-
Saturation of peripheral oxygen
- UTI:
-
Urinary tract infection
- VV-ECMO:
-
Veno-venous extracorporeal membrane oxygenation
- WHO:
-
World Health Organization
References
Su, S. et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 24(6), 490–502 (2016).
Channappanavar, R. & Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 39(5), 529–539 (2017).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 395(10223), 497–506 (2020).
McElvaney, O. J. et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 202(6), 812–821 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 395(10229), 1054–1062 (2020).
Ruan, Q., Yang, K., Wang, W., Jiang, L. & Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Med. 46(5), 846–848 (2020).
Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda (MD): National Institutes of Health (US); 2021.
Bhimraj A MR, Shumaker AH, Baden L, Cheng VC, Edwards KM, Gallagher JC, Gandhi RT, Muller WJ, Nakamura MM, O'Horo JC, Shafer RW, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19: Infectious Diseases Society of America; 2023 [updated 6/26/2023. Version 11.0.0:[Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management.
Jorgensen, S. C. J. & Lapinsky, S. E. Tocilizumab for coronavirus disease 2019 in pregnancy and lactation: a narrative review. Clin. Microbiol. Infect. 28(1), 51–57 (2022).
NationalDrugInformation. Olumiant, INN-baricitinib2019 [cited 2023 22 Apr ]. Available from: https://ndi.fda.moph.go.th/uploads/drug_detail_corporation/doc/word/1163/83bc7196420de5d56f8a01b49109c168-a1.pdf.
Dahms, K. et al. Anakinra for the treatment of COVID-19 patients: a systematic review and meta-analysis. Eur. J. Med. Res. 28(1), 100 (2023).
Elmekaty, E. Z. I. et al. Evaluation of anakinra in the management of patients with COVID-19 infection: A randomized clinical trial. Front. Microbiol. https://doi.org/10.3389/fmicb.2023.1098703 (2023).
Ricci, Z., Romagnoli, S., Reis, T., Bellomo, R. & Ronco, C. Hemoperfusion in the intensive care unit. Intensive Care Med. 48(10), 1397–1408 (2022).
Nalesso, F. et al. The supporting role of combined and sequential extracorporeal blood purification therapies in COVID-19 patients in intensive care unit. Biomedicines. 10(8), 2017 (2022).
FoodandDrugAdministration. Emergency use authorization approval documents: Food and Drug Administration; 2020 [Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidothermeddev.
Esmaeili Vardanjani, A. et al. Early hemoperfusion for cytokine removal may contribute to prevention of intubation in patients infected with COVID-19. Blood Purif. 50(2), 257–260 (2021).
Asgharpour, M. et al. Effectiveness of extracorporeal blood purification (hemoadsorption) in patients with severe coronavirus disease 2019 (COVID-19). BMC Nephrol. 21(1), 356 (2020).
WHO Guidelines Approved by the Guidelines Review Committee. Clinical management of COVID-19: Living guideline. Geneva: World Health Organization © World Health Organization 2021.; 2022.
Ratanarat, R., Sivakorn, C., Viarasilpa, T. & Schultz, M. J. Critical care management of patients with COVID-19: Early experience in Thailand. Am J Trop Med Hyg. 103(1), 48–54 (2020).
Chan G, StatsNotebook T. Propensity Score Matching - StatsNotebook - Simple. Powerful. Reproducible: StatsNotebook; 2020 [updated 12 Apr 2022. Available from: https://statsnotebook.io/blog/analysis/matching/.
Akil, A., Ziegeler, S., Rehers, S., Ernst, E. C. & Fischer, S. Blood purification therapy in patients with severe COVID-19 requiring veno-venous ECMO therapy: A retrospective study. Int. J. Artif. Organs. 45(7), 615–622 (2022).
Ronco, C. et al. Extracorporeal blood purification and organ support in the critically Ill patient during COVID-19 pandemic: Expert review and recommendation. Blood Purif. 50(1), 17–27 (2021).
Jonny, J. & Violetta, L. Coupled plasma filtration adsorption as a potential therapy for critically III Covid-19 patients. J. Med. Chem. Sci. 5(2), 197–203 (2022).
AL Shareef, K. & Bakouri, M. Cytokine blood filtration responses in COVID-19. Blood Purif. 50(2), 141–149 (2020).
Supady, A. et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. The Lancet Respiratory Medicine. 9(7), 755–762 (2021).
Surasit, K. & Srisawat, N. The efficacy of early additional hemoperfusion therapy for severe COVID-19 patients: A prospective cohort study. Blood Purif. 51(11), 879–888 (2022).
Alavi Darazam, I. et al. Efficacy of Hemoperfusion in Severe and Critical Cases of COVID-19. Blood Purif. 52(1), 8–16 (2023).
Mikaeili, H. et al. The early start of hemoperfusion decreases the mortality rate among severe COVID-19 patients: A preliminary study. Hemodial Int. 26(2), 176–182 (2022).
Goligher, E. C. et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med. 46(12), 2314–2326 (2020).
Brochard, L., Slutsky, A. & Pesenti, A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 195(4), 438–442 (2017).
Venet, F. & Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nature Rev. Nephrol. 14(2), 121–137 (2018).
Gando, S. & Akiyama, T. Disseminated intravascular coagulation is associated with poor prognosis in patients with COVID-19. Sci. Rep. 14(1), 12443 (2024).
Ostermann, M. & Koyner, J. L. Extracorporeal blood purification is appropriate in critically Ill patients with COVID-19 and multiorgan failure: Commentary. Kidney. 3(3), 423–425 (2022).
Ankawi, G. et al. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Critical Care. 22(1), 262 (2018).
Kashani, K. & Forni, L. G. Extracorporeal blood purification is appropriate in critically Ill patients with COVID-19 and multiorgan failure: CON. Kidney 3(3), 419–422 (2022).
Agrawal, U. et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: Pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. The Lancet. 400(10360), 1305–1320 (2022).
Jara, A. et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 385(10), 875–884 (2021).
Premikha, M. et al. Comparative effectiveness of mRNA and inactivated whole-virus vaccines against coronavirus disease 2019 infection and severe disease in Singapore. Clin. Infect. Dis. 75(8), 1442–1445 (2022).
Naorungroj, T. et al. Characteristics, outcomes, and risk factors for in-hospital mortality of COVID-19 patients: A retrospective study in Thailand. Front Med (Lausanne). 9, 1061955 (2022).
Soleimani, A., Taba, S. M. M., Hasibi Taheri, S., Loghman, A. H. & Shayestehpour, M. The effect of hemoperfusion on the outcome, clinical and laboratory findings of patients with severe COVID-19: A retrospective study. New Microbes New Infect. 44, 100937 (2021).
Rosas, I. O. et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. New Engl. J. Med. 384(16), 1503–1516 (2021).
CentersforDiseaseControlandPrevention. SARS-CoV-2 Variant Classifications and Definitions: Centers for Disease Control and Prevention; 2023 [updated 20 Mar 2023. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html.
Ronco, C. & Reis, T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 16(6), 308–310 (2020).
Author information
Authors and Affiliations
Contributions
Research idea and study design: RR, SC; data acquisition: SC, NT, ST; data analysis/ interpretation: all authors; statistical analysis: SC, TN; supervision or mentorship: RR, TN. Each author contributed important intellectual content during manuscript drafting or revision, and accepts responsibility for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chiewroongroj, S., Ratanarat, R., Naorungroj, T. et al. Efficacy of additional hemoperfusion in hospitalized patients with severe to critical COVID-19 disease. Sci Rep 14, 17651 (2024). https://doi.org/10.1038/s41598-024-68592-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68592-4
- Springer Nature Limited