Abstract
Non-alcoholic fatty liver disease (NAFLD) has become the first major chronic liver disease in developed countries. 10–20% of NAFLD patients will progress to non-alcoholic steatohepatitis (NASH), and up to 25% of NASH patients may develop cirrhosis within 10 years. Therefore, it is critical to find key targets that may treat this disease. Here, we identified C5aR1 as a highly-expressed gene in NASH mouse model through analyzing Gene Expression Omnibus (GEO) database and confirmed its higher expression in livers of NASH patients than that of NAFL patients. Meanwhile, we verified its positive correlation with patients’ serum alanine transaminase (ALT) and aspartate transaminase (AST) levels. In vivo and in vitro experiments revealed that knocking down C5aR1 in liver significantly reduced liver weight ratio and serum ALT and AST levels and attenuated inflammatory cell infiltration and cell apoptosis in the liver of NASH mice as well as enhanced the efferocytotic ability of liver macrophages, suggesting that C5aR1 may play a crucial role in the efferocytosis of liver macrophages. Furthermore, we also found that the expression levels of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing protein 3 (NLRP3), caspase-1, IL-1β and other inflammation-related factors in the liver were significantly reduced. Our work demonstrates a potential mechanism of how C5aR1 deficiency protects against diet-induced NASH by coordinating the regulation of inflammatory factors and affecting hepatic macrophage efferocytosis.
Similar content being viewed by others
Introduction
Due to rapid economic development and changes in lifestyles, such as the intake of high-calorie foods and high-sugar drinks and a lack of exercise and physical activity, non-alcoholic fatty liver disease (NAFLD) has become a major chronic disease of contemporary society1,2,3. This disease progresses from simple non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), and as the disease progresses, NASH can worsen to fibrosis, cirrhosis, or hepatocellular carcinoma4. NASH, with a prevalence of 3–6%, is the most critical stage in the progression of NAFLD. Compared with NAFL patients, NASH patients have a 30- to 50-fold or even greater risk of developing end-stage liver disease, such as cirrhosis or liver cancer5. Thus, prevention and treatment of NASH have become critical. Fatty liver is caused by excessive fat accumulation in hepatocytes (defined as the presence of lipid droplets in > 5% of hepatocytes)6,which is is a priming factor in the development of NAFLD7. NASH is the more severe form of fatty liver and is characterized by the presence of liver injury, inflammation, and fibrosis8. The mechanism underlying the progression of fatty liver to NASH has been studied extensively to identify a therapeutic target for the pharmacological intervention of NASH; however, few medications are currently used in the treatment of NASH9,10.
Macrophages play a key role in the development and progression of NASH11. In NASH, a large number of rapidly produced proinflammatory macrophages are needed, which is beneficial to the patient in the early stage. However, the persistence of proinflammatory macrophages can aggravate inflammation and fibrosis, and the cytokines produced by macrophages, such as IL-6, TNF, and IL-1β, can directly target hepatocytes and promote steatosis, inflammation and hepatocytic damage12. In both patients and mice with NASH, macrophages often cluster or aggregate in the liver, especially in areas of bulla steatosis, and these cell aggregates promote the progression of NAFL to NASH13.
In studies regarding NAFLD, it has been found that the serum concentration of complement factor C5a in obese children, especially in obese children with NAFLD, was greater than that in control children. C5a concentration was positively correlated with body mass index, waist circumference, diastolic blood pressure, triglyceride content and insulin resistance14. As part of the complement system, complement component 5a (C5a), produced via the complement pathway, binds to its receptor, component 5A receptor 1 (C5aR1), and activates the C5a/C5aR1 axis, thus playing a proinflammatory role in the innate immune response15. Numerous studies16,17,18,19,20,21 have shown that targeting this process can be used to treat a variety of inflammation-related diseases, including cancer, respiratory disease, cardiovascular disease, and liver disease.
In our study, the GEO database(GSE154892,website: https://www.ncbi.nlm.nih.gov/geo/) was analysed to identify the C5aR1-encoding gene, which is abnormally highly expressed in the livers of NASH model mice, and human sample analysis also indicated that the expression level of C5aR1 in the livers of NASH patients was significantly greater than that in the livers of NAFL patients and was positively correlated with patient serum alanine transaminase (ALT) and aspartate transaminase (AST) levels. Furthermore, we investigated the protective effect of C5aR1 knockdown and its possible underlying mechanism in NASH development.
Results
The expression of C5aR1 is significantly increased in mice with NASH
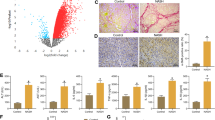
To investigate the key genes that may be involved in the pathogenesis of non-alcoholic liver inflammation, we analysed microarray data from the livers of normal mice and mice with NASH in the GEO database and constructed a cluster heatmap with expression differences greater than twofold. C5aR1 expression was significantly upregulated in the NASH model mice (Fig. 1A). Then, the expression of C5aR1 in the liver tissues of mice fed a High fat methionine choline deficiency diet(HFMCD) diet for 6 weeks or 8 weeks was determined, and the results indicated that the expression of C5aR1 in the livers of mice with HFMCD diet-induced NASH for 6 weeks was significantly greater than that in the livers of control mice (Fig. 1B,C). Both C5aR1 mRNA and protein expression levels in the livers of mice with HFMCD-induced NASH at 8 weeks were significantly greater than those in the livers of control mice (Fig. 1B,D).
Expression of C5aR1 in the liver of NASH mice. (A) The heat map showed some genes whose alteration factor was greater than 2 in the chip data (GSE154892); (B) qRT-PCR verified the expression level of C5aR1 in liver after 6 and 8 weeks of normal diet and HFMCD diet (n = 5/n = 4); (C) Western-blot analysis and quantification of liver protein levels of C5aR1 after 6 weeks of normal diet and HFMCD diet (n = 5); (D) Western-blot analysis and and quantification of C5aR1 expression in liver after 8 weeks of normal diet and HFMCD diet (n = 4). (E) Immunohistochemistry staining and quantification of the expression of C5aR1 in liver tissue of NASH mice. All measurement data were expressed as mean ± standard error (mean ± S.E.M.), and independent sample t test was used for comparison between the two groups. **P ≤ 0.01, ***P ≤ 0.001.
Furthermore, immunohistochemistry was used to determine the distribution of C5aR1 expression in the different groups of mice after 6 and 8 weeks of HFMCD feeding. The results indicated that C5aR1 was mainly expressed in nonparenchymal cells, and the expression of C5aR1 in nonparenchymal liver cells in HFMCD-induced NASH mice was significantly greater than that in the control group. Moreover, C5aR1 expression in the liver nonparenchymal cells of mice fed an HFMCD for 8 weeks was significantly greater than that in the liver nonparenchymal cells of mice fed an HFMCD for 6 weeks (Fig. 1E).
Liver C5aR1 expression is significantly increased in NASH patients
To gain insight into the potential role of C5aR1 in human NASH, we analysed liver samples from human subjects with NAFL and NASH. The results showed that liver C5aR1 mRNA expression levels were significantly greater in NASH patients than in NAFLD patients, regardless of severity (Fig. 2A), consistent with our findings in mice. Next, we sought to assess whether there was a correlation between liver C5aR1 expression and serum ALT and AST levels in these human subjects. Studies have shown that liver C5aR1 mRNA expression is positively correlated with serum ALT and AST levels (Fig. 2B,C). Single-cell sequencing data from the Human Protein Atlas (HPA) revealed that C5aR1 was expressed mainly in Kupffer cells among nonparenchymal cells (Fig. 2D). Immunofluorescence results showed that C5aR1 (red) in the liver of NASH patients colocalised with that of CD68 (green), confirming its expression in Kupffer cells (Fig. 2E).
Expression and correlation analysis of C5aR1 in human. (A) qRT-PCR was used to detect the expression of C5aR1 in the liver samples of patients with NALF (n = 5) and NASH (n = 39). t test was used for comparison between the two groups; (B) The correlation between the expression level of C5aR1 and the serum ALT level in patients with NALF (n = 5) and NASH (n = 39), Pearson correlation coefficient = 0.668; (C) The correlation between the expression level of C5aR1 and the serum AST level in patients with NALF (n = 5) and NASH (n = 39),Pearson correlation coefficient = 0.671; (D) Single-cell sequencing showed the distribution of C5aR1 in different cells of the liver; (E) The expression of CD68 macrophages (green) and C5aR1 (red) in the liver of NASH patients was detected by immunofluorescence. *P ≤ 0.05.
Liver-specific knockdown of C5aR1 expression can significantly reduce indices of liver inflammation in mice
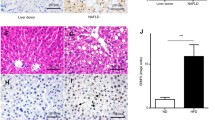
To determine whether C5aR1 is involved in regulating the occurrence and development of NASH, the purified adeno-associated viruses AAV-NC and AAV-shC5aR1 were injected into mice via the tail vein at a concentration of 2 × 1011 vg/mouse, and the mice were subsequently fed a HFMCD diet (Fig. 3A). Four weeks later, the mice were killed, the blood and livers were collected, and total liver proteins were extracted for Western blot analysis. Antibody detection of C5aR1 expression showed that knockdown of C5aR1 expression significantly reduced the protein expression level of C5aR1 (Fig. 3B), indicating successful knockdown. The livers of the mice decreased in size after C5aR1 knockdown, and the liver weight ratio decreased significantly (Fig. 3C,D). Moreover, after C5aR1 knockdown, the serum ALT and AST levels of the mice were decreased by 41.2% and 18.9%, respectively (Fig. 3E,F), which were significantly improved compared with those in the control group. The levels of the proinflammatory factors TNF-α, IL-6 and IL-1β in the liver were also significantly decreased (Fig. 3G). These results suggest that C5aR1 knockdown can significantly reduce liver injury and inflammation induced by an HFMCD diet. In addition, H&E staining, Oil red staining of livers showed that C5aR1 knockdown dramatically improved the degree of inflammatory cell infiltration, vacuole-like degeneration of hepatocytes and lipid deposition in the liver when comparing to the control group (Fig. 3H). Meanwhile, Sirius Red staining show an alleviated fibrosis after knockdown of C5aR1 (Fig. 3H). Immunohistochemical results from the paraffinised sections of mouse livers indicated that the number of F4/80+ macrophages in the liver tissues of mice with C5aR1 knockdown was significantly greater than that in the liver tissues of control mice (Fig. 3I).
Mouse knockdown of C5aR1 reduces liver inflammation. (A) AAV-NC/AAV-shC5aR1 mouse protocol; (B) Western-blot and corresponding quantification of C5aR1 protein levels in liver tissue of AAV-shC5aR1 mice after 4 weeks HFMCD diet (n = 4); (C,D) Liver photos and ratio of liver/body weight of AAV-NC/AAV-shC5aR1 mice fed with HFMCD diet for 4 weeks; (E,F) Blood ALT and AST levels after 4 weeks of HFMCD in AAV-NC/AAV-shC5aR1 mice (n = 4); (G) Expression levels of TNF-α, IL-6 and IL-1β. In AAV-NC/AAV-shC5aR1 mice (n = 4). (H) H&E staining, Oil red staining and Sirius Red staining and corresponding quantification were preformed in livers of AAV-NC/AAV-shC5aR1 mice fed with 4 weeks of HFMCD diet. (I) F4/80 immunohistochemistry and quantification were preformed in livers of AAV-NC/AAV-shC5aR1 mice fed with 4 weeks of HFMCD diet. All Measurement data were expressed as mean ± standard error (mean ± S.E.M.), and independent sample t test was used for comparison between the two groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Cellular knockdown of C5aR1 can significantly reduce the expression of inflammatory genes
To determine the function of C5aR1 at the cellular level, we first treated Raw264.7 cells with lipopolysaccharide (LPS), palmitic acid (PA) + LPS or free fatty acid(FFA) + LPS for 6 h and then determined the expression level of C5aR1 in the cells by qRT-PCR22. The results showed that LPS, PA + LPS and FFA + LPS significantly upregulated the expression of C5aR1 (Fig. 4A). Furthermore, Raw264.7 was treated with C5aR1 siRNA for 18 h and then added with 100 µM PA and 25 ng/mL LPS for 6 h to detect the mRNA and protein expression levels of C5aR1. Figure 4B shows a successful knockdown of C5aR1 in mRNA level while Fig. 4C indicates a successful knockdown in protein level. We then examined the expression levels of inflammatory factors and found that knockdown of C5aR1 dramatically reduced the expression of the inflammatory factors TNF-α and IL-1β in Raw264.7 cells treated with PA + LPS (Fig. 4D,E).
Knockdown of C5aR1 proinflammatory factor in Raw264.7 cells. (A) The expression of C5aR1 was detected in Raw264.7 cells treated with 100 ng/mL LPS, 100 µM PA and 25 ng/mL LPS and FFA (OA:PA = 500:250 μmol/L) for 24 h. (B) Raw264.7 was treated with C5aR1 siRNA for 18 h and then added with 100 µM PA and 25 ng/mL LPS for 6 h to detect the expression levels of C5aR1; (C) Knockdown efficiency of C5aR1-siRNA in protein level and quantification; (D,E) Detection of TNF-α and IL-1β levels after Raw264.7 treated with C5aR1 siRNA for 18 h and then added with 100 µM PA and 25 ng/mL LPS for 6 h. All Measurement data were expressed as mean ± standard error (mean ± S.E.M.), and independent sample t test was used for comparison between the two groups.*P ≤ 0.05, **P ≤ 0.01.
Liver-specific knockdown of C5aR1 expression can significantly promote exocytosis
To verify the effect of C5aR1 on the efferocytosis of liver macrophages, TUNEL staining was performed on liver tissues from mice following C5aR1 knockdown, and the results showed that the number of TUNEL-positive cells in liver sections from C5aR1 knockdown mice was significantly lower than that in liver sections from control mice (Fig. 5A). Western blot analysis and quantification revealed that the expression level of cleaved caspase3 in the liver tissues of mice was significantly decreased after C5aR1 knockdown (Fig. 5B). Both TUNEL and Western blot analyses showed that C5aR1 knockdown significantly reduced the apoptosis of liver cells. Furthermore, F4/80 immunofluorescence and TUNEL staining were used to determine cellular efferocytosis in the liver tissues. The results showed that F4/80 expression and apoptotic cells in the livers of C5aR1 knockdown mice were strongly colocalised and were yellow, indicating that the number of apoptotic cells engulfed by liver macrophages was significantly greater than that in the control group (Fig. 5C).
Knockdown of C5aR1 promotes the efferocytosis of liver macrophages. (A) Liver TUNEL staining of AAV-NC/AAV-shC5aR1 mice on HFMCD diet for 4 weeks (n = 4); (B) Western blots and quantification of the protein expression levels of C-caspase3 (Cleaved caspase3) levels (n = 4) in livers of AAV-NC/AAV-shC5aR1 mice after 4 weeks of HFMCD diet; (C) Macrophage efferocytosis capacity was determined by liver F4/80 immunofluorescence and TUNEL staining in AAV-NC/AAV-shC5aR1 mice on HFMCD diet for 4 weeks (n = 4). (D) The expression level of NLRP3 in the liver of AAV-NC/AAV-shC5aR1 mice (n = 4); (E) Western blots and quantification of the protein expression levels of Caspase-1 and IL-1β in liver of AAV-NC/AAV-shC5aR1 mice (n = 4). (F) A schematic sketch of the methodology showing how C5aR1 affects and inflammation in liver. All measurement data were expressed as mean ± standard error (mean ± S.E.M.), and independent sample t test was used for comparison between the two groups. **P ≤ 0.01.
To further explore the mechanism by which C5aR1 regulates cellular efferocytosis, we reviewed the literature and found that C5aR1 expression is necessary for the assembly of the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome. In diabetic osteoporosis, NLRP3 can inhibit cellular efferocytosis by promoting the expression of inflammatory factors. Therefore, we first determined the expression level of NLRP3. NLRP3 expression was significantly downregulated in the liver tissues of mice after C5aR1 knockdown (Fig. 5D). Further examination revealed that both caspase-1 and IL-1β protein expression levels were significantly downregulated (Fig. 5E), suggesting that C5aR1 may regulate the efferocytosis of liver macrophages through the NLRP3/caspase-1/IL-1β inflammatory pathway. The above observations demonstrate a potential mechanism of how C5aR1 deficiency protects against diet-induced NASH by coordinating the regulation of inflammatory factors and affecting hepatic macrophage efferocytosis (Fig. 5F).
Discussion
NAFLD is the most common liver disease caused by overnutrition and obesity23. Steatosis alone in NAFLD patients is not associated with a short-term increase in morbidity or mortality, but progression to NASH significantly increases the risk of cirrhosis, liver failure, and hepatocellular carcinoma24. Therefore, controlling progression at this stage of NASH is critical. Since there are currently no specific drugs for treating NASH, this in-depth study investigated the molecular mechanism of C5aR1 in the occurrence and development of NASH, aiming to identify a new drug target for the clinical treatment of NASH.
Complement (C) proteins are a group of activated, enzymatically active proteins present in human and animal serum and tissue fluid that can bind to corresponding receptors and mediate the biological effects of complement16. The complement system plays a vital role in resisting invasion by pathogens and repairing tissue damage. When attacked by external pathogens, the complement system is activated in a cascade, and the activated fragments C5a and C5b are produced by the cleavage of intermediate C5 molecules to perform their biological functions. The effect of C5a is mainly achieved through its interaction with its receptor, complement component 5a receptor 1 (C5aR1). In this study, we collected liver samples from patients with NAFLD and NASH to verify the association of C5aR1 expression with NASH, and the results revealed that C5aR1 mRNA expression levels were dramatically greater in the livers of NASH patients than in those of NAFLD patients (Fig. 2A) and were positively correlated with serum alanine transaminase (ALT) and aspartate transaminase (AST) levels (Fig. 2B). However, as it is not ethical to collect liver tissues from healthy people as negative controls, which is a limitation of our study.
C5aR1, also known as CD88 (cluster of differentiation 88), is mainly expressed in macrophages, granulocytes, monocytes, dendritic cells, and bone marrow-derived suppressor cells of various tumour tissues and plays a key role in the innate immune response. Numerous studies have shown that targeting this process can treat a variety of inflammation-related diseases, including cancer, respiratory disease, cardiovascular disease, and liver disease. In the present study, the livers of mice decreased in size after C5aR1 knockdown, and the liver weight ratio decreased significantly (Fig. 3C,D). Moreover, the serum ALT and AST levels and the levels of the proinflammatory factors TNF-α, IL-6 and IL-1β in the liver were also significantly decreased in the mice (Fig. 3G), indicating that C5aR1 knockdown can significantly ameliorate liver injury and inflammation. In addition, C5aR1 knockdown improved the degree of inflammatory cell infiltration, vacuolar degeneration of hepatocytes, and lipid deposition as well as fibrosis in the liver (Fig. 3H).
Liver macrophages include bone marrow-derived monocytes (BMDMs) and Kupffer cells (KCs) that reside in the liver25. Under stable conditions, KCs account for 80–90% of liver macrophages and can phagocytose blood-derived antigens and senescent red blood cell fragments, thereby playing an important role in liver innate immunity and homeostasis26. Intrinsic KCs expressed F4/80hi and CD11bint, while BMDMs expressed F4/80int and CD11bhi. Single-cell sequencing results from the Human Protein Atlas (HPA) indicated that C5aR1 was mainly expressed in nonparenchymal KCs (Fig. 2D), and immunofluorescence results from our study showed that C5aR1 and CD68 were clearly colocalised. In addition, Fig. 2E shows C5aR1 expression in KCs. Hence, we determined the number of F4/80+ macrophages in the liver tissue of C5AR1-knockdown mice and found that the number of F4/80+ macrophages was significantly lower in the C5AR1-knockdown group than in the control group, suggesting the alleviation of liver inflammation (Fig. 3I).
C5aR1 mainly plays a proinflammatory role by regulating the expression of TNF-α, IL-1β and other proinflammatory factors, and NLRP3 is a key link in its proinflammatory effect. In 2016, Giuseppina Arbore et al.27 reported in Science that the NLRP3 inflammasome is assembled in human CD4 + T-cells and activates the secretion of IL-1β dependent on caspase-1 expression, thus promoting interferon-γ production and T-cofactor 1 differentiation in an autocrine manner. NLRP3 assembly requires C5aR1 stimulation. NLRP3, the most widely studied inflammasome in the NOD-like receptor family, is a multiprotein complex that responds to physiological and pathogenic stimuli in the body. Inflammasome activation plays a key role in both innate and adaptive immunity and is important for pathogen or damaged cell clearance. However, overactivation of the inflammasome can also drive the progression of autoimmune diseases28. Activation of the NLRP3 inflammasome can control the maturation and secretion of IL-1β, IL-18 and other inflammatory factors and cause liver inflammation, which is a key factor in the progression of NAFLD to NASH29. The results of the in vitro experiments in this study showed that LPS, PA + LPS and FFA + LPS enhanced the expression of C5aR1 in Raw264.7 cells (Fig. 4A), while C5aR1 knockdown decreased the levels of the inflammatory factors TNF-α and IL-1β (Fig. 4D,E). Previous studies have shown that high glucose levels can induce the expression of NLRP3 inflammation-related proteins (ASC, caspase-1, IL-18, IL-1β, and NLRP3) and inflammatory factors in osteoclasts and inhibit efferocytosis30.
Efferocytosis refers to the process in which apoptotic cells (ACs) are cleared before inflammatory mediators such as cell membrane rupture, cell necrosis, and the release of hazardous model molecules within the cell occur31. Hundreds of millions of apoptotic cells are generated in human tissues and organs during normal growth and development as well as under pathological conditions such as inflammation and ageing. Under normal circumstances, phagocytes phagocytose and eliminate them in a timely manner without causing inflammation in surrounding tissues32. However, if the efferocytosis ability of phagocytes is reduced, apoptotic cells undergo secondary necrosis, release various intracellular autoantigens and inflammatory mediators, and aggravate inflammation. Therefore, efferocytosis is of great physiological significance for the stability of the internal environment and the repair of tissue damage33. There is much evidence confirming that in many chronic inflammatory diseases, including NASH, exocytosis is defective, resulting in tissue damage and chronic inflammation34. Recent studies have shown that NASH-associated macrophages are severely deficient in their ability to engulf apoptotic liver cells. This deficiency is caused by the production of the proinflammatory cytokines TNF-α and IL-1β in the liver due to prolonged excessive nutrient intake. TNF-α and IL-1β activate the metalloproteinase ADAM17 in liver macrophages, which leads to the cleavage of the TREM2 protein and ultimately leads to the failure of macrophages to clear apoptotic, lipid-rich hepatocytes in a timely manner, thus laying the foundation for the pathological transformation of benign fatty liver to NASH35. Therefore, we explored the effect of C5aR1 on the efferocytosis of liver macrophages. We found that inhibiting the expression of C5aR1 can significantly improve the apoptosis of liver cells and can enhance apoptotic cell phagocytosis by liver macrophages, suggesting enhanced efferocytosis of liver macrophages. C5aR1 is necessary for NLRP3 inflammasome assembly, and NLRP3 is capable of inhibiting efferocytosis by promoting the expression of inflammatory factors. In this study, we demonstrated that C5aR1 knockdown can remarkably downregulate NLRP3 mRNA expression levels (Fig. 5D) as well as caspase-1 and IL-1β protein expression levels (Fig. 5E) in mouse livers, suggesting that C5aR1 may regulate the efferocytosis of liver macrophages through the NLRP3/caspase-1/IL-1β inflammatory pathway.
In conclusion, our study reveals that C5aR1 expression is abnormally elevated in diet-induced mouse models of NASH and NASH patients and was strongly positively correlated with serum ALT and AST levels in NASH patients. Specifically, knocking down C5aR1 expression in the liver significantly reduced the liver weight ratio and serum ALT and AST levels and improved the degree of inflammatory cell infiltration in the liver in a mouse model of NASH. After C5aR1 knockdown, the number of apoptotic cells in the livers of mice decreased, and the efferocytotic ability of liver macrophages increased, suggesting that C5aR1 may play a crucial role in the efferocytosis of liver macrophages. Furthermore, we also found that the expression levels of NLRP3, caspase-1, IL-1β and other inflammation-related factors in the liver were significantly reduced.
Previous studies on C5aR1 have focused mostly on chronic kidney disease, cardiovascular disease and cancer, but in this study we innovatively investigated the role of C5aR1 in NASH to elucidate a new mechanism by which C5aR1 regulates liver macrophage efferocytosis, which is an innovative notion for NASH research.
Materials and methods
Collection and analysis of clinical samples
Liver and serum samples from patients with NAFL and NASH at Yancheng First People's Hospital were collected and stored at − 80 °C. In this study, informed consent was obtained from all subjects and/or their legal guardian(s), and this research was approved by the ethics supervision committee.
RT-qPCR and Western blot analysis
Total RNA was extracted from liver tissues and cells using TRIzol reagent. Reverse transcription was performed using a PrimeScript™ RT kit(Takara, Japan). qPCR was performed with a SYBR Green kit, with 36B4 serving as an internal reference. The primer sequences are shown in Table 1. The transfected cells were lysed using cell lysis buffer (PRO-PREP™), shaken on ice for 30 min, and centrifuged at 14,000 × g for 15 min at 4 °C. The total protein concentration was calculated using a BCA protein assay kit (Beyotime Biotechnology, P0010). The following antibodies were used: anti-C5aR1 (Proteintech, 21316-1-AP); anti-β-actin (Proteintech, 66009-1-lg); anti-caspase-1 (Santa Cruz, sc-56036); anti-IL-1β (Santa Cruz, sc-12742); anti-tubulin (Proteintech, 66031-1-lg); and anti-caspase-3 (Proteintech, 19677-1-lg).
HFMCD diet feeding and AAV8 injection
The C57BL/6J mice used in this study were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). The mice were housed in a pathogen-free barrier facility at Jiangsu Vocational College of Medicine under a 12-h light/dark cycle at 25 °C with free access to food and water. All animal experiments were approved by the institutional animal care and use committee of Jiangsu Vocational College of Medicine. A mouse NASH model was established by feeding male mice aged 6–8 weeks an HFMCD diet (60% kcal fat with low methionine and no choline; A06071302; Research Diets, New Brunswick, USA)36 (RD Inc, A06071302) for 4 weeks. Control wild type (WT) mice were fed with normal diet(ND).
For AAV8 transfection, AAV-scramble/AAV-shC5aR1 (2 × 1012 vg/mouse; Obio Technology, Shanghai, China) was delivered by tail vein injection for 4 weeks, after which the mice were sacrificed for analysis. The sequence of the antisense shC5aR1 was as follows (5′–3′): GCCTACATCAACTGCTGTGTT. The experimental procedures were conducted in accordance with the Standard Operating Procedures for Laboratory Animal Center of JSYCMC and approved by the animal ethics committee of Jiangsu Vocational College of Medicine.
H&E staining and immunofluorescence
H&E staining
Liver tissue was fixed overnight in 4% paraformaldehyde (PFA), treated with paraffin coated, and cut into 3 μm slices, after which haematoxylin and eosin (H&E) staining was performed.
Immunofluorescence
Liver tissue was fixed with 1% PFA, dehydrated overnight in 20% sucrose solution, coated with tissue freezing medium (Leica), cut into 40 μm slices, sealed with 5% goat serum (0.3% Triton X-100), incubated with the following antibodies: anti-C5aR1 and anti-F4/80; incubated at room temperature for 3 h; washed with PBS several times; incubated with a FITC-, Cy3- or Cy5-conjugated secondary antibody for 2 h; and stained with 4,6-diamino-2-phenylindoles (DAPI, Invitrogen). After the slides were sealed with an anti-fluorescence quenching sealant, the images were observed and collected under a confocal laser microscope.
In vivo cell efferocytosis test (fluorescence TUNEL + immunofluorescence analysis of paraffin sections)
The paraffin-embedded sections were dewaxed. After equilibration at room temperature, the reaction solution was added (TDT enzyme, dUTP, buffer 1:5:50), the sections were washed with PBS several times, and the sections were uniformly covered with 3% BSA and incubated at room temperature for 30 min. After the slices were slightly dried, the tissue was covered with the corresponding secondary antibody, incubated at room temperature in the dark for 50 min, and washed with PBS several times. DAPI was added to the tissue section, which was then incubated at room temperature in the dark for 10 min and washed with PBS several times. After the slices were slightly dried, the slides were sealed with anti-fluorescence quenching solution, and the images were observed and collected under a confocal laser microscope. Nuclei stained with DAPI were blue under ultraviolet excitation, FITC fluorescein was used to label the TUNEL kit, positive apoptotic cells were green, and the F4/80 primary antibody was red.
Detection of other indices
Inflammation indices and the levels of chemokines, such as TNF-α, IL-6, and IL-1β, in tissue homogenates or serum were determined by ELISA kits, and the methods used for determination were performed according to the instructions (Sigma-Aldrich Company).
Serum ALT and AST levels were determined using a Hitachi 7180 automatic biochemical analyser. TUNEL staining was performed using a FITC fluorescein labelling TUNEL kit (Servicebio), and the positively stained apoptotic nuclei were green.
Statistical analysis
The correlations between the mRNA expression levels of C5aR1 in different liver tissues and the serum ALT and AST levels in patients were analysed using GraphPad Prism 6 (Version 6.01, GraphPad Software, Inc. URL link: https://www.graphpad.com/). Measurement data were expressed as mean ± standard error (mean ± S.E.M.), and independent sample t test was used for comparison between the two groups. A difference was considered statistically significant when P < 0.05.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request. GEO database (GSE154892) we analyzed can be obtained in the following link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE154892.
References
Younossi, Z. et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15(1), 11–20 (2018).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology. 77(4), 1335–1347 (2023).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64(1), 73–84 (2016).
Xu, X. et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target Ther. 7(1), 287 (2022).
Hu, J. et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 10(21), 9702–9720 (2020).
Brunt, E. M. & Tiniakos, D. G. Histopathology of nonalcoholic fatty liver disease. World J. Gastroenterol. 16(42), 5286–5296 (2010).
Buzzetti, E., Pinzani, M. & Tsochatzis, E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 65(8), 1038–1048 (2016).
Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology. 147(4), 765-783.e4 (2014).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24(7), 908–922 (2018).
Ratziu, V., Francque, S. & Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. J. Hepatol. 76(6), 1263–1278 (2022).
Wang, Q. et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 77(2), 312–325 (2022).
Govaere, O. et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 76(5), 1001–1012 (2022).
Daemen, S. et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 34(2), 108626 (2021).
Hu, W. et al. Serum complement factor 5a levels are associated with nonalcoholic fatty liver disease in obese children. Acta Paediatr. 107(2), 322–327 (2018).
Silva de França, F. et al. C5a–C5aR1 axis activation drives envenomation immunopathology by the Snake Naja annulifera. Front. Immunol. 12, 652242 (2021).
Ding, P. et al. C5aR1 is a master regulator in colorectal tumorigenesis via immune modulation. Theranostics. 10(19), 8619–8632 (2020).
Medler, T. R. et al. Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy. Cancer Cell. 34(4), 561-578.e6 (2018).
Carvelli, J. et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 588(7836), 146–150 (2020).
Jiang, Y. et al. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 11(1), 39 (2019).
Niyonzima, N. et al. Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci. Immunol. 6(66), eabf2489 (2021).
McCullough, R. L. et al. Differential contribution of complement receptor C5aR in myeloid and non-myeloid cells in chronic ethanol-induced liver injury in mice. Mol. Immunol. 75, 122–132 (2016).
Hwangbo, H. et al. Anti-inflammatory effect of auranofin on palmitic acid and LPS-induced inflammatory response by modulating TLR4 and NOX4-mediated NF-κB signaling pathway in RAW2647 macrophages. Int. J. Mol. Sci. 22(11), 5920 (2021).
Kanwal, F. et al. Preparing for the NASH epidemic: A call to action. Gastroenterology. 161(3), 1030-1042.e8 (2021).
Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet. 397(10290), 2212–2224 (2021).
Guilliams, M. & Scott, C. L. Liver macrophages in health and disease. Immunity. 55(9), 1515–1529 (2022).
Wang, T. & Ma, C. The hepatic macrophage pool in NASH. Cell Mol. Immunol. 18(8), 2059–2060 (2021).
Arbore, G. et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 352(6292), aad1210 (2016).
Wang, L. & Hauenstein, A. V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Aspects Med. 76, 100889 (2020).
Coll, R. C., Schroder, K. & Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43(8), 653–668 (2022).
Mao, Y. Apoptotic cell-derived metabolites in efferocytosis-mediated resolution of inflammation. Cytokine Growth Factor Rev. 62, 42–53 (2021).
Ge, Y., Huang, M. & Yao, Y. M. Efferocytosis and its role in inflammatory disorders. Front. Cell Dev. Biol. 10, 839248 (2022).
Doran, A. C., Yurdagul, A. Jr. & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 20(4), 254–267 (2020).
Gerlach, B. D. et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 33(12), 2445-2463.e8 (2021).
Wang, X. et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity. 56(1), 58-77.e11 (2023).
Gawrieh, S. et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am. J. Gastroenterol. 114(10), 1626–1635 (2019).
Li, Y. et al. DRAK2 aggravates nonalcoholic fatty liver disease progression through SRSF6-associated RNA alternative splicing. Cell Metab. 33(10), 2004-2020.e9 (2021).
Funding
This work was supported by grants from the Medical Research Project of Jiangsu Health and Health Commission (H2023069 to S.X), Medical Research Project of Yancheng Health and Health Commission (to S.X), China International Medical Exchange Foundation Medical road Hengrui Diabetes metabolism Research Fund (Z-2017-26-2202-4), Huaian City natural science research project (HAB202302 to Y.Q.), Jiangsu Province Engineering Research Center for Cardiovascular and Cerebrovascular Disease and Cancer Prevention and Control (2022), Jiangsu Higher Education Institution Innovative Research Team For Science and Technology (2023).
Author information
Authors and Affiliations
Contributions
X.S. and Y.Q. designed the experiments. X.S., Z.W.X., D.X.N., C.Y.P., S.X.P. performed the experiments. X.S., Z.W.X., Y.L.C., Y.Q. analyzed data. Y.L.C., Y.Q. contributed to discussion and wrote the manuscript. All authors approved the final manuscript. Y.L.C. and Y.Q. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, X., Zheng, W., Du, X. et al. The role of C5aR1-mediated hepatic macrophage efferocytosis in NASH. Sci Rep 14, 17232 (2024). https://doi.org/10.1038/s41598-024-68207-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68207-y
- Springer Nature Limited