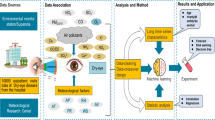
Abstract
Although previous studies have suggested that meteorological factors and air pollutants can cause dry eye disease (DED), few clinical cohort studies have determined the individual and combined effects of these factors on DED. We investigated the effects of meteorological factors (humidity and temperature) and air pollutants [particles with a diameter ≤ 2.5 \(\upmu\)m (PM2.5), ozone (O3), nitrogen dioxide (NO2), and carbon monoxide (CO)] on DED. A retrospective cohort study was conducted on 53 DED patients. DED was evaluated by Symptom Assessment in Dry Eye (SANDE), tear secretion, tear film break-up time (TBUT), ocular staining score (OSS), and tear osmolarity. To explore the individual, non-linear, and joint associations between meteorological factors, air pollutants, and DED parameters, we used generalized linear mixed model (GLMM) and Bayesian kernel machine regression (BKMR). After adjusting for all covariates, lower relative humidity or temperature was associated with a higher SANDE (p < 0.05). Higher PM2.5, O3, and NO2 levels were associated with higher SANDE and tear osmolarity (p < 0.05). Higher O3 levels were associated with lower tear secretion and TBUT, whereas higher NO2 levels were associated with higher OSS (p < 0.05). BKMR analyses indicated that a mixture of meteorological factors and air pollutants was significantly associated with increased SANDE, OSS, tear osmolarity, and decreased tear secretion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Dry eye disease (DED) is a multifactorial condition characterized by an abnormal tear film composition and inflammation of the ocular surface, leading to visual impairment1,2. The worldwide prevalence of DED was approximately 11.59% in 20213. Owing to its high prevalence, DED is considered a crucial public health issue in Asian populations4. It can result from reduced tear production and/or excessive evaporation and is associated with factors such as using contact lenses and LASIK surgery5. Recent studies have also reported that climatic factors and environmental pollutants, such as indoor gases and plastics, can influence ocular surface diseases, including DED6,7.
Particulate matter (PM), nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), and carbon monoxide (CO) are the most dangerous air pollutants8. Air pollutants and meteorological factors increase the risk of cardiorespiratory disease and mortality rates9,10,11. Because these substances are in direct contact with the ocular surface, they can also cause ocular surface diseases. Previous studies have suggested the involvement of meteorological factors (e.g., temperature and humidity) in DED development7,12. Some epidemiological studies have suggested that DED can be caused by low humidity and high temperature12,13. By contrast, other studies have identified no such association14, thus resulting in inconsistent results. In addition, these studies have demonstrated cross-sectional associations. Several clinical studies, including ours, have reported associations between particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5), O3, NO2, and higher ocular surface disease index (OSDI) scores, corneal fluorescein staining (CFS), and lower tear film break-up time (TBUT)15,16,17. Beyond the conventional clinical indicators of DED used in previous studies, whether tear osmolarity can be utilized as another clinical indicator of DED is unclear18. Therefore, we investigated whether tear osmolarity and other indicators are sensitive to meteorological factors and air pollutants.
Atmospheres undergo frequent changes depending on humidity and temperature, and they contain a complex mixture of air pollutants from various sources (e.g., automobile exhaust gases, coal-fired power fuels, and blowing dust from construction sites). Hence, atmospheric meteorological variables and pollutants exist in complex combinations rather than as individual substances19. Studies on air pollution in Korea from 2015 to 2020 have revealed the coexistence of dew and pollutants (e.g., particulate matter with an aerodynamic diameter of less than 10 μm (PM10), PM2.5, CO, SO2, SO4, O3, and NO2)20, indicating concurrent exposure of the human body to meteorological factors and air pollutants. Furthermore, some animal experimental studies have reported the synergistic interaction of air pollutants when acting commonly on inflammatory responses and subsequent genetic changes occurring on the ocular surface, causing a substantial impact. Both NO2 and PM2.5 have been reported to decrease the density of conjunctival goblet cells, and interleukins (IL)-1β, IL-6, tumor necrosis factor (TNF), and conjunctival secretory mucin expressions were significantly increased in dendritic cells on exposure to both NO2 and O321,22,23. However, previous studies have only explored the combined effects of meteorological factors and air pollutants in a single field. To our knowledge, no clinical cohort study has examined the effects of meteorological factors and air pollutant mixtures on DED.
Therefore, this study aimed to investigate the association between meteorological factors (relative humidity and temperature) and air pollutants (PM2.5, O3, NO2, and CO) when exposed individually or in combination with DED.
Materials and methods
Study design and participants
This retrospective cohort study was conducted on patients with DED who sought care at the Department of Ophthalmology, Gachon University Gil Medical Center, between February 2019 and August 2021. All the participants resided in Incheon Metropolitan City and maintained their residential addresses throughout the study. The study protocol received approval from the Institutional Review Board of Gachon University Gil Medical Center (Institutional review board approval no.: GBIRB2023-173), adhered to the principles of the Declaration of Helsinki, and all participants provided written informed consent.
We included adult patients with DED (≥ 19 years) diagnosed based on the criteria outlined by the Korean Corneal Disease Study Group, requiring the presence of at least one of the following two objective dry eye disease (DED): a tear break-up time (TBUT) of ≤ 10 s or positive ocular surface staining of Oxford grade ≥ 11,2. They were prescribed 0.05% cyclosporine twice daily, and artificial tears were used as required during every visit. Among the initially recruited patients with DED, we excluded those who wore contact lenses, had received ocular surgery within 3 months before study enrollment, had a history of prior refractive surgery or other ocular surface disorders, had systemic rheumatic conditions that might impact the ocular surface, or had been prescribed glaucoma medications.
Meteorological factors and ambient air pollutant data
This study considered two meteorological parameters (relative humidity and temperature) and four air pollutants (PM2.5, O3, NO2, and CO) from February 2019 to August 2021. The Incheon metropolitan city where all participants resided has the area of 964.5 km2 and includes 17 monitoring stations in locations such as Songrim, Yeonhui, Gojan, Seongnam, Songhae, Geomdan, Songdo, Gyesan, Unseo, Nonhyeon, Dongchun, Sinheung, Wondang, Bupyeong, Guwol, Sungui, and Cheongna. No values were missing in the measurements obtained at the monitoring sites. We linked the meteorological and air pollution data from the monitoring station nearest each participant’s residential address. Hourly data for the week preceding ocular examinations were averaged using meteorological variables and air pollution factor data. This specific timeframe was selected based on our findings from a previous study17, which suggested that the week preceding the ocular examination had the most pronounced influence on dry eye signs and symptoms.
Measurement of clinical DED parameters
An experienced ophthalmologist (DH Kim) conducted clinical examinations of all patients to assess DED symptoms and signs during follow-up, with at least three follow-ups. DES were assessed using the Symptom Assessment Questionnaire in Dry Eyes (SANDE) score24. Tear secretion was measured using the Schirmer’s test without anesthesia, and TBUT was determined as the duration between the last eye blink and the moment of tear breakup after fluorescein instillation. Ocular staining scores (OSS) were assessed using the Oxford Grading Scale25. Tear osmolarity was quantified using TearLab® (TearLab™ Corp., San Diego, CA, USA) following the manufacturer's instructions26.
Statistical analysis
The patients' characteristics at baseline of the first visit are described as frequencies (%) or means (± standard deviations [SDs]). We tested the pairwise correlations between meteorological factors and air pollutant concentrations using Pearson's correlation analysis. Paired t-tests were used to compare the observed DED values between days with the lowest and highest concentrations of meteorological factors and air pollutants among the days visited by participants throughout the follow-up period.
The individual effects of meteorological factors (relative humidity and temperature) and air pollutants (PM2.5, O3, NO2, and CO) on changes in DED parameters (SANDE, TBUT, OSS, tear secretion, and tear osmolarity) were evaluated using a generalized linear mixed model (GLMM) with a random intercept for subject ID. The models were adjusted for age, sex, eye laterality, and follow-up duration.
The combined effects of meteorological factors and air pollutants on the changes in DED parameters were assessed using Bayesian kernel machine regression (BKMR). BKMR excels in managing multi-pollutant combinations and non-linear, non-additive associations between exposure and response. It also identifies key influential variables27. However, to overcome the computational limitations of BKMR with a random intercept, we used a two-stage modeling strategy28. In the first stage, linear mixed-effects models with random intercepts were fitted to estimate subject-specific DED parameters while considering correlated exposures and DED parameters within each participant over time. In the second stage, BKMR was used to explore the associations between the averaged air pollutant mixtures and subject-specific DED parameter levels from stage 1, considering correlated exposures and non-linear relationships. To determine the importance of each exposure variable in the overall mixture effect, we calculated posterior inclusion probabilities (PIPs) ranging from 0 to 1, with a set threshold of 0.529. All BKMR models were adjusted for identical covariates as in the GLMM and run with 20,000 iterations using a Markov chain Monte Carlo procedure.
All statistical analyses were performed using the SPSS (version 20.0; IBM Corporation, Armonk, NY, USA) and R software (version 4.3.1., R Development Core Team). The BKMR analyses were conducted using the “bkmr” package in the R software. Statistical significance was determined at P-value < 0.05 with a two-sided test.
Ethics approval
The authors declare that all methods were conducted in accordance with the relevant guidelines and regulations. All procedures contributing to this work were performed in accordance with the ethical standards of the relevant national and institutional committees on human experimentation and the Helsinki Declaration of 1975, as revised in 2008.
Results
Baseline characteristics of study participants
Table 1 presents the baseline patient characteristics. Altogether, 53 patients (17 men and 36 women) and their both eyes (i.e., 106 eyes) were included in this study. The participants’ mean age (± SD) was 55.1 (± 12.0) years. The mean follow-up period (± SD) was 14.6 (± 3.2) months. The mean (± SD) of the period interval between visits was 7.3 (± 3.8) months, and the mean (± SD) number of visits was 3.3 (± 0.4) times. The mean (± SDs) SANDE, TBUT, OSS, tear secretion, and tear osmolarity of participants at their first visit were 63.2 (± 24.4) points, 4.3 (± 1.3) s, 1.0 (± 0.6) points, 10.2 (± 6.5) mm, and 299.6 (± 14.4) mOsm/L, respectively. The mean (± SDs) of relative humidity, temperature, PM2.5, O3, NO2, and CO, measured 1 week before the initial visit, were 65.6 (± 10.1) %, 12.4 (± 9.2) °C, 17.2 (± 7.7) µg/m3, 22.6 (± 9.2) ppb, 26.4 (± 10.1) ppb, and 655.5 (± 150.7) ppb, respectively.
Correlation plots between meteorological factors (relative humidity and temperature) and air pollutants (PM2.5, O3, NO2, and CO) at baseline are presented in supplemental Table 1. Significant correlations were observed between relative humidity and temperature (r = 0.662). Among the air pollutants, significant correlations were observed between PM2.5 and NO2 (r = 0.688), O3 and NO2 (r = 0.327), and NO2 and CO (r = 0.332).
Comparing DED parameters between the lowest and highest concentrations of meteorological factors and air pollutants throughout the study period
Figure 1 presents a comparison of the DED parameters between the lowest and highest concentrations of meteorological factors and air pollutants across all days of the participant visit. The participant group visiting on days with the highest humidity and temperature exhibited significantly lower SANDE scores compared to those visiting on days with the lowest humidity and temperature (57.4 vs 68.4 points for humidity; 60.2 vs 69.5 points for temperature). Similar trends were observed in OSS (0.9 vs 1.1 points for humidity; 0.9 vs 1.1 points for temperature) and tear osmolarity (295.2 vs 302.6 mOsm/L for humidity; 294.3 vs 302.9 mOsm/L for temperature). Conversely, participant group visiting on days with the highest levels of PM2.5 and NO2 demonstrated significantly higher SANDE scores compared to those visiting on days with the lowest levels (73.6 vs 63.4 points for PM2.5; 69.7 vs 58.4 points for NO2). Moreover, for NO2, the participant group visiting on days with the highest levels exhibited significantly lower TBUT (4.0 vs 4.6 s) and significantly higher OSS (1.1 vs 0.8 points) and tear osmolarity (302.5 vs 295.6 mOsm/L) compared to those visiting on days with the lowest levels. Regarding O3, the participant group visiting on days with the highest levels showed significantly lower tear secretion (9.3 vs 11.0 mm) and tear osmolarity (296.1 vs 301.1 mOsm/L) compared to those visiting on days with the lowest levels.
Comparison of dry eye disease (DED) parameters between the groups of the lowest and highest concentrations of meteorological factors and air pollutants across all days of participant visitations. SANDE, symptom assessment in dry eye; TBUT, tear film breakup time; OSS, ocular staining score; PM2.5, particulate matter less than 2.5 \(\upmu\)m in aerodynamic diameter; O3, ozone; NO2, nitrogen dioxide; CO, carbon monoxide. *p < 0.05 based on paired t-test.
Individual effects of meteorological factors and air pollutants on DED parameters
Figure 2 illustrates the effects of meteorological factors and air pollutants on DED parameters in a GLMM analysis after adjusting for age, sex, eye laterality, and follow-up time. A 1% increase in relative humidity revealed a significant association with a decrease in the SANDE score (β (95% confidence interval [CI]) = − 0.431 (− 0.847, − 0.014)). A 1 °C increase in temperature demonstrated a significant association with decreases in the SANDE score (β (95% CI) = − 0.617 (− 1.063, − 0.170)), OSS (β (95% CI) = − 0.018 (− 0.031, − 0.005)), and tear osmolarity (β (95% CI) = − 0.285 (− 0.507, − 0.063)). In addition, a 1 μg/m3 increase in PM2.5 and a 1 ppb increase in O3 and NO2 indicated a significant association with increases in SANDE score (β (95% CI) = 0.601 (0.088, 1.115) for PM2.5, 0.580 (0.138, 1.023) for O3, and 0.621 (0.223, 1.019) for NO2) and tear osmolarity (β (95% CI) = 0.580 (0.138, 1.023) for PM2.5, 0.269 (0.046, 0.492) for O3, and 0.323 (0.126, 0.522) for NO2). Moreover, a 1-ppb increase in O3 indicated a significant association with decreases in tear secretion (β (95% CI) = − 0.150 (− 0.253, − 0.045)) and TBUT (β (95% CI) = − 0.030 (− 0.058, − 0.002)). A 1-pbb increase in NO2 demonstrated a significant association with an increase in OSS (β (95% CI) = 0.016 (0.004, 0.028)). CO was not associated with DED indicators.
Individual effects of meteorological factors and air pollutants on DED parameters estimated by generalized linear mixed model analyses. All models were adjusted for age, sex, eye laterality, and follow-up time. DED, dry eye disease; SANDE, symptom assessment in dry eye; TBUT, tear film breakup time; OSS, ocular staining score; PM2.5, particulate matter less than 2.5 \(\upmu\)m in aerodynamic diameter; O3, ozone; NO2, nitrogen dioxide; CO, carbon monoxide.
Joint effects of meteorological factors and air pollutants on DED parameters
Figure 3 shows the posterior mean and 95% credible intervals (95% CrIs) of the estimated changes in DED parameters when all meteorological parameters and air pollutants were set at the 50th percentile. The overall combination of meteorological factors and air pollutants was significantly associated with an increased SANDE score, OSS, and tear osmolarity when the overall combination was at or below the 70th percentile compared to the median value. Compared to the 50th percentile, participants with an overall mixture in the 70th percentile experienced increases of 10.00 points (95% CrIs = 5.06, 14.94), 0.19 points (95% CrIs = 0.03, 0.34), and 2.98 mOsm (95% CrIs = 0.80, 5.16) in SANDE score, OSS, and tear osmolarity, respectively. However, the overall mixture was associated with reduced tear secretion when the overall mixture was at or below the 50th percentile compared to when the mixture was at the observed median value. Participants with an overall mixture in the 50th percentile experienced decreases of 4.63 mm (95% CrIs = 2.43, 6.84) in tear secretion compared to those in the 30th percentile.
Joint effects of air pollutants on DED parameters estimated by Bayesian kernel machine regression. The Y-axis illustrates the estimated shift in the outcome risk when chemicals at specific percentiles are compared to those at their median. All models were adjusted for age, sex, and eye laterality. SANDE, symptom assessment in dry eye; TBUT, tear film breakup time; OSS, ocular staining score.
Table 2 presents the PIPs of the meteorological factors and air pollutants on the DED parameters. The groupPIPs of air pollutants in the SANDE score (0.7974), tear secretion (0.7556), and tear osmolarity (0.3522) were higher than those of meteorological factors (0.6660 in the SANDE score, 0.6592 in tear secretion, and 0.1610 in tear osmolarity). NO2 demonstrated the highest conditionalPIP (condPIP) (0.4653) in the SANDE score, whereas O3 exhibited the highest condPIP for tear secretion (0.5519) and tear osmolarity (0.6706). The meteorological parameters exhibited higher groupPIPs (0.6910) than the air pollutants in the OSS, surpassing the 0.5 threshold. The temperature had the highest condPIP (0.7667) in the OSS.
The exposure–response relationship of meteorological factors and air pollutants estimated using univariate exposure–response functions displays in supplementary Fig. 1. Lower humidity and temperature and higher O3, NO2, and CO concentrations were associated with an increased SANDE score. Non-linear associations were observed between lowered humidity and increased concentrations of PM2.5 and O3 in tear secretion. Higher PM2.5, O3, and NO2 concentrations were inversely associated with TBUT. In OSS, lowered temperature and increased concentrations of NO2 and CO demonstrated increased associations, whereas PM2.5 and O3 demonstrated decreased associations. Tear osmolarity indicated an increased association with humidity or CO but a decreased association with temperature or PM2.5.
The interactions within the mixture using bivariate exposure–response functions illustrates in supplementary Fig. 2. When all other chemicals were held at their median exposure levels, meteorological factors interacted with each other and the four air pollutants, thereby increasing the SANDE score and OSS and decreasing tear secretion and TBUT. In tear osmolarity, only the humidity interacts with PM2.5, increasing tear osmolarity.
Discussion
This retrospective hospital-based cohort study demonstrated that, after adjusting for all covariates, lower temperatures and higher exposure to PM2.5, O3, and NO2 were associated with worsened ocular symptoms and increased tear osmolarity. Additionally, exposure to a mixture of meteorological factors and air pollutants increased the SANDE score, OSS, and tear osmolarity and reduced tear secretion. Finally, significant interaction effects were observed between humidity, temperature, and air pollutants on the SANDE score, tear secretion, TBUT, OSS, and tear osmolarity.
Several previous studies have reported associations between meteorological factors, air pollutants (PM2.5, O3, NO2, and CO), and DED. In the Dry Eye Assessment and Management (DREAM) study conducted among US adults, CFS significantly increased with lower humidity, and TBUT significantly decreased with higher NO2 concentrations7. In a study conducted on the Taiwanese population from 2004 to 2013, CO, NO2, and temperature were significantly associated with higher DED diagnoses, whereas relative humidity was significantly associated with lower DED diagnoses30. Another cross-sectional study involving Taiwanese women who participated in the Taiwan Biobank Study from 2012 to 2019 has reported that NO2 and temperature increased the risk of dry eye syndrome31. In a cross-sectional study targeting residents of 32 Chinese cities in 2013, O3, PM2.5, and SO2 significantly influenced the prevalence of DED32. In a cohort study conducted among residents of Hangzhou, China, from 2014 to 2016, an increase in NO2 concentration of 10 µg/m3 was reported to significantly affect outpatients with DED33. In a study conducted in South Korea, our earlier cohort study among residents of Incheon revealed that O3 and PM2.5 were associated with increased ocular discomfort17. Additionally, high O3 levels and low humidity had a significant impact on the diagnosis of DED in the general Korean population34. These studies support our covariate-adjusted findings by adjusting for key covariates such as sex and age, particularly given that the associations of O3 with tear osmolarity in our study varied depending on covariate adjustment. Meanwhile, in this study, no significant associations with CO were observed, which might be attributed to the lower concentrations (655.5 ppb) than those in a previous study (9,000 ppb) that reported significant associations30. Therefore, further studies are required to investigate the effects of CO on DED at different exposure concentrations.
The impact of meteorological factors and air pollutants on DED can be explained through mechanisms elucidated in various animal experimental results and clinical reports. First, temperature is generally reported to be associated with an increase in DED30,31. Moreover, both excessively low and high temperatures compared to the optimal range of 20 °C to 32 °C can negatively impact DED35. In colder conditions, tear evaporation can occur more rapidly, and reduced blinking may hinder tears from evenly covering the ocular surface, potentially worsening DESs35,36. Therefore, unlike previous studies that observed the effects in a sub-tropical climate ranging from 20 to 30 °C, this study observed the effects at a lower temperature of < 18 °C. Therefore, this may have negatively influenced the DED development. Second, PM2.5 induces oxidative stress on the ocular surface. Exposure to PM10 and PM2.5 in mice has been demonstrated to reduce tear film stability and damage conjunctival goblet cells and corneal epithelial microvilli, leading to inflammatory responses37,38. In this study, exposure to PM2.5 increased the SANDE score and tear osmolarity. Third, O3 is an oxidizing agent, and because of its smaller particle size compared to that of proteins and lipids, it effortlessly penetrates the cornea and lacrimal glands, leading to increased levels of inflammatory cytokines39. Several experimental studies have reported elevated levels of various cytokines (IL-1α, IL-6, and TNF-α mRNA levels) mediating inflammation in corneal epithelial cells exposed to O339,40. Furthermore, in our previous study, O3 caused considerable ocular discomfort and lower tear secretion than relatively larger-sized PM2.517. Finally, NO2 is a gas that does not dissolve well in water and damages the tissues by generating reactive nitrogen-derived free radicals. Furthermore, nitrogen oxides produce nitric acid upon contact with water, thus stimulating tissues and inducing inflammatory responses41.
In this study, positive correlations were observed between the meteorological factors and air pollutants, suggesting the simultaneous exposure of humans to these factors. Additionally, the BKMR results indicated that the interaction of mixtures of meteorological parameters and air pollutants with each other significantly increased the SANDE scores, OSS, and tear osmolarity while decreasing tear secretion. Since few studies have observed concomitant meteorological parameters and air pollutants exposure on DED, we can only compare the results of this study with those of studies that used multi-pollutant models. A study conducted on residents of Hangzhou, China, has reported that simultaneous exposure to PM, SO2, NO2, and CO, after adjusting for relative humidity and temperature, significantly impacted the increase in DED outpatient cases33. Another study conducted on residents of Urumqi, China, has demonstrated that when considering simultaneous exposure to humidity, temperature, PM, SO2, NO2, CO, and O3 in a multi-pollutant model, an increase in NO2 had a significant impact on the increasing DED outpatient cases42. In our previous study, using a multi-pollutant model considering simultaneous exposure to PM2.5, PM10, and O3 after adjusting for temperature, O3 and PM2.5 were associated with increased OSDI17. However, most studies have been limited to cross-sectional designs and have not calculated the contributions of individual chemicals to DED despite the possible non-linear relationship between air pollutants and DED. Furthermore, they did not obtain any information on the interactions among these chemicals. This study is significant because it utilized repeated clinical cohort data and BKMR analyses without losing information for all patients, enabling the observation of the contributions of air pollutants to DED and the interactions among these substances.
To our knowledge, epidemiological studies confirming the effects of exposure to meteorological parameters and air pollutant mixtures on DED have not yet been conducted. Nevertheless, some in vivo, animal, and clinical experiments have confirmed the impact of simultaneous exposure to air pollutants and climatic factors on inflammatory responses and oxidative stress, which partially explains the results of the present study. First, climatic factors and air pollutants share pathways of inflammatory responses that occur on the ocular surface. Ambient temperature and relative humidity are associated with genes that control inflammation43. PM2.5, PM10, and NO2 positively correlated with OSDI, goblet cell density, and tear cytokine concentrations (IL-6)37. In vivo experimental studies using human conjunctival epithelial cells have revealed that nuclear factor-κB-mediated inflammatory responses increased on the ocular surface exposed to O339. The common inflammatory pathways on the ocular surface indicate that simultaneous exposure to these chemicals may increase the risk of developing DED. Second, some studies have identified an interaction between climatic factors and air pollutants, which lead to increased systemic inflammatory responses. Temperature and relative humidity interact to produce stronger effects on DNA methylation and control inflammation43. Short- or long-term exposure to PM2.5 and NO2 has been demonstrated to interact, resulting in increased production of inflammatory cytokines44,45. Additionally, since O3 is an oxidative gas, it can enhance the absorption of particles like PM in various epithelial cell barriers and ultimately induce systemic inflammation46,47. However, the mechanisms by which these climatic factors and air pollutants interact with ocular surface inflammation remain unclear and should be investigated in future studies.
This study had several limitations. First, we did not consider meibomian gland dysfunction to be a significant risk factor for DED. Second, we did not conduct laboratory examinations to assess inflammation related to the tear film, ocular surface, and lacrimal gland. Third, air pollution exposure affects patients differently in indoor and outdoor environments. However, since individual air pollution levels did not vary significantly with each visit and we statistically accounted for inter-individual exposure differences, the results of this study can be considered without disregarding inter-individual exposure differences. Finally, this study had a relatively small sample size. Nevertheless, we believe that this study is significant because this well-designed retrospective clinical cohort study presents the individual and joint effects of meteorological factors and air pollutants on DED using systematic statistical methods.
Conclusion
Lower temperatures and higher exposures to PM2.5, O3, and NO2 were associated with worsened ocular symptoms and increased tear osmolarity after adjusting for all covariates. Furthermore, exposure to a combination of humidity, temperature, and air pollutants significantly increased the SANDE score, OSS, and tear osmolarity while significantly decreasing tear secretion. Meteorological factors interacting with air pollutants worsen ocular symptoms and tear secretion. Further prospective clinical cohort studies involving multiple centers, various geographic regions, larger participant pools, and extended follow-up durations are necessary to elucidate this phenomenon.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Karolinska Institutet.
References
Hyon, J. Y. et al. Korean guidelines for the diagnosis and management of dry eye: Development and validation of clinical efficacy. Korean J. Ophthalmol. 28, 197–206 (2014).
Wolffsohn, J. S. et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 15, 539–574 (2017).
Papas, E. B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol. Opt. 41, 1254–1266 (2021).
Cai, Y., Wei, J., Zhou, J. & Zou, W. Prevalence and incidence of dry eye disease in Asia: A systematic review and meta-analysis. Ophthalmic Res. 65, 647–658 (2022).
NEI (National Eye Institute). Facts about dry eye. Available: https://nei.nih.gov/health/dryeye/dryeye. (2013).
Alves, M. et al. TFOS lifestyle report: impact of environmental conditions on the ocular surface. Ocul. Surf. 29, 1–52 (2023).
Berg, E. J. et al. Climatic and environmental correlates of dry eye disease severity: A report from the dry eye assessment and management (DREAM) Study. Transl Vis. Sci. Technol. 9, 25–25 (2020).
World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment. (2006).
Chen, J. & Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 143, 105974 (2020).
Hayes, R. B. et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 49, 25–35 (2020).
Huangfu, P. & Atkinson, R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 144, 105998 (2020).
Galor, A., Kumar, N., Feuer, W. & Lee, D. J. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology. 121, 972-973.e1 (2014).
Um, S. B., Kim, N. H., Lee, H. K., Song, J. S. & Kim, H. C. Spatial epidemiology of dry eye disease: Findings from South Korea. Int. J. Health Geogr. 13, 1–9 (2014).
Bakkar, M. M., Shihadeh, W. A., Haddad, M. F. & Khader, Y. S. Epidemiology of symptoms of dry eye disease (DED) in Jordan: A cross-sectional non-clinical population-based study. Cont. Lens Anterior Eye. 39, 197–202 (2016).
Hao, R. et al. Impact of air pollution on the ocular surface and tear cytokine levels: A multicenter prospective cohort study. Front. Med. 9, 909330 (2022).
Kim, Y., Paik, H. J., Kim, M. K., Choi, Y. H. & Kim, D. H. Short-term effects of ground-level ozone in patients with dry eye disease: A prospective clinical study. Cornea. 38, 1483–1488 (2019).
Kim, Y., Choi, Y. H., Kim, M. K., Paik, H. J. & Kim, D. H. Different adverse effects of air pollutants on dry eye disease: Ozone, PM2.5, and PM10. Environ. Pollut. 265, 115039 (2020).
Lemp, M. A. et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 151, 792–798 (2011).
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D. & Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525(7569), 367–371 (2015).
Allabakash, S., Lim, S., Chong, K. S. & Yamada, T. J. Particulate matter concentrations over South Korea: impact of meteorology and other pollutants. Remote Sens. 14, 4849 (2022).
Song, F., Hao, S., Gu, Y., Yao, K. & Fu, Q. Research advances in pathogenic mechanisms underlying air pollution-induced ocular surface diseases. Adv. Ophthalmol. Pract. Res. 1, 100001 (2021).
Yang, Q. et al. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 117, 109177 (2019).
Mainka, A. & Żak, M. Synergistic or antagonistic health effects of long-and short-term exposure to ambient NO2 and PM2.5: A review. Int. J. Environ. Res. Public Health. 19, 14079 (2022).
Schaumberg, D. A. et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 5, 50–57 (2007).
Bron, A. J., Evans, V. E. & Smith, J. A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 22, 640–650 (2003).
Benelli, U., Nardi, M., Posarelli, C. & Albert, T. G. Tear osmolarity measurement using the TearLab™ Osmolarity System in the assessment of dry eye treatment effectiveness. Cont. Lens Anterior Eye 33(2), 61–67 (2010).
Bobb, J. F. et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 16, 493–508 (2015).
Wang, X. et al. Exposure to heavy metals and hormone levels in midlife women: The Study of Women’s Health Across the Nation (SWAN). Environ. Pollut. 317, 120740 (2023).
Bobb, J. F., Claus Henn, B., Valeri, L. & Coull, B. A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health. 17, 1–10 (2018).
Zhong, J. Y., Lee, Y. C., Hsieh, C. J., Tseng, C. C. & Yiin, L. M. Association between dry eye disease, air pollution and weather changes in Taiwan. Int. J. Environ. Res. Public Health. 15, 2269 (2018).
Chung, C. J. et al. Exposure to ambient NO2 increases the risk of dry eye syndrome in females: An 11-year population-based study. Int. J. Environ. Res. Public Health 18, 6860 (2021).
Yu, D. et al. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: A prevalence study in China. J. Transl. Med. 17, 1–9 (2019).
Mo, Z. et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 246, 183–189 (2019).
Hwang, S. H. et al. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 134, 503–510 (2016).
Abusharha, A. A., Pearce, E. I. & Fagehi, R. Effect of ambient temperature on the human tear film. Eye Contact Lens. 42, 308–312 (2016).
Hirata, H. & Meng, I. D. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Investig. Ophthalmol. Vis. Sci. 51, 3969–3976 (2010).
Jing, D. et al. Evidence of air pollution-related ocular signs and altered inflammatory cytokine profile of the ocular surface in Beijing. Sci. Rep. 12, 18359 (2022).
Tan, G. et al. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 8, 17828–17828 (2018).
Lee, H., Kim, E. K., Kang, S. W., Kim, J. H. & Hwang, H. J. Effects of ozone exposure on the ocular surface. Free Radic. Biol. Med. 63, 78–89 (2013).
Lee, H., Kim, E. K., Kim, H. Y. & Kim, T. I. Effects of exposure to ozone on the ocular surface in an experimental model of allergic conjunctivitis. PLoS ONE 12, e0169209 (2017).
Tummanapalli, S. S. et al. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 21, 37–51 (2021).
Liang, K. et al. Association between air pollution exposure and daily outpatient visits for dry eye disease: A time-series study in Urumqi, China. Atmosphere 14, 90 (2022).
Bind, M. A. et al. Effects of temperature and relative humidity on DNA methylation. Epidemiology 25, 561 (2014).
DeMarini, D. M. Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: A review. Mutagenesis. 28, 485–505 (2013).
Huang, Y. C. T., Rappold, A. G., Graff, D. W., Ghio, A. J. & Devlin, R. B. Synergistic effects of exposure to concentrated ambient fine pollution particles and nitrogen dioxide in humans. Inhal. Toxicol. 24, 790–797 (2012).
Crobeddu, B., Aragao-Santiago, L., Bui, L. C., Boland, S. & Baeza-Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ Pollut. 230(125), 133 (2017).
Mauderly, J. L. & Samet, J. M. Is there evidence for synergy among air pollutants in causing health effects?. Environ. Health Perspect. 117, 1–6 (2009).
Funding
This work was supported by Korea Environment Industry & Technology Institute (KEITI) through Core Technology Development Project for Environmental Diseases Prevention and Management funded by Korea Ministry of Environment (MOE) (Grant No. RS-2022-KE002107).
Author information
Authors and Affiliations
Contributions
Y.H.C. (first author): Conceptualization, methodology, formal analysis, and writing of the original draft; M.S.S.: Data curation; Y.L., H.J.P, J.S.S: Data curation and methodology; Y.H.C. (co-corresponding author): Methodology, supervision, writing, review, and editing; D.H.K: Conceptualization, methodology, supervision, writing, review, and editing. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, YH., Song, MS., Lee, Y. et al. Adverse effects of meteorological factors and air pollutants on dry eye disease: a hospital-based retrospective cohort study. Sci Rep 14, 17776 (2024). https://doi.org/10.1038/s41598-024-68070-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68070-x
- Springer Nature Limited