Abstract
Recent reports have shown that pre-treatment low muscle mass may lead to poorer outcomes for cancer patients. We explored the correlation between Visceral Adipose Tissue (VAT), Subcutaneous Adipose Tissue (SAT), and Muscle Mass (MM) as measured by CT scans, and overall survival (OS) following diagnosis of colorectal cancer (CRC). We conducted a retrospective review of medical records and CT scans of patients diagnosed with CRC between 2007 and 2018. Demographics, pathology, and clinical parameters were collected. Using Image-J software, we measured VAT, SAT, and MM. Survival rates were analyzed using Kaplan–Meier curves, and prognostic factors were assessed using multivariate Cox regression. Analysis included 408 patients with a mean age of 56.9 years and a median follow-up of 93.3 months. Colon and rectum/rectosigmoid colon cancers were equally distributed. The 5-year OS rate was 67.8%. There was no significant difference in OS rates based on SAT or VAT. However, higher MM was associated with a improved 5-year OS rate. Factors such as age, stage, grade, and surgery were also associated to OS rates. These findings suggest that higher muscle mass may lead to better outcomes for CRC patients, highlighting the potential impact of exercise and nutritional interventions on patient outcomes.
Similar content being viewed by others
Introduction
Incidence of CRC: Jordan vs. worldwide
Colorectal cancer (CRC) ranks as the third most common type of cancer worldwide, accounting for 10.0% of cases according to the latest GLOBCAN statistics1. Mortality rates are equal between male and female patients, with both genders accounting for 9.3% and 9.4% of recorded deaths, respectively.
According to the Global Burden of Disease (GBD), the incidence of CRC is higher in developed countries. However, it is steadily increasing in low- and middle-income countries, posing growing financial and health challenges in maintaining and optimizing quality health services2. The latest report from the Jordan Cancer Registry of 2019 revealed that CRC is the second most prevalent type of cancer in Jordan, accounting for 11.6% of cancer diagnoses, surpassed only by breast cancer (20.3%). Interestingly, CRC appears to affect males more than females, with 13.7% of male cancer cases being CRC compared to 9.7% for females.3. In terms of mortality, CRC stands as the second leading cause of cancer-related death in males at 11%, trailing behind lung cancer. Among females, CRC ranks third in terms of mortality at 10.4%, following breast cancer and leukemia2.
Risk factors and BMI
Several risk factors have been associated with a higher likelihood of developing CRC, including obesity, physical inactivity, smoking, unhealthy lifestyle, and genetic factors among others4,5,6,7.
Body mass index (BMI) is a commonly used measure of body fat calculated based on a person's weight and height (kg/m2)8. Although BMI is widely used to measure obesity9, it is not an accurate indicator of body fat as it does not account for fat distribution or the weight of bones and muscles10. Two types of fat have been linked with obesity; visceral fat and subcutaneous fat, both of which cannot be measured using BMI. Studies have shown that visceral fat, which is the metabolically active form of fat, contributes to the secretion of proinflammatory cytokines and adipokines (tumor necrosis factor and interleukin-6) which induce the high risk of CRC carcinogenesis11,12. On the other hand, subcutaneous fat is linked to favourable outcome in CRC patients13 and can be used as positive metabolic profile for glucose and lipid levels.
Unfortunately, BMI cannot distinguish between increased visceral fat, subcutaneous fat, or muscle mass14, making it an inadequate tool for assessing cancer risk. While BMI is commonly used in medical practice to assess patient obesity and overall risk, its limitations in accurately assessing body composition make it an imperfect tool for predicting cancer risk.
CRC and muscle mass
Several studies have demonstrated the importance of muscle mass in predicting survival rates and outcomes for CRC patients15,16,17. A recent comprehensive review15 showed that the frequency of sarcopenia (low muscle mass) in CRC patients ranges between 12 and 60%.
Factors associated with sarcopenia can be either patient-related, such as physical inactivity, malnutrition, and body composition, or cancer-related, including weight loss and muscle mass deterioration resulting from treatment18. Sarcopenia has also been used as a biomarker to predict chemotherapy tolerance and toxicity in CRC patients18 and several studies have used it to predict surgical complications, reduced survival, and poor quality of life in CRC patients19,20.
Patients with low muscle mass are best identified by computed tomography (CT), as it is considered the gold standard method to measure the mass and quality of muscles in addition to other body composition factors21.
In addition to low muscle mass, elevated levels of fat distribution, particularly abdominal visceral fat, represent significant risk factors in CRC. This is primarily attributed to visceral fat's capability to promote the abdominal tumorigenic environment, thereby increasing the likelihood risk of CRC development through various mechanisms, including enhanced cancer cell proliferation, angiogenesis, and the induction of a protumorigenic microenvironment12. Furthermore, visceral fat contributes to systemic chronic inflammation by releasing proinflammatory cytokines and tumour necrosis factor-alpha22.
Aim
The objective of this study was to explore how Visceral Adipose Tissue (VAT), Subcutaneous Adipose Tissue (SAT), and Muscle Mass (MM) correlate with overall survival (OS) in colorectal cancer (CRC) patients treated at King Hussein Cancer Center (KHCC), a leading comprehensive cancer center in Jordan. Utilizing CT scans, we measured muscle mass, visceral fat, and subcutaneous fat, and investigated its relationship with demographic variables (such as age and gender) and clinical indicators (including cancer stage, grade, and primary treatment) to patient survival.
Materials and methods
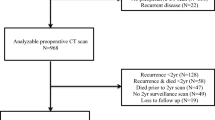
We screened a total of 2280 patients with CRC who visited KHCC between 2007 and 2018. Inclusion criteria required patients to have a confirmed diagnosis of CRC regardless of the stage, and an available CT scan at the lower edge of L3 vertebral level before treatment initiation. Patients who had CT scans after treatment interventions, types of cancer other than CRC, CT scans at different levels than L3, or low-quality CT scans were excluded from the study. Patients with missing BMI measures at diagnosis were also excluded. The slice thickness of the CT scan used for eligible patients was 3 mm, which is sufficient to provide higher resolution images with more details.
Image-J software (version 1.52a), which is a Java-based image processing program, developed by the U.S. National Institutes of Health (NIH) and available for free in the public domain (https://imagej.nih.gov/ij/), was used to measure the visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and muscle mass (MM) in square centimeters (cm2) (Fig. 1). The instructions on how to use ImageJ were followed as mentioned in the instruction manual23. Three physicians were trained to measure VAT, SAT, and MM from the CT scans using the same ImageJ version and measuring technique. The threshold values applied to measure the CT scans were determined through visual inspection and experimentation with different thresholds until optimal separation of VAT, SAT, and MM was achieved.
CT scans for CRC patients at the lower edge of L3 level, at different thresholds using ImageJ. (A) CT scan of CRC patient at lower edge of L3 level. (B) The area in red is the fat (visceral and subcutaneous) after setting the threshold between (10–90). The visceral fat was selected and measured at the indicated threshold. (C) Visceral fat was cut out, leaving subcutaneous fat alone to be measured at the same threshold (10–90). (D) The area in red is the muscle area after setting the threshold between (90–170). The area was selected and measured at the indicated threshold.
Demographic, pathological, and clinical parameters were collected for the patients. Additionally, height and weight at diagnosis were collected to calculate BMI. To evaluate the agreement and reproducibility among the measurements obtained by the three physicians, 78 CT scans were independently measured three times by different physicians. Subsequently, the intraclass correlation coefficient (ICC) was calculated to evaluate the consistency among the measurements obtained by the three physicians.
Receiver Operating Characteristic (ROC) curves were generated to determine cutoff points for VAT, SAT, and MM based on gender. Survival rates were estimated using the Kaplan–Meier method and compared between groups using the Log-rank test. Multivariate Cox regression was used to assess prognostic factors. A significance criterion of P ≤ 0.05 was used in the analysis, and all analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
The data collected in this study adhered to HIPAA-compliant standards and was ethically approved by the Institutional Review Board (IRB) at King Hussein Cancer Center (study number: 17 KHCC 99). Informed consent was waived due to retrospective nature of study design.
Results
Agreement and reproducibility
The results of the two-way mixed effects, absolute agreement ICC for VAT, SAT, and MM were 0.88, 0.76, and 0.75, respectively, with a 95% confidence interval (CI) (Table 1). ICC values exceeding 0.70 indicate good reliability24. These findings suggest that the measurements obtained by the three physicians were consistent and reproducible.
Descriptive analysis
Of 2280 screened CRC patients, 408 patients diagnosed at KHCC between 2007 and 2018 met our inclusion criteria and were included in the analysis.
The mean age at diagnosis was 56.9 ± 13.2 (± SD) years, median follow-up was 93.3 months (range: 40.9–182), and 233 (57.1%) were male patients. Of these patients, 216 (52.9%) had colon cancer and 192 (47.1%) had rectum or rectosigmoid cancer. A total of 350 (85.8%) patients’ cancers were grade 1 or 2. Of the measured CT scans, 210 (51.5%) patients had high VAT, 188 (46.1%) had high SAT, and 228 (55.9%) had high MM. Table 2 shows the descriptive analysis of the demographic of CRC in the sample cohort.
Table 3 summarizes the cut-off points for the area under the curve (AUC) for MM, VAT and SAT, stratified according to gender. The ROC curves are for MM, VAT and SAT for male and females are represented in the supplementary materials (Figs. S1–S6).
Survival analysis
At the follow-up time in March 2022, 145 patients (35.4%) of the patients had died. The median survival of the entire cohort was 96.3 months (95% CI 63.1, 72.2) and the 5-year OS rate was 67.8% (Fig. 2).
Patients who had surgery had a higher survival rate compared with those who did not have surgery (80.7% vs 33.6% P < 0.0001). Tumors of grades 1 and 2 were compared with tumors of grades 3 and 4 and showed a significantly better survival rate (71.0% vs. 46.7% P = 0.0015). Additionally, significant differences in survival rates between age groups and TNM stages were observed (P = 0.0014 and P < 0.0001 respectively) (Table 4).
High MM was associated with a better 5-year overall survival rate in CRC patients (71.8% vs. 62.7%, P = 0.0224) (Fig. 3). In contrast, SAT and VAT were not associated with OS when comparing high to low values (67.9% vs 67.6% P = 0.74), (67.9% vs 67.6%, P = 0.6351), respectively. Moreover, there was no association between BMI and cancer site groups and OS (P = 0.6085, P = 0.5062, and P = 0.5793 respectively) (Table 4).
Using a multivariate Cox regression adjusting for age, stage, grade, surgery, VAT, SAT, and MM, no significant association was observed for VAT, SAT, or grade with a five-year OS rate (P = 0.474, 0.863, 0.101 respectively). However, there was an association between age, stage, surgery, and MM with a five-year OS rate (P = 0.016, < 0.0001, 0.0001, 0.040 respectively) (Table 5).
Discussion
In this study, we investigated the relationship between Visceral Adipose Tissue (VAT), Subcutaneous Adipose Tissue (SAT), and Muscle Mass (MM) measured using CT scans with OS in CRC patients treated at KHCC in Jordan. Our findings showed a significant association between high muscle mass and improved OS rates in CRC patients, aligning with previous research emphasizing the positive impact of increased MM on survival outcomes in cancer patients25,26,27. This analysis revealed that OS in CRC patients was influenced by multiple predictive factors, including surgical intervention, tumour grade, TNM stage, and age at diagnosis, all of which demonstrated significant associations with survival outcomes28,29.
The 5-years OS rate of 67.8% (95% CI 63.1–72.2) of our population was similar to other studies conducted in Brazil 63.5%30 and a population-based study in 9 European countries 71·1% (95% CI 70·7–71·4)31 which can be attributed to similarities in the high level of quality of care provided to CRC patients. A previously published study utilizing data from the Jordan’s cancer registry during the period of 2005–2010 reported a 5-years OS of 58.2% for CRC patients32. The notable increase in survival rates between their study and our findings suggests positive progress in the level of care and advancements in treatment modalities over time.
To accurately measure MM, VAT, and SAT, our study employed a CT scan-based method33 using ImageJ software. Three independent physicians measured the images to ensure data accuracy and reliability.
A study conducted in biliary duct cancer patients showed that high MM was associated with a high survival rate (HR 0.46, 95% CI 0.22–0.95, P = 0.037)25 which is similar to our results (HR 0.997, 95% CI 0.994–1, P = 0.0397). Another meta-analysis27 on rectal cancer patients with sarcopenia showed a poor survival rate (HR 2.10, 95% CI 1.33–3.32, P = 0.001) compared to patients with high MM, which is in line with our results that high muscle mass provides survival benefit for patients with CRC.
Moreover, a higher level of muscle mass is usually linked to a favourable inflammatory profile due to the release of myokines. These myokines play a key role in the anti-inflammatory effects of physical activity, helping to counteract metabolic disturbances and the effects of adipokines34,35. In addition to the pivotal role of physical exercise on increasing the muscle mass, it positively influences metabolic pathways and energy homeostasis by enhancing aerobic capacity, insulin sensitivity, glucose control, and oxidative capacity, among other adaptations35. All these factors contribute to the survival of CRC and MM relationship, which is usually altered during cancer35.Based on our knowledge and existing literature highlighting the adverse effects of VAT in the abdominal region, we explored the association between VAT and OS in CRC patients. Interestingly, our findings indicated no significant impact of high VAT (HR 1.001, 95% CI 1.003–0.4738, P = 0.4738) on OS, similar to results from a study on Korean CRC patients (HR 0.656; 95% CI 0.402–1.071; P = 0.092)36. However, this is contrary to other studies reporting a significant association between increased VAT (HR 2.61, 95% CI 1.155–5.924, P = 0.020) and poor survival outcomes37,38.
We also investigated the association between SAT and OS in CRC patients, and our findings did not indicate a significance on OS (HR 1.000, 95% CI 0.999–1.001, P = 0.8633). Our results are similar to the above-mentioned study37, (HR 1.18, 95% CI 0.614–2.036, P = 0.715) but contrary to the study on Korean CRC patients among other studies (HR 0.505, 95% CI 0.266–0.957; P = 0.036)36,38.
This discrepancy may be attributable to the relatively small sample size and the heterogeneity of the CRC patient population in our study. Genetic factors specific to the Middle Eastern population may also contribute to this variation5,6. These include IL-17 polymorphisms which play a crucial role in inflammation, and autoimmune diseases and are responsible for CRC growth and invasion5,39. Moreover, CRC driver mutations like RAS and BRAF mutations vary in their prevalence in Middle Eastern compared to Western countries7,40. However, this data was not available for the population of patients we studied and further research is needed to study these variable and their interplay with body composition and effect on CRC outcome.
Additionally, we rigorously controlled for potential confounding factors such as age at diagnosis, gender, tumour grade, and TNM stage, enhancing the validity and reliability of our findings. Our findings in the multivariate analysis revealed that only the age at diagnosis, TNM stage group, surgery, and MM were significant independent predictors of the patient’s survival. Our results are similar to findings in other Middle Eastern populations41,42 and analyses based on the SEER database43. Tumour grade did not emerge as a significant predictor of CRC survival in multivariate analysis, which is contrary to other published research in Western populations like the US and Canada using their National Cancer Database44 and Eastern populations such as Korean CRC patients45.
It is important to18 acknowledge the limitations of our study, primarily due to its retrospective design which resulted in missing data that could contribute important insights to the question of body composition and its association with CRC outcome. This includes the absence of lifestyle data encompassing physical activity and diet, both of which have the potential to impact body composition, fat, and muscle distribution, and could be associated with OS in CRC patients46,47, in addition to their role in cancer prevention48. Unfortunately, these data were not present in our electronic medical records at the time of diagnosis nor during the patient’s treatment course.
While the integration of artificial intelligence (AI) is rapidly growing in the radiology field -making CT scan analysis of body composition faster, more accurate, and potentially more informative- the need for human oversight remains important to make medical judgement49. Our study would have benefited from using AI to quantify the measurements of SAT, VAT and MM by improving the consistency and reducing human error in measurements. However, this would require training of the AI model by introducing massive amount of medical data using advanced computing power, which is not readily available in our healthcare setting.
Future research with larger sample sizes and more diverse patient cohorts is needed to better understand the association between body composition and CR outcome. Such studies would help validate our findings and elucidate the underlying mechanisms involved, thereby informing clinical practice to improve CRC outcomes and enhance the quality of life for patients. Additionally, investigations exploring genetic factors and genetic variations across different populations may help to answer questions specific to the Middle Eastern population and provide further insights into the relationship between body composition and survival outcomes in CRC patients.
Conclusion
This study provides evidence supporting the association between high muscle mass and improved OS rates in CRC patients. Additionally, TNM stage, surgical intervention, and age at diagnosis were identified as significant independent predictors of OS. Notably, VAT and SAT did not demonstrate a significant association with OS. These findings highlight the importance of assessing body composition, especially muscle mass as a valuable prognostic indicator in CRC patients.
Furthermore, integrating routine physical activity and promoting healthy lifestyle habits in the management of CRC patients hold potential benefits. Future studies can explore the impact of exercise and nutritional interventions on body composition and OS rates, contributing to the development of comprehensive treatment strategies.
In addition, nutritional counselling can be a crucial aspect of CRC patient care. Exploring the effects of tailored nutritional interventions, including increased protein intake, on body composition and muscle mass, OS rates, and quality of life, can provide valuable insights into optimizing patient outcomes.
By addressing these areas, we can emphasize the significance of physical activity and healthy lifestyle choices, and incorporate nutritional counselling into the comprehensive care of CRC patients. These factors can have the potential to advance the field and contribute to improved survival rates, enhanced quality of life, and better overall outcomes for individuals with CRC.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author (A.A).
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Xi, Y. & Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 14(10), 101174 (2021).
Health JMo. Cancer Incidence in Jordan. Cancer Incidence in Jordan 45 (2018).
Lewandowska, A., Rudzki, G., Lewandowski, T., Stryjkowska-Góra, A. & Rudzki, S. Risk factors for the diagnosis of colorectal cancer. Cancer Control 29, 10732748211056692 (2022).
Al Obeed, O. A. et al. IL-17 and colorectal cancer risk in the Middle East: Gene polymorphisms and expression. Cancer Manag. Res. 10, 2653–2661 (2018).
Oukkal, M. et al. Middle East and North Africa registry to characterize rate of RAS testing status in newly diagnosed patients with metastatic colorectal cancer. Turk. J. Gastroenterol. 34(2), 118–127 (2023).
Garawin, T., Lowe, K., Kafatos, G. & Murray, S. The prevalence RAS and BRAF mutations among patients in the Middle East and Northern Africa with metastatic colorectal cancer. J. Clin. Oncol. 34, e15077 (2016).
Shaukat, A., Dostal, A., Menk, J. & Church, T. R. BMI is a risk factor for colorectal cancer mortality. Dig. Dis. Sci. 62(9), 2511–2517 (2017).
Simillis, C. et al. A systematic review and meta-analysis assessing the impact of body mass index on long-term survival outcomes after surgery for colorectal cancer. Eur. J. Cancer 172, 237–251 (2022).
Humphreys, S. The unethical use of BMI in contemporary general practice. Br. J. Gen. Pract. 60(578), 696–697 (2010).
Donohoe, C. L., Doyle, S. L. & Reynolds, J. V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 3, 12 (2011).
Lee, J. Y. et al. Visceral fat accumulation is associated with colorectal cancer in postmenopausal women. PLoS ONE 9(11), e110587 (2014).
Kim, J. M. et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin. Nutr. 40(11), 5631–5638 (2021).
Shuster, A., Patlas, M., Pinthus, J. H. & Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 85(1009), 1–10 (2012).
Vergara-Fernandez, O., Trejo-Avila, M. & Salgado-Nesme, N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J. Clin. Cases 8(7), 1188–1202 (2020).
van Roekel, E. H. et al. Associations of adipose and muscle tissue parameters at colorectal cancer diagnosis with long-term health-related quality of life. Qual. Life Res. 26(7), 1745–1759 (2017).
Meyer, H. J., Strobel, A., Wienke, A. & Surov, A. Prognostic role of low-skeletal muscle mass on staging computed tomography in metastasized colorectal cancer: A systematic review and meta-analysis. Clin. Colorectal Cancer 21(3), e213–e225 (2022).
Cespedes Feliciano, E. M., Avrutin, E., Caan, B. J., Boroian, A. & Mourtzakis, M. Screening for low muscularity in colorectal cancer patients: A valid, clinic-friendly approach that predicts mortality. J. Cachexia Sarcopenia Muscle 9(5), 898–908 (2018).
Tsaousi, G. et al. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur. J. Cancer Care 26(6), e12491 (2017).
Heus, C., Cakir, H., Lak, A., Doodeman, H. J. & Houdijk, A. P. J. Visceral obesity, muscle mass and outcome in rectal cancer surgery after neo-adjuvant chemo-radiation. Int. J. Surg. 29, 159–164 (2016).
Ní Bhuachalla, É.B. et al. Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: Is nutritional screening capturing hidden malnutrition? J. Cachexia Sarcopenia Muscle 9(2), 295–305 (2018).
Dusserre, E., Moulin, P. & Vidal, H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta 1500(1), 88–96 (2000).
Gomez-Perez, S. L. et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: A step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J. Parenter. Enteral Nutr. 40(3), 308–318 (2016).
Koo, T. K. & Li, M. Y. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropract. Med. 15(2), 155–163 (2016).
Limpawattana, P. et al. The impact of skeletal muscle mass on survival outcome in biliary tract cancer patients. PLoS ONE 13(10), e0204985 (2018).
Lopez, P. et al. Associations of fat and muscle mass with overall survival in men with prostate cancer: A systematic review with meta-analysis. Prostate Cancer Prostat. Dis. 25(4), 615–626 (2022).
Zhu, Y., Guo, X., Zhang, Q. & Yang, Y. Prognostic value of sarcopenia in patients with rectal cancer: A meta-analysis. PLoS ONE 17(6), e0270332 (2022).
Joachim, C. et al. Overall survival of colorectal cancer by stage at diagnosis: Data from the Martinique Cancer Registry. Medicine 98(35), e16941 (2019).
Andreoni, B. et al. Surgical outcomes for colon and rectal cancer over a decade: Results from a consecutive monocentric experience in 902 unselected patients. World J. Surg. Oncol. 5, 73 (2007).
Aguiar Junior, S. et al. Survival of patients with colorectal cancer in a cancer center. Arq. Gastroenterol. 57(2), 172–177 (2020).
Cardoso, R. et al. Overall and stage-specific survival of patients with screen-detected colorectal cancer in European countries: A population-based study in 9 countries. Lancet Reg. Health 21, 100458 (2022).
Sharkas, G. F. et al. Colorectal cancer in Jordan: Survival rate and its related factors. J. Oncol. 2017, 3180762 (2017).
Goulart, A., Malheiro, N., Rios, H., Sousa, N. & Leão, P. Influence of visceral fat in the outcomes of colorectal cancer. Dig. Surg. 36(1), 33–40 (2019).
Eckel, J. Myokines in metabolic homeostasis and diabetes. Diabetologia 62(9), 1523–1528 (2019).
Thyfault, J. P. & Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 63(8), 1464–1474 (2020).
Kim, J.-M. et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin. Nutr. 40(11), 5631–5638 (2021).
Basile, D. et al. Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clin. Nutr. 40(1), 286–294 (2021).
Park, J. W. et al. Impact of visceral fat on survival and metastasis of stage III colorectal cancer. Gut Liver 16(1), 53–61 (2022).
Kolls, J. K. & Lindén, A. Interleukin-17 family members and inflammation. Immunity 21(4), 467–476 (2004).
Saharti, S. KRAS/NRAS/BRAF mutation rate in saudi academic hospital patients with colorectal cancer. Cureus 14(4), e24392 (2022).
Alyabsi, M., Sabatin, F., Ramadan, M. & Jazieh, A. R. Colorectal cancer survival among Ministry of National Guard-Health Affairs (MNG-HA) population 2009–2017: Retrospective study. BMC Cancer 21(1), 954 (2021).
Zare-Bandamiri, M., Khanjani, N., Jahani, Y. & Mohammadianpanah, M. Factors affecting survival in patients with colorectal cancer in Shiraz, Iran. Asian Pac. J. Cancer Prev. 17(1), 159–163 (2016).
Xie, Y. et al. Impact of tumor site on lymph node status and survival in colon cancer. J. Cancer 10(11), 2376–2383 (2019).
Alese, O. B. et al. Predictive and prognostic effects of primary tumor size on colorectal cancer survival. Front. Oncol. 11, 728076 (2021).
Lee, J. M. et al. Impact of tumor sidedness on survival and recurrence patterns in colon cancer patients. Ann. Surg. Treat. Res. 96(6), 296–304 (2019).
Yun, Z. et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open 3(2), e002270 (2013).
Dashti, S. G. et al. Physical activity and the risk of colorectal cancer in Lynch syndrome. Int. J. Cancer 143(9), 2250–2260 (2018).
Chan, A. T. & Giovannucci, E. L. Primary prevention of colorectal cancer. Gastroenterology 138(6), 2029–43.e10 (2010).
Paudyal, R. et al. Artificial intelligence in CT and MR imaging for oncological applications. Cancers 15(9), 2573 (2023).
Funding
This research was supported by funds from the Intramural Research Grants Program at King Hussein Cancer Center (Award Number: 17 KHCC 99).
Author information
Authors and Affiliations
Contributions
Conceptualization: (A.A), (M.A.S). Methodology: (A.A), (H.A), (M.A.S). Writing – Original Draft: (H.A). Writing – Review & Editing: (H.A), (A.A), (A.T), (F.A). Data collection: (H.A), (M.A.S), (F.A), (K.A.J), (Z.A.J), (O.A). Formal statistical analysis: (A.T). Data management: (H.A.K). All authors have critically revised the manuscript for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abaza, H., Taqash, A., Shattal, M.A. et al. Association between muscle mass and overall survival among colorectal cancer patients at tertiary cancer center in the Middle East. Sci Rep 14, 20836 (2024). https://doi.org/10.1038/s41598-024-68503-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68503-7
- Springer Nature Limited