Abstract
Claudin 18.2 has emerged as a viable therapeutic target in gastric cancer (GC), but little is known about the heterogeneity of its expression in GC. This study investigated the heterogeneity of claudin 18.2 expression in 166 patients with metastatic GC whose surgical or paired primary–metastatic specimens were available. The prevalence of claudin 18.2 positivity (moderate-to-strong expression in ≥ 75% by the 43-14A clone) was 47.0%. Claudin 18.2-positive tumors exhibited more frequent peritoneal metastasis and a lower incidence of hepatic and distant lymph node involvement. Survival outcomes were comparable between patients with claudin 18.2-positive and -negative tumors. Intratumoral heterogeneity was noted in 38.5% of surgical specimens. Paired primary-metastatic site analysis revealed that 25.2% of patients had discordant results for claudin 18.2 positivity. Across different metastatic organ categories, peritoneal lesions showed the highest positivity rate (44.3%) and positive concordance rate (31.4%), whereas liver lesions had the lowest positivity rate (17.9%) and concordance rate (12.8%). In conclusion, claudin 18.2 expression exhibits intratumoral and intrapatient spatial heterogeneity in metastatic GC. Claudin 18.2 positivity is associated with more frequent peritoneal metastasis, and peritoneal lesions are more likely to have positively concordant claudin 18.2 results with the primary site than other metastatic sites.
Similar content being viewed by others
Introduction
Gastric cancer ranks as the fourth leading cause of cancer-related deaths globally and stands as the fifth most prevalent form of malignancy1. Over the past decade, considerable strides have been made in systemic chemotherapy, particularly with the advent of targeted therapies and immunotherapeutic agents, benefiting patients with advanced gastric cancer. Notably, for tumors expressing human epidermal growth factor 2 (HER2), studies such as ToGA have underscored the survival advantage of combining trastuzumab with chemotherapy in the initial treatment phase2. Additionally, research like the Destiny-Gastric-01 trial has revealed the survival benefits of trastuzumab–deruxtecan over standard chemotherapy options in later stages3. Furthermore, recent phase 3 trials have demonstrated that incorporating immune checkpoint inhibitors (ICIs) alongside chemotherapy extends overall survival in patients with advanced gastric cancer lacking HER2 expression during their first-line treatment4,5.
Claudin 18.2 serves as a critical tight junction protein engaged in tissue permeability, paracellular transport, and signal transduction. While predominantly found at tight junctions within normal gastric epithelium, its presence becomes pronounced on the surface of gastric adenocarcinoma cells during malignant transformation, rendering it an appealing target for anti-cancer interventions6. Two pivotal phase 3 trials, namely SPOTLIGHT7 and GLOW8, have showcased that the addition of zolbetuximab, a monoclonal antibody targeting claudin 18.2, to standard chemotherapy, significantly extends overall survival in patients with unresectable or metastatic gastric and gastroesophageal junction cancer, particularly in the first-line treatment setting. Recently, a novel antibody–drug conjugate targeting claudin 18.2 (CMG901) showed promising results in the second- and later-line setting with a confirmed objective response rate of 32.6%9, further highlighting claudin 18.2 as a viable therapeutic target in gastric cancer.
One of the distinct histopathologic features of gastric cancer is a substantial intratumoral and intrapatient spatial heterogeneity10,11. This heterogeneity may pose a challenge in assessing therapeutic target expression as well as improving efficacy outcomes with targeted or immunotherapeutic agents. For instance, in the GASTHER-1 study12, a subset of patients who were initially diagnosed with HER2-negative gastric cancer based on endoscopic specimens exhibited a HER2-positive status upon repeated evaluation using tumor tissues acquired by endoscopy or biopsy of metastatic sites. This highlights the diagnostic challenge of evaluating HER2 expression due to intratumoral and intrapatient spatial heterogeneity. Subsequently, this heterogeneity of HER2 status was associated with unfavorable clinical outcomes with trastuzumab-based chemotherapy12, pointing to the challenge in therapeutic efficacy posed by the heterogeneity. These findings emphasize the significance of biomarker expression heterogeneity in guiding treatment decisions and predicting clinical outcomes.
However, little is known about the heterogeneity in the expression pattern of claudin 18.2 in different tumor sites in metastatic gastric cancer. As this intratumoral and spatial heterogeneity of claudin 18.2 expression could potentially affect the efficacy of claudin 18.2-directed treatments, this is a clinically relevant issue that deserves investigation. In the current study, we aimed to investigate the heterogeneity in the expression patterns of claudin 18.2 expression using the same detection method employed in phase 3 trials7,8 across different tumor sites in metastatic gastric cancer.
Results
Patient characteristics according to claudin 18.2 positivity
Among the overall study population (n = 166) (Fig. 1A), 78 patients (47.0%) had claudin 18.2-positive tumors. The clinicopathological characteristics according to the claudin 18.2 positivity of all 166 patients are summarized in Table 1, and their chemotherapy regimens are summarized in Supplementary Table 1. Baseline features including age, sex, and histologic type were comparable between the claudin 18.2-positive and -negative groups. Of note, peritoneal metastasis was more frequently noted among patients with claudin 18.2-positive tumors than in those with claudin 18.2-negative tumors (65.4% vs. 47.7%, P = 0.033). In contrast, the proportion of patients having liver metastasis was lower in patients with claudin 18.2-positive tumors (19.2% vs. 38.6%, P = 0.010). The same finding was noted for the proportion with distant lymph node (LN) metastasis (39.7% vs. 60.2%, P = 0.013). Neither mismatch repair (MMR) status nor PD-L1 CPS score showed a significant difference between the two groups.
Scheme of the study population. (A) Clinical characteristics and prognostic value of claudin 18.2 positivity in the overall study population (n = 166). (B) Intratumoral heterogeneity of claudin 18.2 expression was investigated among those who underwent surgical resection of the primary tumor (n = 39). (C) Intrapatient spatial heterogeneity of claudin 18.2 expression across different metastatic sites in paired cases of primary and metastatic tumors (n = 135).
Survival outcomes according to claudin 18.2 positivity
During a median follow-up period of 40.8 months (range 9.6–95.4 months) in surviving patients, progression-free survival (PFS) was comparable between those with claudin 18.2-positive (median 6.6 months) and claudin 18.2-negative tumors (median 6.3 months) (hazard ratio [HR] 0.97, 95% confidence interval [CI] 0.70–1.34 P = 0.86) (Fig. 2A). OS was also similar between the two groups (median 14.4 months and 13.8 months for claudin 18.2-positive and -negative tumors, respectively (HR 1.12, 95% CI 0.81–1.54, P = 0.51) (Fig. 2B).
Intratumoral heterogeneity of claudin 18.2 expression
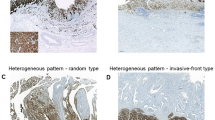
Intratumoral heterogeneity of claudin 18.2 expression was evaluated in 39 patients whose surgical specimens were available (Fig. 1B). Seven (17.9%) patients did not exhibit any claudin 18.2 expression at all. A homogeneous expression pattern was observed in 17 patients (43.6%). Heterogeneous expression of claudin 18.2 was noted in 15 patients (38.5%): the superficial pattern accounted for 12.8% (n = 5), the invasive-front pattern accounted for 7.7% (n = 3), and the random pattern accounted for 17.9% (n = 7), respectively (Supplementary Fig. 1).
Claudin 18.2 expression across primary and metastatic sites
Next, among 135 patients whose tumor tissues from both the primary and metastatic sites were available (Fig. 1C), the concordance and levels of claudin 18.2 expression were evaluated. Figure 3A shows the representative immunohistochemical (IHC) staining patterns exhibiting concordant or discordant claudin 18.2 expression between primary and metastatic sites. There were 34 patients (25.2%) whose tumors were positively concordant for claudin 18.2 expression, while 67 (49.6%) had negatively concordant claudin 18.2 expression. The remaining one-quarter of the patients (n = 34, 25.2%) exhibited discordant claudin 18.2 positivity between primary and metastatic sites, with 14 and 20 patients showing claudin 18.2 positivity only in the primary and metastatic site, respectively (Table 2). Notably, among the 20 cases exhibiting discordance with claudin 18.2 positivity only in metastatic tumors, all of the specimens from the primary site consisted of biopsy specimens, not surgical specimens.
Categorization of paired cases into 4 groups based on the concordance of claudin 18.2 expression determined by immunohistochemical (IHC) staining. (A) Representative examples of immunohistochemical staining for each group. (B) Percentage of tumor cells exhibiting moderate-to-strong staining patterns for claudin 18.2 in each group.
Figure 3B depicts the paired percentages of tumor cells having moderate-to-strong claudin 18.2 expression in the primary and metastatic sites in each patient category for claudin 18.2 concordance.
Heterogeneity of claudin 18.2 expression in different metastatic sites
The claudin 18.2 expression patterns in primary and metastatic sites were then examined among different tumor sites (Fig. 4). Among the organ categories, peritoneal lesions exhibited the highest rate for claudin 18.2-positivity (n = 31 of 70 cases, 44.3%), whereas liver lesions had the lowest rate for claudin 18.2 positivity (n = 7 of 39 cases, 17.9%).
Peritoneal lesions showed a relatively high rate of positive concordance for claudin 18.2 (31.4.%), whereas liver had the lowest positive concordance rate (12.8%) (Fig. 4). The proportion of cases with discordant results of claudin 18.2 positivity between primary and metastatic sites ranged from 5.1 to 20.0% across different organ categories.
Discussion
In the current study, we systematically investigated the intratumoral and intrapatient spatial heterogeneity of claudin 18.2 in metastatic gastric cancer. The rate of claudin 18.2 positivity was 47.0% in overall stage IV gastric cancer, which is comparable to the rates reported in phase 3 studies7,8. Patients with claudin 18.2-positive tumors exhibited more frequent peritoneal involvement with a lower incidence of hepatic involvement and distant LN metastasis. Claudin 18.2-positivity was not prognostic, which aligns with an earlier observation in a metastatic setting13. About 40% of patients whose primary tumors were evaluated with surgical specimens exhibited intratumoral heterogeneity of claudin 18.2 expression. Paired analysis of the primary and metastatic sites revealed that one-quarter of patients had discordant results of claudin 18.2 positivity. Across different metastatic organ categories, peritoneal lesions showed the highest rate of claudin 18.2 positivity (44.3%), with a relatively high positive concordance rate for claudin 18.2 positivity between primary and metastatic sites (31.4%).
To our knowledge, this study is the first to explore the heterogeneous profiles of claudin 18.2 expression among metastatic gastric cancer, with the achievement of higher clinical relevance by applying the same method for claudin 18.2 evaluation used in recent phase 3 trials7,8. While previous studies have explored the heterogeneity of claudin 18.2 expression in gastric cancer, their applicability to metastatic disease remains limited. This limitation arises from two key factors. First, prior studies primarily focused on earlier stage (stage I–III) gastric cancer, where surgical specimens were available for analysis14,15,16. Second, the use of tissue microarrays15 and antibodies not employed in the pivotal SPOTLIGHT and GLOW trials15,16 limits the generalizability of their findings to future practice. Our study addresses these limitations by directly comparing claudin 18.2 expression between primary and metastatic sites within the same patient. Additionally, we investigated differential expression patterns across various metastatic organs. This approach provides novel and practical insights into understanding the heterogeneity of claudin 18.2 expression in metastatic gastric cancer. Moreover, given that the acquisition of tumor tissues from metastatic sites is practically challenging, our results could serve as a unique reference for claudin 18.2 expression across different tumor sites.
In accordance with previous studies reporting intratumoral heterogeneity in resectable gastric cancer14,15,16, heterogeneous claudin 18.2 expression was also noted in the primary site of metastatic gastric cancer in surgical specimens. Notably, there have been limited data on the intrapatient spatial heterogeneity of claudin 18.2 expression, especially in metastatic gastric cancer. A previous Japanese study that employed the same claudin 18.2 clone (43-14A) but a different cut-off (≥ 40% tumor cells) reported 18% discordant results for claudin 18.2 positivity between the primary and regional LN lesions in a localized resectable setting17. Our results reaffirm the concept that heterogeneity of claudin 18.2 expression is also present in metastatic gastric cancer, supporting the concept that intratumoral and spatial heterogeneity is one of the key features of gastric cancer10,11.
The clinical relevance of our study is based on the concept that the heterogeneity of claudin 18.2 expression in gastric cancer might impact the future application of claudin 18.2-targeted therapy as in the case for HER2. The GASTHER-1 study showed that a proportion of patients (i.e., 5–8%) revealed ‘recued HER2 positivity’ with additional biopsies and HER2 testing in patients initially categorized as HER2-negative12. Subsequently, rescued HER2 positivity, which represents heterogeneous HER2 expression per se, was associated with a less prominent benefit of trastuzumab-based chemotherapy12. Similarly, heterogeneous PD-L1 expression among primary and metastatic tumors has also been reported18, indicating its potential to differentially affect efficacy outcomes of ICI-based treatments. Likewise, our study raises an important question as to how these claudin 18.2 heterogeneities might impact the efficacy of zolbetuximab-based chemotherapy in gastric cancer patients. On the other hand, there remains a possibility that zolbetuximab-based chemotherapy may still be efficacious for patients with heterogeneous claudin 18.2 expression. Indeed, various cut-off values for claudin 18.2 positivity are currently being employed for novel claudin 18.2-directed treatments. Therefore, it will be important to identify patient candidates who initially had negative results for claudin 18.2 but are likely to show claudin 18.2 expression on repeated evaluation and therefore could be candidates for zolbetuximab-based chemotherapy. Future studies should evaluate the diagnostic and therapeutic relevance of additional evaluation of claudin 18.2 for ‘rescued claudin 18.2 positivity.’
It also should be noted that there was no paired case whose primary tumors from surgically resected specimens showed claudin 18.2 negativity while the corresponding metastatic tumor exhibited claudin 18.2 positivity. Although there were not enough cases to draw any definite conclusion, this raises the possibility that the minor tumor clones expressing claudin 18.2 (i.e. < 75%) in the primary tumor were not sufficient enough to make the metastatic site claudin 18.2 positive (i.e. ≥ 75%). However, there were cases that showed claudin 18.2 negativity in the primary tumor with endoscopically acquired specimens but claudin 18.2 positivity in the metastatic site. This indicates that this discrepancy may be caused by underrepresented evaluation of the primary tumor with a limited amount of tumor tissue acquired by endoscopic biopsy. Therefore, this further points to the potential clinical utility of claudin 18.2 evaluation based on repeated biopsies, which warrants further studies.
In our analysis, claudin 18.2 positivity was associated with a higher incidence of peritoneal metastasis and a lower incidence of liver/LN metastasis. As this was not seen in previous reports, although a trend for more frequent peritoneal metastasis has been reported13,19, these associations between claudin 18.2 positivity and metastasis to the peritoneum, liver, and LN need to be confirmed in future studies. On the other hand, peritoneal lesions showed the highest positive rate for claudin 18.2 (44.3%) with a relatively high positive concordance rate (31.4%) compared to other organ categories. These findings somewhat accord with the previous findings that claudin 18.2 positivity was associated with Borrmann type 4 and diffuse histological type14, which frequently involve peritoneal metastasis. While the SPOTLIGHT and GLOW studies7,8 did not present a subgroup analysis of the patients with peritoneal metastasis, these potential associations between claudin 18.2 and peritoneal metastasis suggest a need to explore the efficacy of zolbetuximab-based chemotherapy specifically in this patient subgroup.
There are some limitations to be considered in the current study. The retrospective nature of our study, its single center-based analysis, and the absence of a validation cohort may limit the interpretation and generalizability of our data. As the study population was chosen based on the availability of surgical specimens or paired primary–metastatic tumors, the study population may not fully recapitulate the characteristics of all patients treated with palliative chemotherapy. In addition, the relatively heterogeneous chemotherapy regimens, which would differentially affect treatment outcomes, may limit the interpretation of the prognostic value of claudin 18.2 positivity. Nevertheless, it was not the main goal of the current study to investigate the clinical relevance of claudin 18.2 expression patterns in the context of specific treatments. A limited number of patients for each organ category is also one of the limitations of this study.
In conclusion, claudin 18.2 expression exhibits intratumoral and intrapatient spatial heterogeneities in metastatic gastric cancer. Claudin 18.2 positivity is associated with more frequent peritoneal metastasis, and peritoneal lesions are more likely to have positively concordant claudin 18.2 results with the primary site than other metastatic sites. The clinical relevance of claudin 18.2 heterogeneity to the efficacy of claudin 18.2-directed treatments in gastric cancer patients should be evaluated in future studies.
Patients and methods
Study population
This retrospective study included 166 patients with metastatic gastric adenocarcinoma treated with systemic chemotherapy from January 2012 to December 2022 at Asan Medical Center, Seoul, Korea, whose surgical and paired primary–metastatic specimens were available (Fig. 1A). Among the 166 patients, 122 received palliative chemotherapy without gastrectomy, while the remaining 44 underwent surgery including open and closure followed by chemotherapy. Of the surgical group, 16 patients had metastatic disease identified during initial assessment, and 28 patients developed recurrent disease during follow-up.
Among the whole study population (n = 166), 39 patients had surgical specimens from the primary site before chemotherapy administration and were evaluated for intratumoral heterogeneity of claudin 18.2 expression (Fig. 1B), and 135 had paired tumor tissue specimens from primary and metastatic sites, allowing for the assessment of claudin 18.2 expression in both primary and metastatic sites (Fig. 1C).
Clinical data including patient age, sex, Eastern Cooperative Oncology Group (ECOG) performance score, histopathological information including the location and gross type of tumor, histologic subtypes, HER2 and Ebstein–Barr virus (EBV) status, profiles of chemotherapy, and survival outcomes were obtained from reviewing the electronic medical records.
This study received approval from the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2023-0154). Informed consent was waived by the IRB of Asan Medical Center following the de-identification of patient information. This study was conducted in compliance with the ethical standards of the latest Declaration of Helsinki.
Claudin 18.2 expression by immunohistochemistry
The immunohistochemistry method employed in the authors’ prior study14 was replicated in the present investigation. For the surgical specimens, representative sections from formalin-fixed paraffin-embedded (FFPE) blocks were selected by pathologists (YS.P., J.S., and E.C.). Immunohistochemistry (IHC) was performed on 4 μm thick FFPE sections, which were deparaffinized and re-hydrated using xylene and ethanol serially. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 10 min, followed by heat-induced antigen retrieval. IHC staining was performed using a claudin18.2 antibody (clone 43-14A, Ventana) with an autostainer (Benchmark XT, Ventana Medical Systems) and an OptiView DAB Detection Kit (Ventana Medical Systems), following the manufacturer’s protocol.
The immunostaining pattern was interpreted as positive only in cases of membranous, linear staining. Granular expression in the cytoplasm or nucleus was disregarded, considering claudin 18.2 is known to be expressed on the cellular surface. Claudin 18.2 expression status was assessed using the methods used in previous phase 3 studies7,8 either in primary or metastatic sites. In paired cases, those having claudin 18.2-positivity on at least one of the paired tissues were deemed claudin 18.2-positive.
Evaluation of the heterogeneity of claudin 18.2 expression
For patients whose surgical or paired primary–metastatic site specimens were available (Fig. 1B,C), intratumoral and intrapatient heterogeneity of claudin 18.2 expression was assessed. Claudin 18.2 positivity was defined as at least 75% of tumor cells showing moderate-to-strong positive staining, following established criteria7,8. We evaluated intratumoral heterogeneity only in cases with claudin 18.2 positivity, as weak staining makes it difficult to assess staining patterns. Intratumoral heterogeneity of claudin 18.2 expression was assessed in surgical specimens from the primary site (i.e., the stomach) (Fig. 1B) which showed claudin 18.2 positivity, as weak staining makes assessment of staining patterns difficult. The expression patterns of claudin 18.2 were classified based on the homogeneity and the pattern of expression within the tumor. The homogeneous pattern was defined as expressed in more than 90% of the area with a moderate-to-strong intensity as previously described, whereas heterogeneous patterns were further categorized into superficial, invasive-front, and random patterns14. The superficial pattern was defined as expression primarily shown in the mucosa; the invasive-front pattern was characterized by prominent expression in the deep invasive components of the tumor; and the random pattern was defined as a pattern in which the distribution of expression was patchy with varying intensities that were evenly distributed.
For the evaluation of claudin 18.2 in different metastatic sites (Fig. 1C), these were categorized into following four groups: peritoneum, liver, lymph node, and others (including ovary, bone, and soft tissue).
Statistical analyses
PFS was defined as the interval of time between the date of initiation of first-line chemotherapy (index date) and the date of progression or death, whichever comes first. OS was defined as the interval of time between the index date and the date of death from any cause. Survival outcomes were analyzed using the Kaplan–Meier method, and then compared among subgroups by using the log-rank test. For the comparison between two-tiered groups, the Chi-square test or Fisher’s exact test for categorical variables and the t-test or Mann–Whitney U test for continuous variables were used as appropriate. All statistical analysis was conducted with R software ver. 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Rha, S. Y. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncol. 24, 1181–1195 (2023).
Sahin, U. et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 14, 7624–7634 (2008).
Shitara, K. et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 401, 1655–1668 (2023).
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 29, 2133–2141 (2023).
Xu, R.-H. et al. A Phase 1a Dose-Escalation, Multicenter Trial of Anti-claudin 18.2 Antibody Drug Conjugate CMG901 in Patients with Resistant/Refractory Solid Tumors (American Society of Clinical Oncology, 2023).
Nakamura, Y., Kawazoe, A., Lordick, F., Janjigian, Y. Y. & Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: An emerging paradigm. Nat. Rev. Clin. Oncol. 18, 473–487 (2021).
Valenza, C. et al. Targeting HER2 heterogeneity in breast and gastrointestinal cancers. Trends Cancer 1, 1 (2023).
Bang, K. et al. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric Cancer 25, 794–803 (2022).
Kubota, Y. et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 8, 100762 (2023).
Kim, H. D. et al. Clinicopathologic features and prognostic value of claudin 18.2 overexpression in patients with resectable gastric cancer. Sci. Rep. 13, 20047 (2023).
Coati, I. et al. Claudin-18 expression in oesophagogastric adenocarcinomas: A tissue microarray study of 523 molecularly profiled cases. Br. J. Cancer 121, 257–263 (2019).
Dottermusch, M., Krüger, S., Behrens, H. M., Halske, C. & Röcken, C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: Results from a large Caucasian cohort study. Virchows Arch. 475, 563–571 (2019).
Rohde, C. et al. Comparison of claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn. J. Clin. Oncol. 49, 870–876 (2019).
Zhou, K. I. et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin. Cancer Res. 26, 6453–6463 (2020).
Kayikcioglu, E., Yüceer, R. O., Cetin, B., Yüceer, K. & Karahan, N. Prognostic value of claudin 18.2 expression in gastric adenocarcinoma. World J. Gastrointest. Oncol. 15, 343–351 (2023).
Author information
Authors and Affiliations
Contributions
E.C participated in methodology, formal analysis, investigation, data curation and writing—original draft. J.S participated in methodology, investigation, data curation and writing—review & editing. M-H.R participated in conceptualization and writing—review & editing. H-D.K and Y.S.P participated in conceptualization, methodology, writing—review & editing and supervision.
Corresponding authors
Ethics declarations
Competing interests
M-H.R. received honoraria from DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo and AstraZeneca, and served as a consultant for DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly and Ono Pharmaceutical. H-D.K. received honoraria from AstraZeneca, Bristol Myers Squibb, Ono Pharmaceuticals, Boryung Pharmaceuticals, and Boostimmune; and served as a consultant for Mustbio. E.C., J.S, and Y.S.P. declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, E., Shin, J., Ryu, MH. et al. Heterogeneity of claudin 18.2 expression in metastatic gastric cancer. Sci Rep 14, 17648 (2024). https://doi.org/10.1038/s41598-024-68411-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68411-w
- Springer Nature Limited