Abstract
In osteoarthritis (OA), extracellular matrix (ECM) digestion by cartilage-degrading enzymes drives cartilage destruction and generates ECM fragments, such as proteoglycan aggrecan (PG) peptides. PG peptides have been shown to induce immunological functions of chondrocytes. However, the role of PG peptides in stimulating catabolic mediators from chondrocytes has not been investigated. Therefore, we aim to determine the effects and its mechanism by which PG peptides induce chondrocytes to produce catabolic mediators in OA. Human chondrocytes were stimulated with IFNγ and various PG peptides either (i) with or (ii) without TLR2 blockade or (iii) with Lactobacillus species-conditioned medium (LCM), a genus of bacteria with anti-inflammatory properties. Transcriptomic analysis, cartilage-degrading enzyme production and TLR2-intracellular signaling activation were investigated. Chondrocytes treated with PG peptides p16-31 and p263-280 increased expression levels of genes associated with chondrocyte hypertrophy, cartilage degradation and proteolytic enzyme production. TLR2 downstream signaling proteins (STAT3, IkBα and MAPK9) were significantly phosphorylated in p263-280 peptide-stimulated chondrocytes. MMP-1 and ADAMTS-4 were significantly reduced in p263-280 peptides-treated condition with TLR2 blockade or LCM treatment. Phosphorylation levels of IkBa, ERK1/2 and MAPK9 were significantly decreased with TLR2 blockade, but only phosphorylation levels of MAPK9 was significantly decreased with LCM treatment. Our study showed that PG peptide stimulation via TLR2 induced cartilage-degrading enzyme production via activation of MAPK, NFκB and STAT3 pathways.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a debilitating joint condition associated with low-grade inflammation1. The pathology of OA encompasses degradation and destruction of the cartilage, which is driven by cartilage-degrading enzymes, such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)2,3. Chondrocytes are cartilage tissue-resident cells that regulate homeostasis of the cartilage via anabolic and catabolic effects on extracellular matrix (ECM) production and cartilage-degrading enzyme (MMP and ADAMTS) production1,4. The production of MMPs and ADAMTS are imbalanced in OA patients, with a predominant of MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5, etc.4,5,6,7.
ECM fragments are generated from degradation by MMPs and ADAMTS, especially type II collagen and proteoglycan aggrecan8,9. ECM fragments are present in the synovial fluid of OA patients and act as danger-associated molecular patterns (DAMPs) to stimulate chondrocytes by binding to innate immune receptors present on the cells2. ECM fragments that stimulate chondrocytes by binding to TLRs include type II collagen, low molecular weight-hyaluronan, biglycan, fibronectin, chondroitin sulfate fragment and aggrecan 32-mer peptide2,3,10,11. Chondrocytes respond to such ECM fragments, by increasing inflammatory cytokines and chemokine production that results in immune cell recruitment12. The aggrecan 32-mer peptide has been described to bind to TLR2, despite its nature of being a protein-based molecule10. In addition, proteoglycan aggrecan peptides have also been shown to stimulate chondrocytes in the presence of IFNγ10,13.
Proteoglycan aggrecan (PG) is the second most abundant ECM in the cartilage produced by chondrocytes14. Its peptides at positions p16-31 and p263-280 are known to stimulate peripheral blood mononuclear cells (PBMCs) of OA patients and upregulate antigen presentation molecules of osteoarthritic chondrocytes13,15. In these studies, the immunological role as an antigen of proteoglycan aggrecan peptide stimulation on chondrocytes have been demonstrated. However, the anabolic and catabolic effects that proteoglycan aggrecan peptide stimulation has upon chondrocytes have not been investigated yet. In addition, probiotic substances have recently received more attention for their ability to reduce the severity of OA16. Lactobacillus species are well-characterized probiotics that have anti-inflammatory effects, alleviate inflammation and pain, and reduce cartilage destruction in OA, both in in vitro and in vivo studies17,18,19,20. Therefore, in this study, we aimed to determine the biological effects on catalytic substance production of chondrocytes that proteoglycan aggrecan peptides may have upon and the role of Lactobacillus species that may inhibit these effects.
Results
PG peptides p16-31 and p263-280 induces signaling genes related to chondrocyte hypertrophy and cartilage destruction in chondrocytes
Chondrocytes maintain their homeostasis by balancing anabolic and catabolic features of ECM production1,3. In OA chondrocytes, chondrocytes express a hypertrophic phenotype that shifts their homeostasis21. PG is a component of the ECM and its breakdown results in fragments that can stimulate chondrocytes13,15. In order to determine the anabolic and catabolic effects of PG peptides, chondrocytes were stimulated with PG peptides p16-31, p263-280 and p2379-2394 in the presence of IFNγ and their transcriptomics were determined via microarray analysis. Our results showed significant changes in gene expression in pathways involved with chondrocyte structure, development, organization, regulation and cell death (Fig. 1A). These pathways were recategorized into 3 subgroups: (i) pathways involved with proteolysis and proteolytic enzymes, (ii) pathways involved with ECM formation and organization and (iii) pathways involved with chondrocyte and cartilage development (Fig. 1B). Heat maps showing upregulation and downregulation of gene expression in chondrocytes stimulated with the various conditions are shown in Fig. 1B.
Determination of chondrocyte and cartilage degradation-related genes of PG peptide-stimulated chondrocytes in the presence of IFNγ. (A) RNA microarray analysis showing GO classification for differentially expressed genes (DEGs) in chondrocytes stimulated with IFNγ and PG peptides (N = 3). (B) Heat maps showing differential gene expression related to proteolytic enzymes, regulation of ECM production and chondrocyte and cartilage development. Each heat map represents log2 (fold change) ratio of DEGs in IFNγ and PG peptides stimulated chondrocytes compared with the unstimulated condition. Color gradients represent level of expression of genes; green denotes upregulation of genes and red denotes downregulation of genes. (C) Bar graphs illustrating differential gene expression related to proteolytic enzymes, regulation of extracellular matrix production and chondrocyte and cartilage development in IFNγ and PG peptide-treated chondrocytes compared with IFNγ-only treated chondrocytes (N = 3). The significant level of fold change is cut off at 1.5 and -1.5 as shown in the black dot line. (D) Schematic diagram of selected DEGs function relates to ECM production, cartilage degradation and chondrocyte hypertrophy.
In order to determine the effects of PG peptide stimulation, we analyzed gene expression levels of chondrocytes that were stimulated in the presence of PG and IFNγ compared to chondrocytes that were treated with IFNγ only (Fig. 1C). Our results showed many cartilage-degrading enzymes being upregulated when chondrocytes were stimulated with PG p16-31 and p263-280, but not p2379-2394 peptides. These included ADAMTS-4, MMP-1, MMP-3, MMP-10, MMP-12 and MMP-14. Nearly all genes related to ECM production regulation and over half of the genes related to chondrocyte and cartilage development revealed a pattern of upregulation and downregulation whereby chondrocytes stimulated with PG p16-31 or p263-280 peptides had an opposing effect compared to chondrocytes stimulated with PG p2379-2394 peptides, a control peptide in which do not stimulate PBMCs in OA patients13,15 (Fig. 1C). Mapping of the upregulated and downregulated genes in this model along the signaling cascades demonstrated reduced ECM production and increased chondrocyte hypertrophy and cartilage degradation (Fig. 1D).
Cartilage-degrading enzymes in the synovial fluid positively correlate with OA pathology and BMI
In OA, chondrocytes are targets of destruction and cartilage pathology determines disease severity22. Therefore, we correlated the levels of cartilage-degrading enzymes with OA severity using the Mankin/Modified Mankin scoring system22, in which categorizes OA severity into mild, moderate and severe (Fig. 2A). Cartilage-degrading enzymes present in synovial fluids of 30 knee OA patients8,21, MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5, were measured via ELISA. We found that MMP-1 was present at the highest amount and was significantly higher than all other cartilage-degrading enzymes tested (Fig. 2B). There was no significant correlation between the levels of cartilage-degrading enzymes with Mankin scores nor with surface destruction scores nor with combined surface and PG degradation scores (Supplementary Fig. 1). However, MMP-9, ADAMTS-4 and ADAMTS-5 significantly correlated with increased PG degradation scores (Fig. 2C). Surprisingly, despite MMP-1 being the most abundant in the synovial fluid, its correlation with OA severity based on the PG degradation scores was not significant (p = 0.1480) (Fig. 2C). In addition, we also found that ADAMTS-4 and ADAMTS-5 had a significant positive correlation with body mass index (BMI) of these OA patients, an indicator of obesity and known risk factor of OA (Supplementary Fig. 2)1. Altogether, these results show that high levels of cartilage-degrading enzymes, especially ADAMTS-4 and ADAMTS-5 are related with the level of cartilage destruction and may be predisposed by obesity (high BMI), a risk factor for OA.
Correlation between cartilage-degrading enzyme levels and cartilage destruction severity or BMI. (A) Histological assessment according to four parameters (cartilage structure, cellularity, safranin O staining and tidemark integrity) of the Mankin scoring system (score 0–14) on OA cartilage section indicates 3 phases: (i) mild OA (surface irregularity and loss some cartilage matrix (scores 0–4)), (ii) moderate OA (vertical clefts through the transitional and radial zone, loss of cartilage matrix, chondrocyte clustering (scores 5–8)) and (iii) severe OA (complete disorganization of surface zone, severe loss of cartilage matrix, chondrocyte clustering and hypocellularity (scores 9–12)). (B) Bar graphs showing concentration levels of cartilage degrading enzymes (MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5) in synovial fluid of OA patients (N = 30) determined by ELISA. (C) Scattered plots showing correlation between cartilage-degrading enzyme levels and PG score (scoring from safranin-O staining parameter).
PG p263-280 peptide may be a ligand for TLR2 on chondrocytes
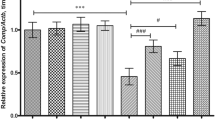
Chondrocytes are able to uptake DAMPs via innate immune receptors, such as Toll-like receptors (TLRs)2,3. Multiple TLRs are expressed in human chondrocytes3. However, only TLR2 and TLR4 are increased in OA chondrocytes23. Both TLR2 and TLR4 recognize ECM fragments such as fibronectin, biglycan, and lubicin2,3. TLR2 have been reported to recognize the 32-mer aggrecan peptide10 and lacking TLR2 on chondrocytes reduces OA severity24. Therefore, we determined whether TLR2 may act as a receptor for taking PG peptides that are known to stimulate chondrocytes13,15. Isolated chondrocytes from knee OA patients were cultured with different PG peptides, the 32-mer peptide and type II collagen (Col2) peptide. Messenger RNA (mRNA) expression of transcription factors (HOXA11, SOX5 and RUNX2) were determined via RT-PCR. Our results showed that the PG p263-280 peptide, but not at other positions, significantly upregulated HOXA11 (direct comparison) (Supplementary Fig. 3), in which TLR2 blockade significantly reduced its expression (p = 0.0498) (Fig. 3A). The expression level of HOXA11 in chondrocytes when treated with Col2 peptide were comparable to when treated with PG p263-280 peptide. However, TLR2 blockade on Col2-stimulated chondrocytes did not reduce HOXA11 expression (Fig. 3A). These findings suggest that TLR2 may act as a specific receptor for PG p263-280 peptide.
Effects of TLR2-blockade on expression levels of chondrocyte development and cartilage degradation-related genes in PG peptide-stimulated chondrocytes. Knee OA chondrocytes (N = 10) were treated with 50 ng/ml of IFNγ and 10 µg/ml of PG peptides (p16-31, p263-280 or p2379-2394), the aggrecan 32-mer peptide or Col2 peptide in the presence of 20 ng/ml of anti-TLR2 antibodies and expression levels of chondrocyte development and cartilage degradation-related genes were determined by quantitative realtime PCR. Comparison of expression levels of chondrocyte differentiation and development (HOXA11, SOX5 and RUNX2) (A), cartilagenous matrix (COL2A1) (B), and cartilage degradation (IL-6) (C) -related genes from chondrocytes stimulated with IFNγ and PG peptides, aggrecan 32-mer peptide and Col2 peptide with and without anti-TLR2 treated chondrocytes were normalized to GAPDH and interpreted as relative expression levels (fold change) (Y-axis). Data was shown as mean ± SEM and statistical significance was calculated by two-way ANOVA with bonferroni post hoc test (*,p < 0.0500; **, p < 0.0100; ***, p < 0.0010; ****, p < 0.0001).
Chondrocytes that are present in OA are in a hypertrophic state and have reduced levels of type II collagen expression as well as increased levels of pro-inflammatory cytokines25. Therefore, we further tested for these two characteristics of hypertrophy on chondrocytes isolated from OA patients; type II collagen production and IL-6 production. Chondrocytes were similarly treated with PG peptides p16-31, p263-280 and p2379-2394, a 32-mer peptide and Col2 peptide in the presence of IFNγ. Treated chondrocytes in all conditions had significant reduction in type II collagen expression (Fig. 3B). TLR2 blockade resulted in a partial restoration of type II collagen production in PG p263-280-stimulated chondrocytes (Fig. 3B). In conditions that chondrocytes were stimulated with other peptides tested, type II collagen production restoration was not observed (Fig. 3B). In addition, we also observed significant increases in IL-6 production only in chondrocytes stimulated with p263-280 peptide, the aggrecan 32-mer peptide and Col2 peptide (Fig. 3C). TLR2 blockade resulted in a significant reduction of IL-6 production only in PG p263-280-stimulated chondrocytes as well. These results demonstrate the effects that p263-280 peptide has upon chondrocytes and by preventing TLR2 receptor binding, these effects were reduced. The blocking effects of TLR2 suggest that PG p263-280 peptide stimulation is mediated via TLR2.
Higher chondrocyte inhibition of PG p263-280 peptide stimulation via TLR2 blockade than aggrecan 32-mer peptide
Chondrocytes regulate cartilage homeostasis by synthesizing a group of cartilage-degrading enzymes1,4. MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 are reported to have increased levels in OA patients4,5,6,7. Based on our microarray analysis, we further evaluated for MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 levels via ELISA in chondrocytes stimulated with PG peptides, the aggrecan 32-mer peptide and Col2 peptide in the presence of TLR2 blockade. In all stimulated conditions, the levels of MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 all increased, despite not up to significant levels. Interestingly, when TLR2 blockade was applied to these stimulation conditions, there were significant decreases in MMP-1 and ADAMTS-4 production in only PG p263-280 peptide-stimulated chondrocytes (Fig. 4A), suggesting TLR2-mediated PG p263-280 stimulation in driving MMP-1 and ADAMTS-4 production. When we calculated the percentage inhibition of each cartilage-degrading enzyme upon TLR2 blockade in each stimulation condition, our results show that stimulation of chondrocytes with PG p263-280 peptide had the highest percentage of inhibition of MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 production (Fig. 4B). Moreover, the percentage inhibition was also higher than conditions stimulated with the 32-mer aggrecan peptide (Fig. 4B). Direct comparisons of percentage inhibition of MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 between PG p263-280 with p16-31 peptide and PG p263-280 and aggrecan 32-mer peptide stimulation conditions showed that the percentage inhibition of MMP-1, MMP-13 and ADAMTS-4 in the PG p263-280 peptide stimulation condition was signficantly higher than the p16-31 peptide stimulation condition (Supplementary Fig. 4A). The percentage inhibition in the PG p263-280 peptide stimulation was generally higher than the aggrecan 32-mer peptide stimulation condition, with MMP-1 production having significant difference (Supplementary Fig. 4A).
Effects of TLR2 blockade on cartilage-degrading enzyme production in PG peptide-stimulated chondrocytes. Knee OA chondrocytes (N = 10) were treated with 50 ng/ml of IFNγ and 10 µg/ml of PG peptides (p16-31, p263-280 or p2379-2394), the aggrecan 32-mer peptide or Col2 peptide in the presence of 20 ng/ml of anti-TLR2 antibodies. Cartilage degrading- enzymes (MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5) and IL-6 production were determined by ELISA. (A) Bar graphs showing comparison of MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 production from IFNγ and PG peptide, aggrecan 32-mer peptide and Col2 peptide stimulation with ( +) and without (-) anti-TLR2 antibodies. Y-axis represents cartilage-degrading enzyme concentrations (pg/ml). (B) Bar graphs showing percentage of inhibition of MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 production after TLR2 blockade in each stimulation condition. (C) Bar graph showing comparison of IL-6 production from IFNγ and PG peptide stimulation with ( +) and without (-) anti-TLR2 antibody. Y-axis represents IL-6 concentration (pg/ml). (D) Bar graph showing the percentage of inhibition of IL-6 production after TLR2 blocking in IFNγ and PG peptide-stimulated chondrocytes. Data was shown as mean ± SEM and significant difference of cartilage degrading factor production was calculated by two-way ANOVA with bonferroni post hoc test, while significant difference of percentage of inhibition was calculated by one-way ANOVA with post-hoc Turky HSD (*,p < 0.0500; **, p < 0.0100; ***, p < 0.0010; ****, p < 0.0001).
In addition, there was a significant reduction in IL-6 production upon TLR2 blockade only in PG p263-280 peptide-stimulated condition (Fig. 4C). Moreover, the percentage of inhibition of IL-6 production when stimulated with PG p263-280 peptide was the highest when compared to other stimulation conditions, especially significantly higher than PG p16-31 and p2379-2394 peptide stimulations (Fig. 4D). Direct comparisons in IL-6 production between PG p263-280 with p16-31 peptide and PG p263-280 and aggrecan 32-mer peptide stimulation conditions showed that the percentage inhibition of IL-6 in the PG p263-280 peptide stimulation condition was higher with strong significance than the PG p16-31 peptide stimulation condition (Supplementary Fig. 4B). The percentage of inhibition of IL-6 production in the PG p263-280 peptide stimulation condition was also higher, but with no significance, than the aggrecan 32-mer peptide stimulation condition (Supplementary Fig. 4B).
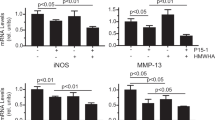
Lactobacilli-conditioned media inhibits production of MMP-1, MMP-13 and IL-6 from chondrocytes
Probiotics have been shown to reduce clinical symptoms of OA in mice models and human trials16,17,18,19. We tested the inhibition effects of 5 strains of Lactobacilli-conditioned media (LCM) with chondrocytes in the presence of PG peptide stimulation. These strains included Lactobacillus gasseri-L10, L. rhamnosus-L34, L. casei-L39, L. salivarius-B60 and L. plantarum-XB726,27,28. All 5 strains inhibited MMP-1 production significantly as depicted by significant decreases in enzyme production when compared with chondrocytes that were treated with deMan, Rogosa and Sharpe (MRS) media and those that were not exposed to any media (Fig. 5A). For MMP-13 production, chondrocytes in all conditions had significant decreases in enzyme production when treated with the 5 strains of Lactobacilli species when compared with chondrocytes alone. However, chondrocytes stimulated with PG p16-31 peptide had significant decreases in MMP-13 production in conditions when all 5 strains of LCM were treated compared to when treated with MRS media alone (Fig. 5A). Treatment with all 5 strains of LCM significantly reduced MMP-1 and MMP-13 production in chondrocytes with and without PG peptide stimulation in a dose-dependent manner (Supplementary Fig. 5). There were no significant changes in MMP-9, ADAMTS-4 and ADAMTS-5 production among chondrocytes that were stimulated with the various conditions regardless of the strain of Lactobacilli species that were used to generate conditioned media (Fig. 5A). Interestingly, when we measured IL-6 production, there were signficant reduction of IL-6 levels in chondrocytes that were stimulated with PG p16-31 and p263-280 peptides and that of the 32-mer peptide10 (Fig. 5A). Our observation demonstrates that cartilage-degrading enzyme production in chondrocytes is independent of peptide stimulation, which is in contrast to IL-6 production (Fig. 5A). Next, we investigated the properties of LCM that inhibited cartilage-degrading enzyme and IL-6 production by treating LCM with heat and proteinase K. Our results show that treatment of all 5 strains of LCM with heat and proteinase K restored the production of MMP-1 significantly (Fig. 5B and C). Significant restoration was also observed with MMP-13 production in only heat-treated Lactobacilli salivarius-B60 conditioned media (Fig. 5B).
Inhibition effect of Lactobacillus-conditioned medium on cartilage degraded mediators released from chondrocytes after IFNγ with PG peptides treatment. Chondrocytes isolated from OA patients (N = 4) were treated with 10% of Lactobacillus-conditioned medium (LCM), L. gasseri-L10 (LG-L10), L. rhamnosus-L34 (LR-L34), L. casei-L39 (LC-L39), L. salivarius-B60 (LS-B60) and L. plantarum-XB7 (LP-XB7) before stimulating with IFNγ and either PG peptides (p16-31, p263-280 and p2379-2394) or control peptides (32-mer and Col2). The cultured medium was collected for cartilage degraded mediators measurement by ELISA. (A) Comparison of MMP-1, MMP-9, MMP-13, ADAMTS-4, ADAMTS-5 and IL-6 production from IFNγ and PG peptides stimulated chondrocytes between LCM-treated and untreated conditions (B) Bar graphs show the MMP-1 and MMP-13 production in 10% LCM-treated chondrocytes (without IFNγ and PG peptides stimulation) compared with that of chondrocytes treated with 10% of heated LCM. (C) Bar graphs show the MMP-1 and MMP-13 production in 10% LCM-treated chondrocytes (without IFNγ and PG peptides stimulation) compared with that of chondrocytes treated with 10% of proteinase K-treated LCM. Data was shown as mean ± SEM and statistical significance was calculated by two-way ANOVA with Bonferroni post hoc test (*,p < 0.0500; **, p < 0.0100; ***, p < 0.0010; ****, p < 0.0001).
Intracellular signaling of peptide stimulated chondrocytes
TLR2 signaling pathway involves downstream signaling and activation of transcription factors and signaling proteins that includes STAT3, NFκB, ERK1/2, p38 and MAPK93. Therefore, we measured the level of phosphorylation of these mediators in chondrocytes stimulated with PG peptides, aggrecan 32-mer peptide and Col2 peptide. The level of phosphorylation of STAT3, IkBα, ERK1/2, p38 and MAPK9 (JNK2) was determined via flow cytometry. The gating strategy of phosphorylated transcription factors and signaling proteins are depicted in Supplementary Fig. 6. Chondrocytes stimulated with p263-280 peptide and aggrecan 32-mer peptide had the highest percentage of chondrocytes expressing phosphorylated STAT3, IkBα, p38 and MAPK9 (JNK2) at 30 min (Supplementary Fig. 7). Therefore, in all subsequent experiments, we evaulated phosphorylation of downstream signaling of TLR2 at 30 min.
Our findings showed that chondrocytes stimulated with PG p263-280 peptide had significantly higher levels of p-STAT3, p-IkBα and p-MAPK9 than chondrocytes stimulated with the control PG p2379-2394 peptide15 (Fig. 6A). p-p38 also exhibited a similar trend, but with no statistical significance (Fig. 6A). In contrast, chondrocytes stimulated with PG p2379-2394 peptide gave the highest percentage of p-ERK1/2 (Fig. 6A). These findings suggest that PG p263-280 peptide induces chondrocyte stimulation via STAT3, NFκB and MAPK (p38 and MAPK9 (JNK2)) pathways, supporting the notion of TLR2-mediated PG p263-280 peptide stimulation in chondrocytes. We further applied TLR2 blockade or treatment with LCM from L. salivarius-B60 cultures and measured the levels of phosphorylated transcription factors and signaling proteins.
Effects of TLR2 blockade and Lactobacillus-conditioned media treatment on intracellular signaling activation in PG peptide-stimulated chondrocytes. Chondrocytes isolated from OA patients (N = 5) were treated with 20 μg/ml of anti-TLR2 antibodies or 10% of LCM, L. salivarius-B60 for 1 h before stimulating with IFNγ and PG peptides (p16-31, p263-280 and p2379-2394) or control peptides (32-mer and Col2 peptides). Intracellular phosphorylated proteins; p-STAT3, p-IkBα, p-ERK1/2, p-p38 and p-MAPK9 (JNK2) were determined by flow cytometry. (A) Bar graph showing the fold change of mean fluorescent intensity of phosphorylated proteins of stimulated chondrocyte which was divided by that of unstimulated chondrocytes. Statistical significance was calculated by one-way ANOVA with post-hoc Turky HSD. (B) Comparison of mean fluorescence intensity (MFI) of phosphorylated protein expression after stimulating with IFNγ and PG peptides between no blocking and anti-TLR2 antibody treatment (upper panel) or between no blocking and L. salivarius-B60 LCM treatment in each individual. Statistical significance was calculated by paired-T test. (C) Bar graphs showing the comparison of percentage of phosphorylated protein- expressing chondrocytes in no blocking, anti-TLR2 antibody treatment and L. salivarius-B60 LCM treatment conditions after stimulation with IFNγ and PG peptides or control peptides. Data was shown as mean ± SEM and statistical significance was calculated by two-way ANOVA with Bonferroni post hoc test (*,p < 0.0500; **, p < 0.0100; ***, p < 0.0010; ****, p < 0.0001).
Representative flow cytometric histograms showed a decrease in p-STAT3, p-IkBα, and p-MAPK9 in PG p263-280 peptide-stimulated chondrocytes treated with anti-TLR2 antibodies or LCM from L. salivarius-B60 compared to conditions without any treatment (Supplementary Fig. 8). TLR2 blockade of peptide-stimulated chondrocytes resulted in significant decreases of MFI values of p-IkBα, p-ERK1/2 and p-MAPK9 only in PG p263-280 peptide-stimulated chondrocytes (Fig. 6B). In comparison, L. salivarius-B60 LCM treatment resulted in significant decreases in MFI values of only p-MAPK9 in PG p16-31 and p263-280 peptide-stimulated chondrocytes (Fig. 6B). The percentages of chondrocytes that expressed p-STAT3, p-IkBα, p-p38 and p-MAPK9 in PG p263-280 peptide stimulation condition was higher (despite with no statistical significance) when compared to other peptide stimulation conditions (Fig. 6C). After treatment with anti-TLR2 antibodies or L. salivarius-B60 LCM, only the percentage of p-MAPK9 in PG p263-280 peptide-stimulated conditions were significantly reduced (Fig. 6C) These results emphasize the stimulated downstream signaling pathway(s) as a result of p263-280 peptide stimulation in chondrocytes.
Discussion
The cartilage remains the target of destruction in OA pathology. Chondrocytes, as cartilage-residing cells, respond to DAMPs that are present in the synovial fluid by secreting pro-inflammatory cytokines and cartilage-degrading enzymes2. When cartilage is digested by cartilage-degrading enzymes, type II collagen and PG fragments are released into the synovial fluid29. In OA, type II collagen peptides were found in both synovial fluid and serum, whereas PG peptides were only detected in synovial fluid29. PG composes of three globular domains (G1, G2 and G3). The major cleavage sites for ADAMTS-4 and ADAMTS-5 are located in the interglobular domain (IGD) between G1 and G2 domains. After degradation, neoepitope aggrecan fragments, particularly those in the G1 domain, are generated and released into the synovial fluid29,30. The PG p263-280 peptide induced TNFα and IFNγ production in peripheral blood T cells of OA patients and stimulated their proliferation15. Our previous study showed that the PG p263-280 peptide induced inflammatory cytokine production and promoted antigen presentation function of chondrocytes in the presence of IFNγ13. The immunological properties of G1 domain aggrecan fragments are also observed in other forms of arthritis, such as in ankylosing spondylitis patients, in which T cells produced IFNγ in response to G1 aggrecan peptide stimulation31.
In this study, we demonstrated that certain DAMPs, such as peptide fragments of proteglycan aggrecan, are able to stimulate chondrocytes and induce chondrocyte phenotypes that favour cartilage destruction via TLR2. The PG peptides at positions p16-31 and p263-280 are known to stimulate PBMCs of OA patients at high levels15, whereas the p2379-2394 peptide stimulated at a low level and therefore, was used as a control throughout this study. We have shown that the PG p16-31 and p263-280 peptide induced upregulation of proteolytic enzymes and responded in opposing effects to the PG p2379-2394 peptide with regards to genes that are related ECM production regulation and chondrocyte and cartilage development. MMP-9 was shown to upregulate when chondrocytes were stimulated with the p263-280 PG peptide. This was in relation to MMP-9 having a significant relation with PG degradation score (adapted from the Mankin scoring system). In addition, ADAMTS-4 and ADAMTS-5 also had a significant correlation with PG degradation scores and body mass indices (BMI) of OA patients. Cartilage breakdown in OA is caused by MMPs and ADAMTS activity8,9. MMP-1 and MMP-13 mostly digest type II collagen, whereas ADAMTS-4 and ADAMTS-5 digest PG8. MMP-9 degrades both PG and type II collagen9. These findings are consistent with our findings that the level of MMP-9, ADAMTS-4 and ADAMTS-5 in synovial fluid were significantly positively correlated to the PG degradation scores despite low levels of ADAMTS-4 and ADAMTS-5. Degradation of PG by ADAMTS-4 and ADAMTS-5 is a hallmark of early stage of OA32. However, synovial fluids used in this study are considered to be in the late stages of OA, which may explain the low levels of ADAMTS-4 and ADAMTS-5 observed. The hypertrophic state of chondrocytes are induced by ECM fragments2,24 and is reflected from high levels of MMP-13 present in the synovial fluid as in our findings21. The presence of the PG p16-31 and p263-280 sequences within the host is evident from its natural sequence within the PG structure33. However, the presence of the fragmented peptides (p16-31, p263-280 and others) in the synovial fluid have not been demonstrated yet. It is worth noting that endogenous ligands are present mostly in pathological conditions rather than at physiological states34. Apoptotic cells eg. hypertrophic chondrocytes, are also able to release endogenous TLR ligands35. Indirect evidence that chondrocytes may engulf/process/endocytose/generate the PG p263-280 peptide is from the work of de Jong H et al. demonstrating antigen-specific T cell responses in PBMCs from rheumatoid arthritis (RA) and OA patients to the PG p263-280 peptide fragment15. In addition, chondrocytes are also capable of presenting antigens to T cells13,36. Further experiments would be required to validate the true origin of generating the PG p263-280 peptide/peptide containing the PG p263-280 binding “epitope”.
TLR2 is an innate immune receptor expressed on phagocytes, antigen-presenting cells and chondrocytes3,37. TLR2 itself is also a pattern recognition receptor (PRR) that recognizes PAMPs (eg. peptidoglycan)38. Bacterial components (including peptidoglycan) have been detected in synovial fluid of OA patients39 and may provide TLR2 their initial source of TLR2 ligand(s). Blocking of TLR2 on chondrocytes significantly prohibited HOXA11 and IL-6 expression while restoring type II collagen expression. Moreover, TLR2 blockade also significantly reduced MMP-1 and ADAMTS-4 expression. The highest percentage of inhibition of enzyme production was observed in all cartilage-degrading enzymes tested (MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5) when chondrocytes were stimulated with the PG p263-280 peptide fragment with blockade. This inhibition effect was also higher than when chondrocytes were stimulated with the 32-mer peptide10, suggesting a potential role for PG p263-280 peptide stimulation via TLR2 in driving OA pathogenesis. Therefore, in the progression of OA, TLR2 would act as a common receptor to both TLR2 ligands that are obtained from microbes and also generated from within the cartilage itself via ECM degradation.
Many DAMPs that are ligands of TLR2 are byproducts of ECM degradation2,3,10. The aggrecan 32-mer peptide, fibronectin, chondroitin sulfate fragments, biglycan, lubicin and hyaluronan stimulate chondrocytes via TLR2 to produce cytokines and cartilage-degrading enzymes2,10,11,40,41,42,43. The 32-mer aggrecan peptide increased expressions of MMP-13, ADAMTS-5, IL-6 and decreased expression levels of COL2A1 and aggrecan10. Fibronectin fragments enhanced MMP-1, MMP-3 and MMP-13 production44. Chondroitin sulfate fragment, biglycan, lubicin and hyaluronan also increased cytokines, MMPs and ADAMTS production2,11,40,41,42,43. This is obtained by inducing downstream signaling of TLR2 and activation of NFκB11,44.
Binding of DAMPs to TLRs contribute to the progression of OA by triggering inflammation, chondrocyte hypertrophy, senescence and apoptosis, and cartilage degradtion via intracellular signaling transduction3,45. A variety of signaling pathways, including MAPK, NFκB and JAK/STAT pathways, which are the main TLR2 downstream signallings, play a crucial role in the control of inflammatory cytokines and cartilage-degrading enzyme production46,47.
Treatment with various Lactobacillus LCM significantly reduced the production of MMP-1 and MMP-13 in all culture conditions. In addition, LCM of all strains also signficantly reduced IL-6 production when chondrocytes were stimulated with PG peptides p16-31, p263-280 and the 32-mer peptide. The inhibition effects of LCM was abrogated when treated with heat and proteinase, suggesting that the responsible mediator for suppressing cartilage-degrading enzyme production and IL-6 production is a heat-labile protein-based molecule. The significant decrease in MMPs and ADAMTS production by treatment of stimulated chondrocytes with LCM suggests that other mechanisms are in play to suppress production of cartilage-degrading enzymes. Probiotic treatment has been shown to reduce inflammation and disease severity in cases of OA with chronic low-grade inflammation48. In MIA-induced OA mice and OA patients, treatment with Lactobacillus casei or Lactobacillus rhamnosus reduced the production of pro-inflammatory cytokines, MMP-1, MMP-3 and MMP-13 in chondrocytes17,19. Probiotic bacteria are well-known for producing bioactive molecules with anti-inflammatory properties24,49. Further identification of the bioactive molecules responsible for the effects observed in this study and its mechanisms are still needed.
In conclusion, PG p263-280 peptide induced hypertrophic changes and the production of inflammatory cytokines and cartilage-degrading enzymes in chondrocytes via TLR2. The activation of TLR2 by p263-280 peptide induced MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMT5 production through MAPK (p38 and JNK), NFκB and STAT3 signaling pathways. The increased production of MMPs and ADAMTS from chondrocytes further increased ECM degradation and causes a vicious cycle of increased cartilage-degrading enzymes and increased cartilage destruction (Fig. 7). Our findings shed light on the effects of TLR2-mediated PG p263-280 peptide stimulation on cartilage. Inhibition of PG p263-280 binding to TLR2 or treatment with probiotic strains of Lactobacilli reduced the production of MMPs and ADAMTS. TLR2 antagonist or intraarticular injection of LCM-generated bioactive molecules may be used as an alternate modality in the treatment of OA. These modalities introduce new possibilities for intervening with the progression of cartilage destruction.
Schematic diagram depicting the catabolic mechanism driven by PG p263-280 peptide stimulation in chondrocytes. The degradation cartilage ECM generates aggrecan peptide fragments, in which act as DAMPs that bind to TLR2 on chondrocytes. TLR2 activation induces MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 production via NFκB, MAPK and STAT3 signaling pathways, in which results in an increase in ECM degradation and further cartilage destruction.
Materials and methods
Patient recruitment and specimen collection
Synovial fluid and articular cartilage were collected from knee OA patients (ages 50–80 years) undergoing total knee replacement surgery at King Chulalongkorn Memorial Hospital. Ethical approval was obtained from the ethical committee of Institution Review Board (IRB) at the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No.497/60) and was in accordance with the Helsinki Declaration of 1975, as revised in 2000. All patients provided written informed consent. Samples were obtained from a total of 48 knee OA patients, in which samples from 30 knee OA patients were used for cartilage histopathology scoring and synovial fluid assessment. Samples from the remaining 18 knee OA patients were used for chondrocyte isolation, microarray analysis, functional assays and intracellular signaling assessment (Supplementary Fig. 9).
Cartilage severity determination
Harvested cartilage were immediately fixed in 10% neutral formalin for 72 h and subsequently decalcified with 10% acetic acid for 5 days. Decalcified samples were cut into smaller pieces, dehydrated with ethanol in a conventional gradient and embedded in paraffin. Five micron-thick sections were cut from the sample block and stained with safranin O and Light green (MedChemExpress). Cartilage sections was visualized under light microscope and the severity grading was scored using the Mankin assessment system, with a total score ranging from 0 (normal) to 14 (most severe) based on four parameters: cartilage structure, cellularity, safranin O staining and tidemark integrity22.
Chondrocyte isolation and extraction
Cartilage was cut into small pieces and digested with 1% (w/v) pronase (Sigma-Aldrich) in DMEM containing 10% FBS at 37 °C for 1 h, followed by incubation with 0.3% (w/v) type II collagenase (Worthington) in DMEM containing 10% FBS for 16 h at 37 °C. Isolated cells were filtered through a 40 µm cell strainer and centrifuged at 500 g for 5 min. Cells were washed twice with 10 ml of complete DMEM medium (supplemented with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 1% non-essential amino acid) before resuspending in 10% DMSO in FBS and storing at − 80 °C for further experiments.
Transcriptomic profiling via RNA microarray
Isolated chondrocytes were cultured in a 6-well plate and treated with 50 ng/ml IFNγ (R&D System) with either 10 μg/ml of synthetic proteoglycan aggrecan (PG) peptides (GenScript); p16-31 (QPSPLRVLLGTSLTIP), p263-280 (TTGHVYLAWQAGMDMCSA) or p2379-2394 (LQKRSSRHPRRSRPST) and cultured at 37 °C, 5% CO2 for 6 h. The stimulated chondrocytes were harvested and RNA extraction was performed via the liquid-phase separation method using TRIzol (Ambion) and 1-bromo-3-chloropropane (Sigma-Aldrich) reagents. RNA integrity was measured using an Agilent 2100 Bio-analyzer. RNA samples were converted to cDNA, labeled with cyanine 3 or 5 (Cy3 or Cy5) fluorescent dyes, hybridized to SurePrint G3 Human Gene Expression 8 × 06 K v3 Microarray, and scanned using the Agilent Feature Extraction v11.0. The differential gene expression was analyzed using R 3.5.1 software. Gene ontology annotation was generated using Enrichr tool50. All PG peptides used in this study were tested for endotoxin contamination before using in all experiments. The purity of all PG peptides was greater than 95%, with no endotoxin contamination.
Gene expression determination by quantitative real-time PCR
Total RNA was extracted from PG peptide-stimulated chondrocyte with and without anti-TLR2 treatment using TRIzol (Ambion) and 1-bromo-3-chloropropane (Sigma-Aldrich) and converted to cDNA using SuperScript VILO cDNA synthesis kit (Invitrogen) according to the manufacturer’s instruction. The cDNA (500 ng) template was added into the SYBR Green PCR master mix (Applied biosystem) containing 10 µM forward and reverse primers of target genes. The reaction mixes were incubated at 95 °C for 10 min, followed by 40 cycles of incubation at 95 °C for 15 seconds and 60 °C for 1 min using the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System (Applied biosystem). Gene transcripts were normalized to the expression level of GAPDH and the relative expression levels were calculated from the 2−ΔΔCT equation.
Chondrocyte stimulation and TLR2 blocking assay
Chondrocytes were resuspended in complete DMEM medium and cultured in a 96-well plate (1 × 104 cells/well) for 48 h or until cells were completely attached to the plate. Chondrocytes were treated with 50 ng/ml IFNγ (R&D System) with either 10 μg/ml of synthetic PG peptides (GenScript); p16-31 (QPSPLRVLLGTSLTIP), p263-280 (TTGHVYLAWQAGMDMCSA) or p2379-2394 (LQKRSSRHPRRSRPST) and cultured at 37 °C, 5% CO2 for 24 h. For TLR2 blocking assay, chondrocytes were treated with 20 μg/ml of anti-TLR2 (clone TL2.1, Biolegend) for 1 h prior to PG peptide stimulation. The aggrecan peptide 32-mer (FFGVGGEEDITVQTVTWPDMELPLPRNITEGE) and type II collagen (Col2) peptide (HRGYPGLDG) were used as a positive and negative control for the stimulation assay, respectively. Supernatant were collected for cytokine and cartilage-degrading enzyme evaluation.
Generation of Lactobacillus-conditioned media
Lactobacillus-conditioned media (LCM) was prepared by culturing strains of probiotic bacteria: L. salivarius B60, L. casei L39, L. gasseri L10, L. rhamnosus L34, and L. plantarum XB7, at an OD600 of 0.1 in de Man, Rogosa and Sharpe (MRS) broth for 48 h in an anaerobic condition. Cultures were centrifuged at 3000 g at 4 °C for 10 min. Supernatant was collected and filtered through a 0.22-μm syringe filter and was considered as LCM. LCM were aliquoted into microcentrifuge tubes, dried by speed vacuum at 40 °C for 3 h and resuspended in complete DMEM medium to its original LCM volume.
Lactobacillus-conditioned medium (LCM) treatment on PG peptide-stimulated chondrocytes
Chondrocytes were treated with LCM (10% v/v) with or without stimulation of the PG peptides (p16-31, p263-280, p2379-2394), 32-mer peptide and Col2 peptide. Stimulated cells were incubated at 5% CO2 at 37 °C for 24 h. Supernatant was collected to measure the concentration of cytokines and cartilage-degrading enzymes by ELISA.
Determination of cytokine and cartilage-degrading enzyme levels
Synovial fluid and supernatant of stimulated chondrocyte cultures were collected to determine the levels of IL-6 (Invitrogen) and cartilage-degrading enzymes (MMP-1, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5) (R&D System) via the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction. Briefly, samples at titrated dilutions were incubated for 2 h in a 96-well plate pre-coated with capture antibodies specific to human IL-6, MMP-1, MMP-9, MMP-13, ADAMTS-4 or ADAMTS-5, and blocked with 2% BSA in PBS. After washing with PBS containing 0.05% Tween-20, samples were incubated with biotinylated antibodies towards IL-6, MMP-1, MMP-9, MMP-13, ADAMTS-4 or ADAMTS-5, followed by incubation with streptavidin-conjugated horseradish peroxidase (HRP). Substrate solution was added into each well and the reaction was subsequently inhibited by adding 2N sulfuric acid (H2SO4). The absorbance of the solution was measured by a multifunctional microplate reader (Thermo Scientific). Protein concentration was calculated from subtraction values of 570 nm from that of 450 nm compared to the standard curve.
Intracellular signaling determination by flow cytometry
Isolated chondrocytes were cultured in a 48-well plate (1 × 105 cells/well) and stimulated with 50 ng/ml IFNγ (R&D System) with either 10 μg/ml p16-31, p263-280, p2379-2394, 32-mer or Col2 peptides (GenScript) for 30 min with or without anti-TLR2 antibodies or LCM-LS-B60. Stimulated chondrocytes were harvested and treated with 1.5% formaldehyde for 10 min at room temperature. Treated cells were pelleted and permeabilized using 100% cold methanol for 1 h at 4 °C. Intracellular signaling proteins were labeled with phospho-specific antibodies; anti-human phospho-p38 (Thr180/Try182)-PE/Cy7 (Clone 4NIT4KK, Invitrogen), anti-human phospho-MAPK9 (pJNK2) (Thr183/Tyr185)-APC (Clone SAPKT183Y185-A11, Invitrogen), anti-human phospho-ERK1/2 (Thr202/Try204)-PE/Cy5 (Clone 6B8B69, Biolegend), anti-human phospho-STAT3 (Tyr705)-AF488 (Clone 13A3-1, Biolegend) and anti-human phospho-IkBα (Ser32/Ser36)-PE (Clone RILYB3R, Invitrogen) antibodies for 1 h at room temperature. After washing twice with PBS containing 2% FBS, samples were acquired on the BD-LSR II flow cytometer (BD Biosciences) and data were subsequently analyzed using the FlowJo program (Treestar).
Statistical analysis
All data were analyzed using the statistical software GraphPad Prism version 8.0.2 (GraphPad Software Inc.). Correlation between two parameters was evaluated using Linear regression and Pearson correlation. Statistical significance was assessed by paired T-test, one-way and two-way ANOVA. Bonferroni correction or Turky HSD was used as a post hoc test in case of multiple comparisons. The data were shown as mean ± SEM and statistically significant data was considered at p < 0.05.
Ethical approval
This study was approved by the ethical committee of Institution Review Board (IRB) at the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No.497/60).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary information files).
References
Terkawi, M. A. et al. Low-grade inflammation in the pathogenesis of osteoarthritis: Cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines 10 (2022).
Lambert, C. et al. The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: Perspectives from a review of the literature. Front. Med. (Lausanne) 7, 607186 (2020).
Barreto, G., Manninen, M. & K, K.E. Osteoarthritis and toll-like receptors: When innate immunity meets chondrocyte apoptosis. Biology (Basel) 9 (2020).
Akkiraju, H. & Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 3, 177–192 (2015).
Zeng, G. Q., Chen, A. B., Li, W., Song, J. H. & Gao, C. Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 14, 14811–14822 (2015).
Xin, X. et al. Potential Value of Matrix Metalloproteinase-13 as a Biomarker for Osteoarthritis. Front. Surg. 8, 750047 (2021).
Verma, P. & Dalal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 112, 3507–3514 (2011).
Ashruf, O. S. & Ansari, M. Y. Natural compounds: Potential therapeutics for the inhibition of cartilage matrix degradation in osteoarthritis. Life (Basel) 13 (2022).
Slovacek, H. et al. Interrelationship of MMP-9, Proteoglycan-4, and inflammation in osteoarthritis patients undergoing total hip arthroplasty. Clin. Appl. Thromb. Hemost. 27, 1076029621995569 (2021).
Lees, S. et al. Bioactivity in an aggrecan 32-mer fragment is mediated via Toll-like receptor 2. Arthritis Rheumatol. 67, 1240–1249 (2015).
Jung, Y. K. et al. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 9, 15846 (2019).
Maldonado, M. & Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 284873 (2013).
Sengprasert, P. et al. Upregulation of antigen presentation function and inflammation in chondrocytes by induction of proteoglycan aggrecan peptides (P16–31 and P263–280). Clin. Exp. Rheumatol. 40, 596–607 (2022).
Roughley, P. J. & Mort, J. S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 1, 8 (2014).
de Jong, H. et al. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 69, 255–262 (2010).
Dunn, C. M. & Jeffries, M. A. The microbiome in osteoarthritis: A narrative review of recent human and animal model literature. Curr. Rheumatol. Rep. 24, 139–148 (2022).
Jhun, J. et al. Oral Administration of Lactobacillus rhamnosus ameliorates the progression of osteoarthritis by inhibiting joint pain and inflammation. Cells 10 (2021).
Lei, M., Guo, C., Wang, D., Zhang, C. & Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 8, 697–703 (2017).
So, J. S. et al. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 88, 358–366 (2011).
Sengprasert, P., Kamenkit, O., Tanavalee, A. & Reantragoon, R. The immunological facets of chondrocytes in osteoarthritis: A narrative review. J. Rheumatol. (2023).
Chawla, S. et al. Chondrocyte hypertrophy in osteoarthritis: Mechanistic studies and models for the identification of new therapeutic strategies. Cells 11 (2022).
Pauli, C. et al. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthr Cartil 20, 476–485 (2012).
Kim, H. A. et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 54, 2152–2163 (2006).
Indira, M., Venkateswarulu, T. C., Abraham Peele, K., Nazneen Bobby, M. & Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 9, 306 (2019).
Robinson, W. H. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 580–592 (2016).
Boonma, P., Spinler, J. K., Venable, S. F., Versalovic, J. & Tumwasorn, S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 14, 177 (2014).
Panpetch, W., Spinler, J. K., Versalovic, J. & Tumwasorn, S. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori-stimulated gastric epithelial cells. BMC Microbiol 16, 242 (2016).
Thiraworawong, T. et al. Anti-inflammatory properties of gastric-derived Lactobacillus plantarum XB7 in the context of Helicobacter pylori infection. Helicobacter 19, 144–155 (2014).
Kalogera, S. et al. Relevance of biomarkers in serum vs. synovial fluid in patients with knee osteoarthritis. Int. J. Mol. Sci. 24 (2023).
Germaschewski, F. M. et al. Quantitation OF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthr. Cartil. 22, 690–697 (2014).
Zou, J., Appel, H., Rudwaleit, M., Thiel, A. & Sieper, J. Analysis of the CD8+ T cell response to the G1 domain of aggrecan in ankylosing spondylitis. Ann. Rheum. Dis. 64, 722–729 (2005).
Li, T. et al. The mechanism and role of ADAMTS protein family in osteoarthritis. Biomolecules 12 (2022).
Szanto, S. et al. Induction of arthritis in HLA-DR4-humanized and HLA-DQ8-humanized mice by human cartilage proteoglycan aggrecan but only in the presence of an appropriate (non-MHC) genetic background. Arthritis Rheum. 50, 1984–1995 (2004).
Pollanen, R. et al. Microbial antigens mediate HLA-B27 diseases via TLRs. J. Autoimmun. 32, 172–177 (2009).
Taniguchi, N. et al. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol. Cell. Biol. 27, 5650–5663 (2007).
Kuhne, M. et al. HLA-B27-restricted antigen presentation by human chondrocytes to CD8+ T cells: Potential contribution to local immunopathologic processes in ankylosing spondylitis. Arthritis Rheum. 60, 1635–1646 (2009).
Candia, L., Marquez, J., Hernandez, C., Zea, A. H. & Espinoza, L. R. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: A pathogenic role for innate immunity?. J. Rheumatol. 34, 374–379 (2007).
Oliveira-Nascimento, L., Massari, P. & Wetzler, L. M. The role of TLR2 in infection and immunity. Front. Immunol. 3, 79 (2012).
Holub, M. N. et al. Peptidoglycan in osteoarthritis synovial tissue is associated with joint inflammation. Res Sq (2023).
Avenoso, A. et al. The proteoglycan biglycan mediates inflammatory response by activating TLR-4 in human chondrocytes: Inhibition by specific siRNA and high polymerized Hyaluronan. Arch. Biochem. Biophys. 640, 75–82 (2018).
Campo, G. M. et al. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 80, 480–490 (2010).
Liu-Bryan, R. & Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 62, 2004–2012 (2010).
Barreto, G. et al. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthr. Cartil. 28, 92–101 (2020).
Hwang, H. S., Park, S. J., Cheon, E. J., Lee, M. H. & Kim, H. A. Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res. Ther. 17, 320 (2015).
Lei, L. et al. Effect of cell receptors in the pathogenesis of osteoarthritis: Current insights. Open Life Sci. 17, 695–709 (2022).
Hu, X. et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24, 563–574 (2006).
Li, Z., Huang, Z. & Bai, L. Cell interplay in osteoarthritis. Front. Cell Dev. Biol. 9, 720477 (2021).
Tan, T. C., Chong, T. K. Y., Low, A. H. L. & Leung, Y. Y. Microbiome and osteoarthritis: New insights from animal and human studies. Int. J. Rheum. Dis. 24, 984–1003 (2021).
Jenab, A., Roghanian, R. & Emtiazi, G. Bacterial natural compounds with anti-inflammatory and immunomodulatory properties (mini review). Drug Des. Dev. Ther. 14, 3787–3801 (2020).
Kuleshov, M. V. et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90-97 (2016).
Acknowledgements
We would like to thank the medical staffs and the patients involved in this study.
Funding
This study was supported by the Ratchadaphiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (RA62/112) and the Thailand Science, Research and Innovation Fund (Fundamental Fund; HEAF67300018), Chulalongkorn University. P.S. is a postdoctoral fellowship supported by the Ratchadapisek Somphot Fund for Postdoctoral Fellowship from the Graduate School, Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
P.S. conceptualized the study, performed the experiments, analyzed the results and wrote the manuscript. P.W., O.K., A.S., T.C., J.W. and S.N. performed the experiments and data collection. M.T., S.L., S.T. and A.T. provided intellectual input and revised the manuscript. R.R. conceptualized the study, analyzed the results, wrote the initial draft of the manuscript and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sengprasert, P., Waitayangkoon, P., Kamenkit, O. et al. Catabolic mediators from TLR2-mediated proteoglycan aggrecan peptide-stimulated chondrocytes are reduced by Lactobacillus-conditioned media. Sci Rep 14, 18043 (2024). https://doi.org/10.1038/s41598-024-68404-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68404-9
- Springer Nature Limited