Abstract
Prostate-Specific Antigen (PSA) based screening of prostate cancer (PCa) needs refinement. The aim of this study was the identification of urinary biomarkers to predict the Prostate Imaging—Reporting and Data System (PI-RADS) score and the presence of PCa prior to prostate biopsy. Urine samples from patients with elevated PSA were collected prior to prostate biopsy (cohort = 99). The re-analysis of mass spectrometry data from 45 samples was performed to identify urinary biomarkers to predict the PI-RADS score and the presence of PCa. The most promising candidates, i.e. SPARC-like protein 1 (SPARCL1), Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), Alpha-1-microglobulin/bikunin precursor (AMBP), keratin 13 (KRT13), cluster of differentiation 99 (CD99) and hornerin (HRNR), were quantified by ELISA and validated in an independent cohort of 54 samples. Various biomarker combinations showed the ability to predict the PI-RADS score (AUC = 0.79). In combination with the PI-RADS score, the biomarkers improve the detection of prostate carcinoma-free men (AUC = 0.89) and of those with clinically significant PCa (AUC = 0.93). We have uncovered the potential of urinary biomarkers for a test that allows a more stringent prioritization of mpMRI use and improves the decision criteria for prostate biopsy, minimizing patient burden by decreasing the number of unnecessary prostate biopsies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The screening of prostate cancer (PCa) based on the serum levels of prostate specific antigen (PSA) is limited by low specificity and sensitivity. The low specificity leads to a significant number of prostate carcinoma-free men undergoing unnecessary prostate biopsies and results in an increased incidence of low-risk tumors1,2,3,4,5. The low sensitivity is reflected in the 15% of PCa cases occurring in men with normal serum PSA levels6.
A more efficient method to evaluate the indications not only for biopsies but also for active surveillance (low-risk tumors) would significantly improve the quality of life of patients and reduce health care costs for the diagnosis and treatment of PCa. In fact, adequately targeted active surveillance has been shown to effectively reduce PCa mortality with high long-term survival rates7.
The adoption of multiparametric-magnetic resonance imaging (mpMRI) and the Prostate Imaging Reporting and Data System (PI-RADS)8,9,10 have improved patient selection for biopsy and allowed the use of MRI-based sampling methods to enhance risk stratification9,11. However, the interpretation of some lesions can be equivocal12,13,14,15,16,17,18 and its application is limited by costs and availability in specific regions19.
Current efforts in PCa research are aimed at the discovery of novel biomarkers to improve screening of PCa through the development of standard blood or urine laboratory tests that can benefit a broader number of patients and reduce their discomfort7,8,20,21,22,23,24,25,26,27,28.
An ideal screening test should be characterized by high sensitivity and thus aimed at ruling out the disease if the test result is negative, as it should rarely misclassify patients with the disease as healthy. Such a test would be particularly advantageous if the subsequent diagnostic test can lead to relevant complications for patients. More importantly, the positive result of such a screening test should not be used as a decision criterion for medical procedures, as it would lead to many false positives29.
In this study, we have analyzed mass spectrometry (MS) screening data to identify potential urinary biomarkers that can predict the PI-RADS score. We confirmed the results by ELISA for six biomarkers, and investigated whether their combination with the PI-RADS score improved the early prediction of PCa or clinically significant PCa and corroborated these results in an independent validation cohort.
Materials and methods
Patient selection, urine collection and processing
Patients with suspected PCa due to high serum PSA levels (according to the EAU guidelines) and/or abnormal digital rectal examination (DRE) results were enrolled in this study. Sample aliquots (neat urine) were collected prior to biopsy, aliquoted and stored at − 80 °C until use, without prostate massage. Patient recruitment, urine sample collection and analysis were approved by the Ethics Committee of Canton Zürich (BASEC n° 2016-00829). All men enrolled in this study underwent a transperineal template saturation prostate biopsy with additional fusion-guided targeted biopsy for suspected clinically significant PCa. Most men underwent a mpMRI prior to biopsy. Details of the biopsy and mpMRI techniques are reported in the study by Mortezavi et al.30. All MRI examinations were centrally reviewed by an experienced radiologist. A specialized uro-pathologist assessed each core separately. Evidence of Gleason pattern ≥ 4 was defined as clinically significant PCa (GS ≥ 7).
Re-analysis of mass spectrometry data
A new data analysis was performed on previously acquired whole proteome profiles of urinary samples from PCa patients obtained by DIA LC–MS/MS31. Biognosis AG (Schlieren, Switzerland) had performed mass spectrometry measurements for all urine samples including sample preparation, HPRP fractionation, shotgun LC–MS/MS for spectral library generation, HRM mass spectrometry acquisition, database search of shotgun LC–MS/MS data and spectral library generation. HRM data analysis and Raw Data analysis were performed and described in our previous work31. A new analysis of candidate biomarkers was performed based on MS1 and MS2 protein intensities, using different prioritization criteria and end-points compared to the previous study. Here, we compared the abundance of 2.651 proteins in samples from patients with PI-RADS scores 1–2 to PI-RADS scores 3–5. The analysis was based on a two sample Student’s t-test and p-values were corrected for overall FDR using the q-value approach32. Differently from our previous analysis, ranking for biomarker candidates was carried out by setting thresholds of p-values (< 0.05) and absolute average log2 ratios (> 1.75) for all end-points. In order to discriminate prostate carcinoma-free patients with a negligible number of false negatives (sensitivity > 90%), all proteins that were detected in less than three samples were excluded from further analysis. A receiver operating characteristic (ROC) analysis was performed for the selection of the biomarkers with highest diagnostic performances (AUC > 0.57, specificity > 10% at sensitivity of 100%, Fig. 1D).
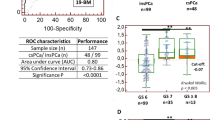
Identification of six putative urinary biomarkers by mass spectrometry for the identification of all grades or clinically significant PCa and for prediction of PI-RADS scores. (A) Left: GS distribution divided in the different groups according to PSA values (cohort of 99 patients). Right: GS distribution divided by the Pi-RADS score groups. mpMRI was performed on 94 out of the 99 patients. (B) Results of the PI-RADS scores. mpMRI was performed on 42 out of the 45 patients of the discovery cohort. (C) Distribution of GS cases grouped by the PI-RADS scores in the validation cohort. mpMRI was performed on 52 out of the 54 patients of the validation cohort. (D) Table summarizing the biomarker selection process. (E) Venn diagram showing the distribution of the candidate biomarkers across the three prediction groups. Out of the 28 proteins shared between the three groups, 6 biomarkers were selected for further studies (AMBP, CD99, HRNR, KRT13, LYVE1 and SPARCL1).
ELISA quantification
Quantification of the target biomarkers was performed by commercial kits, as described in31 for PEDF, HPX, CD99, CANX, FCER2, HRNR, and KRT13. SPARCL1 was quantified by ELISA ab272478 (Abcam, Cambridge, UK), AMBP by ABIN6953623 (antibodies-online GmbH, Germany) and LYVE1 by ab238263 (Abcam, Cambridge, UK), according to the manufacturers’ protocols. Normalization, only applied to the analysis of the single biomarkers in order to compensate for the sample variability in regards to life-style and diet habits of the individual patients, is described in our previous study31. Frozen samples were thawed and assays were performed with urine equilibrated to room temperature. Colorimetric read-out was assessed with the Epoch 2 microplate reader (BioTek, Switzerland) and analysis occurred via Gen5 software (version 2.09, BioTek, Switzerland).
Statistics and data analysis
Statistical significance was calculated with the unpaired non-parametric Mann–Whitney U test (GraphPad prism v9, GraphPad software). For the characterization of individual biomarkers, ROC curve analysis was performed applying the Wilson/Brown method, whereas for combinatorial analysis of non-correlated proteins, a multiple logistic regression was applied. The maximum number of variables, that could be combined in the algorithms avoiding over-fitting results, was calculated by use of five as events per predictor variable (EPV) in the smallest group used in each comparison33.
The correlation matrix and the correlation between mass-spectrometry intensities and ELISA concentrations were assessed with the non-parametric Spearman’s test. Non-parametric Kruskal–Wallis test was performed to check if clinical parameters were significantly different between groups.
Validation analysis
Multiple logistic analysis was performed on the discovery cohort. The resulting algorithm was applied to the validation cohort to calculate the resulting sensitivity and specificity. The algorithm resulting from each specific analysis (performance of each individual biomarker, combination of multiple variables) was applied to calculate the score of each sample of the validation cohort, at a threshold corresponding to a sensitivity of 100% and 90% in the discovery cohort. Subsequently, the samples of the validation cohort were grouped as True Positives, False Positives, True Negatives and False Negatives; these groups were used to calculate the sensitivity and specificity of the validation cohort.
Immunohistochemical staining of prostate tissues
For immunohistochemical evaluation, a representative tissue block of n = 11 prostate adenocarcinoma cases, including periurethral tumor manifestations if available, was selected and stained for specific antibodies (AMPB: dilution 1:50, polyclonal, AB, Sigma-Aldrich, Missouri, USA; SPARCL1: dilution 1:1000, clone EPR22615-277, Abcam, Cambridge, UK; LYVE1: dilution 1:100, polyclonal by R&D Systems, Minneapolis, USA). Staining and detection was performed using an automated Ventana staining system (Roche, Rotkreuz, Switzerland). Semi-quantitative evaluation for each antibody was performed by two experienced pathologists (KB, NJR). Adjacent healthy prostatic tissue was used as control for each patient. For each marker the expression in benign and malignant prostatic glands as well as periglandular stroma was evaluated separately by assigning a four-tiered score: 0 = negative, 1 = weak, 2 = moderate, 3 = strong. Where heterogeneous expression was found, the predominant expression strength was chosen.
Ethics approval and consent to participate
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Canton Zürich, Switzerland (BASEC n° 2016-00829). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Results
Patient characteristics
A total of 99 consecutive patients with suspected PCa were enrolled in this study and underwent prostate biopsy after urine sample collection. 94 patients underwent mpMRI prior to the biopsy (Table 1). The first 45 patients recruited for this study were assigned to the discovery cohort. Their urine samples have been quantified by both mass-spectrometry and ELISA. The samples collected from the 54 subsequently recruited patients were assigned to the validation cohort, and their samples were analyzed only by ELISA. At the beginning of our study, when patients were enrolled for the discovery cohort (Table 2) the PI-RADS was still being established as a decision criterion for biopsy at our institution. Therefore, also patients with a PI-RADS score of 1–2 underwent biopsies, whereas in the validation cohort (Table 3) most patients with low PI-RADS scores (1–2) did not undergo prostate biopsy. The PSA and PI-RADS performances in detecting PCa is shown in Fig. 1A–C.
Urinary biomarkers selected by mass spectrometry screening predict the PI-RADS score and the presence of prostate tumors (discovery cohort)
The comparison of the abundance of 2.651 proteins in samples from patients with PI-RADS scores 1–2 to PI-RADS scores 3–5 resulted in 78 biomarker candidates (Fig. 1D). The same strategy was applied to detect PCa (GS 6–9) and clinically significant tumors (GS 7–9, Supplementary Table 1). Due to the different selection criteria (Fig. 1D), it resulted in a different ranking compared to our previous study. Of the 78 candidates, 28 were found to consistently predict all three conditions (Fig. 1E) and only six of them, Alpha-1-microglobulin/bikunin precursor (AMBP), cluster of differentiation 99 (CD99), Hornerin (HRNR), Keratin 13 (KRT13), Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE1) and SPARC-Like protein 1 (SPARCL1), could be selected for further validation thanks to the availability of commercially ELISA kits. The performances measured by MS of the six biomarkers for the prediction of the PI-RADS score are shown in Fig. 2A and Supplementary Table 2, for the detection of all grade PCa in Supplementary Fig. 1A and Supplementary Table 2, and for detection of clinically significant tumor in Supplementary Fig. 2A and Supplementary Table 2.
Performance of the six candidate biomarkers for the prediction of the PI-RADS score in the discovery cohort. (A) Mass spectrometry quantification of the biomarkers AMBP, CD99, HRNR, KRT13, LYVE1 and SPARCL1 in the discovery cohort. Their performance in predicting the PI-RADS score was assessed with the receiver operating characteristic (ROC) analysis. (B) Validation of the candidate biomarkers with commercially available ELISA kits. The relative concentration of the biomarkers was normalized to two control molecules (CD44 and RNASE231) and results are represented as ROC curves.
The ELISA quantification demonstrates that all six biomarkers have the potential to predict the PI-RADS scores and detect carcinoma-free men with different performances and, mostly, a high sensitivity (Fig. 2B and Supplementary Table 2). The same is true for all grade PCa (Supplementary Fig. 1B and Supplementary Table 2) and clinically significant tumors (Supplementary Fig. 2B and Supplementary Table 2). All the biomarkers show decreased levels in patients with prostate carcinoma compared to controls (Supplementary Fig. 3) and, mostly, higher AUC compared to serum PSA, but not all of them (including serum PSA) show a significantly different distribution. This might be due to a less efficient normalization method compared to mass-spectrometry, or to the limited size of the cohort. The combination of multiple quantitative biomarkers is therefore a key feature of urinary tests. The Spearman’s rank correlation analysis of all variables indicates that they are independent and therefore their combination can improve the performance of the test (Figs. 3A, 4A). Here we took a conservative approach to the combination strategy by limiting the number of variables to four (for PCa prediction) and three (for PI-RADS), in order to avoid over fitting the statistical analysis31, by using five as events per predictor variable (EPV) in the smallest group used in each comparison33.
Combinatorial analysis of the selected biomarkers for the detection of the PI-RADS score in the discovery cohort. (A) Correlation matrix by Spearman's rank test showing the correlation coefficients of the six biomarkers, age and serum PSA. (B) Multiple logistic regression analysis of the ELISA quantification for two possible biomarker combinations with patient age in the discovery cohort. Both combinations (red curves) showed higher diagnostic performances compared to single biomarkers and serum PSA.
Combinatorial analysis of the selected biomarkers for the detection of all grades and clinically significant prostate cancer (PCa) in the discovery cohort. (A) Correlation matrix by Spearman's rank test showing the correlation coefficients of the possible biomarkers, age serum PSA and PI-RADS score. The fact that PI-RADS has low correlation coefficients with the urinary biomarkers explains why the ranking of candidates is different when predicting PI-RADS and PCa, as different biomarkers have different ability to identify false positive and false negative results of the multiparametric-magnetic resonance imaging. (B) For the identification of all grades of PCa in the discovery cohort, the normalization or the combination of the single biomarkers with age markedly improves their performance (AUC) compared to serum PSA. (C) The performance of the biomarkers is further increased when two biomarkers are combined together. (D) The best performance for the identification of all grades of PCa is obtained with the addition of the PI-RADS score (left) or of the PI-RADS score and an additional biomarker (right) as variables in the combination. (E) Similarly, for the identification of GS 7–9 PCa in the discovery cohort, the addition of the PI-RADS score (left) or of the PI-RADS score and an additional biomarker (right) as variables leads to better performing biomarker combinations.
All combinations achieve good performances in terms of AUC for the prediction of the PI-RADS (Fig. 3B, Supplementary Table 3). Positive correlation between the MS intensities and ELISA concentrations is shown in Supplementary Fig. 4. The weak to moderate rank correlation is explained by the fact that the ELISA can detect only a specific epitope of the target protein, whereas the MS detects any peptides present, independently of the integrity of the protein structure. Therefore, a high intensity of different peptides of the target protein might not be reflected in the presence of high levels of a specific epitope. This is particularly significant in urine samples, where proteins are expected to be more likely degraded than in normal tissue.
In the combinatorial ELISA analysis for the detection of all grade PCa and clinically significant tumors, we included the high performing biomarkers PEDF (Pigment Epithelium-Derived Factor), HPX (Hemopexin), CANX (Calnexin precursor) and FCER2 (CD23, Fc Fragment Of IgE Receptor II) identified in our previous study31. As an example, after normalization KRT13 shows an AUC of 0.81 and a specificity of 43.5% (sensitivity of 100%, Fig. 4B) for the identification of PCa. These biomarkers were identified in the same discovery cohort by applying different prioritization criteria and due to their good performance in predicting PCa, we decided to investigate their performance in combination with the new candidates identified here. The combination of two biomarkers improves their performance (Fig. 4C, Supplementary Table 4). These data indicate that such a urine test could potentially spare 39.2–47.8% of carcinoma-free men from an unnecessary prostate biopsy, without the need for a prior mpMRI and without the risk of mis-diagnosing patients affected by tumor.
In addition, individually combined with PI-RADS, each biomarker is able to increase the performance of the PI-RADS score (Supplementary Table 4 and Fig. 4D). The combination of two biomarkers with PI-RADS also achieves high performances, as can be seen for KRT13 + SPARCL1 + PI-RADS with an AUC of 0.89 and a specificity of 31.8% (sensitivity of 100%, Fig. 4D). The additional combination with age improves the AUC to 0.91 with a specificity of 31.8% and 72.7% (at sensitivity of 100% and 90%, respectively, Supplementary Table 4).
For the detection of clinically significant PCa, the biomarkers outperform serum PSA, e.g. PEDF + FCER2 + age achieve an AUC of 0.78 and a specificity of 40.7% at 100% sensitivity (Supplementary Table 5), indicating that potentially 40.7% of individuals carcinoma-free individuals or harbouring an indolent tumor could be spared from performing a prostate biopsy, without missing any clinically significant tumor. The combination with the PI-RADS score markedly improves the performance of the test (Supplementary Table 5). SPARCL1 + PI-RADS shows an AUC of 0.88 and a remarkable specificity of 74.1% (sensitivity of 100%) and adding age or a second biomarker further increases the specificity: SPARCL1 + KRT13 + PI-RADS achieves an AUC of 0.93 and specificity of 77.8% (Fig. 4E and Supplementary Table 5).
Validation analysis confirms the ability of selected urinary biomarkers to predict PI-RADS score and prostate cancer
The results of the application of the algorithms to the validation cohort at values corresponding to the thresholds of 100% and 90% sensitivity confirmed the ability of the selected urinary biomarkers to predict the PI-RADS score (Fig. 5A, Supplementary Table 6). The validation of the prediction of all grades of PCa and of clinically significant tumors are shown in Fig. 5B and C and Supplementary Tables 7 and 8.
Visual representation of the validation method of selected biomarker combinations in an independent cohort. In order to evaluate the prediction potential of the biomarkers, the algorithms trained in the discovery cohort have been applied, at the values corresponding to the threshold of 100% sensitivity, to an independent validation cohort, and by calculating the resulting sensitivity and specificity. Below the threshold, the green dots represent True Negatives and the red the False Positives, above the threshold they represent the False Negatives and True Positives, respectively. In (A) two examples of algorithms predicting the PI-RADS score, in (B) three examples for all grades of PCa and (C) three examples for clinically significant PCa.
SPARCL1 is not expressed in prostatic adenocarcinoma
The immunohistochemical analysis on prostatic tissues from 11 men (out of the 45 patients of the discovery cohort) showed that SPARCL1 protein was not detectable in malignant prostatic glands, whereas the expression in stromal areas varied from moderate to strong. SPARCL1 expression in benign prostatic glands ranged from weak to moderate. LYVE1 and AMBP showed heterogenous expression patterns (Supplementary Table 9). We observed uniform LYVE1 expression in periglandular stroma, while both, benign and malignant prostatic glands were mostly negative. AMBP expression in malignant glands was consistently weak to moderate while periglandular stroma and benign glands mostly demonstrated weak expression (Fig. 6). The expression of KRT13, CD99 and HRNR was described in our previous study31.
Immunohistochemical analysis of LYVE1, SPARCL1 and AMBP. (A) LYVE1, (B) SPARCL1 and (C) AMBP expression in three representative tissue samples. Overview: ×10 magnification; insets: ×40 magnification. Top inset: acinar adenocarcinoma/malignant prostatic glands; bottom inset (dashed lines): benign prostatic glands. Scale bars: overview 200 µm, inset 50 µm.
Discussion
We have identified six biomarkers through MS and ELISA that were able to predict PI-RADS scores with high performance. Our results (Fig. 2A and B, Supplementary Table 2) indicate that potentially, as seen in our cohort, a simple urine test could spare a relevant number of men (in the examples: 30.8%) who would result with a PI-RADS 1–2 from an unnecessary and expensive procedure. These patients would be advised to undergo prostate biopsy and might benefit from an early diagnosis, but would at the same time be spared the mpMRI procedure that, in their specific case, would provide no additional clinical information. This would be especially beneficial for low-income countries.
The data from our cohort show that, based on PI-RADS score alone, 24% of patients with GS 6 tumor lesions (Fig. 1A) would remain undetected. Although GS 6 is considered low risk, several studies have shown that patients with GS 6 can develop metastasis and progress to higher grade PCa34,35,36,37,38,39,40,41. If a highly sensitive urine test could detect and distinguish prostate carcinoma-free men and patients with GS 6, the latter could be managed by active surveillance and potentially also monitored by said urine test, therefore allowing for early detection of progression to a higher grade.
For the detection of PCa of any grade, different combinations of the urinary biomarkers show a higher specificity at a sensitivity of 100% compared to the PI-RADS score, which is a crucial characteristic of a non-invasive screening test, as it should be aimed at identifying individuals not affected by the disease29. Our data show (Fig. 4B and C, Supplementary Table 4) that, in this specific cohort, we could potentially spare up to 47.8% of prostate carcinoma-free men from an unnecessary prostate biopsy, without the need for a prior mpMRI and without running the risk of missing any PCas (sensitivity of 100%, no FN). In addition, the biomarkers can improve the performance of PI-RADS in detecting carcinoma-free men, achieving a remarkable AUC of 0.89 and specificity of 31.8% (at 100% sensitivity, Fig. 4D and Supplementary Table 4).
The early detection of clinically significant PCa is critical, as these patients might benefit from prompt active treatment and, in this regard, it is relevant that the combination of the PI-RADS score with the biomarkers substantially improves its performance (Fig. 4E and Supplementary Table 5, AUC of 0.93, specificity of 77.8%, sensitivity of 100%).
Of note, the biomarkers identified in this study were selected solely based on the MS analysis, irrespective of their function in PCa. Nevertheless, it appears from the literature that, at least some of them, might play a role in the onset or progression of the disease. The role of PEDF, HPX, CD99, CANX, FCER2, HRNR, and KRT13 has been described in our previous study31. SPARCL1 expression has been shown to be decreased in high-grade/metastatic PCa and its loss was shown to increase the migratory and invasive properties of epithelial PCa cells. It also proved to be a significant, independent prognostic marker of disease progression42. As shown by Xiang and colleagues, the over-expression of SPARCL1 decreases the metastatic potential of human PCa (PC3) cells in both in vitro functional assays and in vivo experimental metastasis models43. AMBP plays a role in the regulation of inflammation and has been associated with certain cancers44,45, including PCa46,47. Lima et al.48 showed lower levels of AMBP in an MS analysis of urine samples collected from PCa patients compared to controls. LYVE-1 has been reported as a specific marker for lymphatic endothelium in prostatic tissue, benign prostatic hyperplasia and PCa tissue. Its expression was shown to be significantly lower in prostate tumors than in healthy prostate tissue49, which is consistent with the findings reported here, but its role in the development of PCa remains unclear50,51,52.
In recent years, particular attention has been focused on the glycosylation status of urinary prostatic molecules, which might be affected by infections or metabolic changes related to tumorogenesis53,54,55, but this type of investigation is not within the scope of this study, as it requires a dedicated MS analysis and sample processing and handling55.
All the biomarkers detected in this study show decreased urinary levels in patients with prostate carcinoma. This might appear as surprising findings but, as explained above, it might be in connection to the role of the molecule (loss of tumor suppressor). It was also hypothesized that reduced urinary protein levels might reflect the gland disruption caused by the tumor, resulting in a decrease of prostatic molecules released in the urine56. Since molecules from the blood flow or other tissues can be detected in urine, we have performed an IHC analysis to demonstrate that the six biomarkers can originate from the prostate. Immunohistochemistry supports our findings, but due to the limited number of samples, it was not possible to perform a significant quantitative correlation. For SPARCL1, the results are consistent with the urine analysis and the literature, confirming its role as a tumor suppressor in PCa, as demonstrated by its complete absence in tumor tissues. On the other hand, the expression of LYVE1 and AMBP is heterogeneous and, because of the small size of the cohort, it is not possible to draw a clear conclusion.
This study has some limitations. First, it is a prospective single center study, with a small sample size in both the discovery and validation cohort and an imbalance in terms of prevalence of PI-RADS score (Table 3), due to the fact that, at the time of enrollment for the validation cohort, a relevant number of patients with PI-RADS score 1–2 did not undergo prostate biopsy and consequently their samples could not be analyzed in this study. It is well-established that prediction models are highly dependent on the characteristics of the discovery and validation population and on disease prevalence22,25 and different statistical method can be more or less appropriate in order to avoid or minimize the risk of over-fitting57. Therefore, the imbalance in PI-RADS and the reduced number of true positives of PI-RADS that could be analyzed in the validation cohort has impaired the performance of the algorithm. The small sample size, which is an intrinsic characteristic of exploratory studies, became evident when performing the multiple logistic regression analysis, as the cohort size determines the number of variables that can be combined to improve the model, therefore it was not possible to evaluate the potential of all biomarkers combined together. Here, we chose a conservative approach by using five events per predictor variable (EPV) in the smallest group of comparison33, but we recognize that this method does not allow the definition of the best biomarkers or combination of biomarkers, but rather highlights their predictive potential. The analysis in larger cohorts will be essential to define the best combination of biomarkers.
Despite the limitations, the explorative analysis of our biomarkers was able to yield robust prediction models that were validated in an independent cohort. Moreover, the results were confirmed by two different and independent quantitative technologies. The performances of the biomarkers in terms of sensitivity and specificity reported here are not meant as definitive values, but it is rather intended as a proof-of-concept, outlining the potential of the urinary biomarkers and the reliability and feasibility of a MS screening to identify potential urinary biomarkers. Future studies investigating larger cohorts will be needed to confirm the true screening or diagnostic performance of the biomarkers.
Conclusion
In conclusion, we have shown proof of concept for a urine-based test that can provide many advantages such as easy serial and large volume sample collection, minimal processing, high sample stability (compared to blood)58, and low burden on patients. The biomarkers achieve high AUC and high sensitivity, which is a crucial criterion for screening tests. Future studies on larger cohorts are necessary to demonstrate the real performance of the candidate biomarkers we have identified through MS and, most likely, the final algorithms will include some of the candidates that could not be validated at this time. One could envision that such a urine test could be implemented as first-line screening test to determine which patients would benefit from mpMRI. In cases where mpMRI is not recommended, the urine test alone could serve as a tool to reach decisions for prostate biopsy recommendations with high performance. Alternatively, the urine test could be combined with the PI-RADS score, which would improve the overall predicting performance for PCa of any grade or high grade. Thus, the test could be used to identify prostate cancer-free men or patients who would benefit from Active Surveillance rather than from active treatment, providing a simple, cost-effective and non-invasive method to monitor low-grade PCa.
Data availability
All data presented in this study are available in the manuscript and in the supplementary materials. Additional information are available for bona fide researchers who request it from the authors.
Abbreviations
- AMBP:
-
Alpha-1-microglobulin/bikunin precursor
- AUC:
-
Area under the curve
- CANX:
-
Calnexin precursor
- CD44:
-
Cluster of differentiation 44
- CD99:
-
Cluster of differentiation 99
- csPCa:
-
Clinically significant prostate cancer
- DIA:
-
Data-independent acquisition
- DRE:
-
Digital rectal examination
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPV:
-
Events per predictor variable
- FA:
-
Formic acid
- FCER2:
-
Fc fragment Of IgE receptor II
- FDR:
-
False discovery rate
- FN:
-
False negative
- FP:
-
False positive
- GS:
-
Gleason score
- HE:
-
Hematoxylin–eosin
- HPLC:
-
High-performance liquid chromatography
- HPRP:
-
High-pH reversed-phase chromatography
- HPX:
-
Hemopexin
- HRM:
-
High resolution mass spectrometry
- HRNR:
-
Hornerin
- IHC:
-
Immunohistochemistry
- KRT13:
-
Keratin 13
- LC–MS:
-
Liquid chromatography–mass spectrometry
- LYVE1:
-
Lymphatic vessel endothelial hyaluronan receptor 1
- mpMRI:
-
Multiparametric-magnetic resonance imaging
- MS:
-
Mass spectrometry
- NMR:
-
Nuclear magnetic resonance
- Non-csPCa:
-
Non clinically significant prostate cancer
- PCa:
-
Prostate cancer
- PEDF:
-
Pigment epithelium-derived factor
- PI-RADS:
-
Prostate Imaging Reporting and Data System
- PSA:
-
Prostate specific antigen
- RNASE2:
-
Ribonuclease A family member 2
- ROC:
-
Receiver operating characteristic
- SPARCL1:
-
SPARC-like protein 1
- TN:
-
True negative
- TP:
-
True positive
- UV/VIS:
-
Ultraviolet–visible
References
Draisma, G. et al. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J. Natl. Cancer Inst. 95(12), 868–878 (2003).
Walz, J. et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 100(6), 1254–1258 (2007).
Loeb, S. et al. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol. 65(6), 1046–1055 (2014).
Chun, F. K. et al. Significant upgrading affects a third of men diagnosed with prostate cancer: Predictive nomogram and internal validation. BJU Int. 98(2), 329–334 (2006).
Shariat, S. F., Kattan, M. W., Vickers, A. J., Karakiewicz, P. I. & Scardino, P. T. Critical review of prostate cancer predictive tools. Future Oncol. 5(10), 1555–1584 (2009).
Thompson, I. M. et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N. Engl. J. Med. 350(22), 2239–2246 (2004).
Klotz, L. et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. 33(3), 272–277 (2015).
Polanec, S. H. et al. Can the addition of clinical information improve the accuracy of PI-RADS version 2 for the diagnosis of clinically significant prostate cancer in positive MRI?. Clin. Radiol. 75(2), 157.e151-157.e157 (2020).
Weinreb, J. C. et al. PI-RADS prostate imaging—Reporting and data system: 2015, Version 2. Eur. Urol. 69(1), 16–40 (2016).
Esen, T., Turkbey, B., Patel, A. & Futterer, J. Multiparametric MRI in prostate cancer. BioMed Res. Int. 2014, 296810 (2014).
Turkbey, B. et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 76(3), 340–351 (2019).
Rico, L. et al. PI-RADS 3 lesions: Does the association of the lesion volume with the prostate-specific antigen density matter in the diagnosis of clinically significant prostate cancer?. Urol. Oncol. 39(7), 431.e439-431.e413 (2021).
Zhang, Y., Zeng, N., Zhang, F., Huang, Y. & Tian, Y. How to make clinical decisions to avoid unnecessary prostate screening in biopsy-naïve men with PI-RADs v2 score ≤ 3?. Int. J. Clin. Oncol. 25(1), 175–186 (2020).
Rozas, G. Q., Saad, L. S., Melo, H., Gabrielle, H. A. A. & Szejnfeld, J. Impact of PI-RADS v2 on indication of prostate biopsy. Int. Braz. J. Urol. 45(3), 486–494 (2019).
Borofsky, S. et al. What are we missing? False-negative cancers at multiparametric MR imaging of the prostate. Radiology 286(1), 186–195 (2018).
Kitajima, K. et al. Prostate cancer detection with 3 T MRI: Comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J. Magn. Reson. Imaging 31(3), 625–631 (2010).
Selnæs, K. M. et al. Peripheral zone prostate cancer localization by multiparametric magnetic resonance at 3 T: Unbiased cancer identification by matching to histopathology. Investig. Radiol. 47(11), 624–633 (2012).
de Rooij, M., Hamoen, E. H., Fütterer, J. J., Barentsz, J. O. & Rovers, M. M. Accuracy of multiparametric MRI for prostate cancer detection: A meta-analysis. AJR Am. J. Roentgenol. 202(2), 343–351 (2014).
Gaziev, G. et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 117(1), 80–86 (2016).
Zhu, J. et al. Can the combination of biparametric magnetic resonance imaging and PSA-related indicators predict the prostate biopsy outcome?. Andrologia 52(10), e13734 (2020).
Polanec, S. H. et al. Can the addition of clinical information improve the accuracy of PI-RADS version 2 for the diagnosis of clinically significant prostate cancer in positive MRI?. Clin. Radiol. 75(2), 157 e151-157 e157 (2020).
Roumiguié, M. et al. Independent evaluation of the respective predictive values for high-grade prostate cancer of clinical information and RNA biomarkers after upfront MRI and image-guided biopsies. Cancers (Basel) 12(2), 285 (2020).
Liu, X., Deng, L., Zhou, L. & Peng, W. Combining prostate imaging reporting and data system, histogram analysis, and prostate-specific antigen density to determine the risk of prostate cancer in patients with prostate-specific antigen of 4 to 20 ng/mL. J. Comput. Assist. Tomogr. 43(4), 645–651 (2019).
Fan, Y. H. et al. Prostate Health Index outperforms other PSA derivatives in predicting a positive biopsy in men with tPSA <10 ng/mL: Largest prospective cohort in Taiwan. J. Chin. Med. Assoc. 82(10), 772–777 (2019).
Friedl, A. et al. Prostate-specific antigen parameters and prostate health index enhance prostate cancer prediction with the in-bore 3-T magnetic resonance imaging-guided transrectal targeted prostate biopsy after negative 12-core biopsy. Urology 110, 148–153 (2017).
Porpiglia, F. et al. In-parallel comparative evaluation between multiparametric magnetic resonance imaging, prostate cancer antigen 3 and the prostate health index in predicting pathologically confirmed significant prostate cancer in men eligible for active surveillance. BJU Int. 118(4), 527–534 (2016).
Druskin, S. C. et al. Combining Prostate Health Index density, magnetic resonance imaging and prior negative biopsy status to improve the detection of clinically significant prostate cancer. BJU Int. 121(4), 619–626 (2018).
de la Calle, C. M. et al. Clinical utility of 4Kscore(®), ExosomeDx™ and magnetic resonance imaging for the early detection of high grade prostate cancer. J. Urol. 205(2), 452–460 (2021).
Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front. Public Health 5, 307 (2017).
Mortezavi, A. et al. Diagnostic accuracy of multiparametric magnetic resonance imaging and fusion guided targeted biopsy evaluated by transperineal template saturation prostate biopsy for the detection and characterization of prostate cancer. J. Urol. 200(2), 309–318 (2018).
Alijaj, N. et al. Identification of urine biomarkers to improve eligibility for prostate biopsy and detect high-grade prostate cancer. Cancers (Basel) 14(5), 1135 (2022).
Storey, J. D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 100(16), 9440–9445 (2003).
Vittinghoff, E. & McCulloch, C. E. Relaxing the rule of ten events per variable in logistic and cox regression. Am. J. Epidemiol. 165(6), 710–718 (2006).
Grossman, D. C. et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA 319(18), 1901–1913 (2018).
Tosoian, J. J. et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J. Clin. Oncol. 29(16), 2185–2190 (2011).
Eggener, S. E. et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J. Urol. 189(1 Suppl), S19-25 (2013).
Popiolek, M. et al. Natural history of early, localized prostate cancer: A final report from three decades of follow-up. Eur. Urol. 63(3), 428–435 (2013).
Bul, M. et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur. Urol. 63(4), 597–603 (2013).
Dall’Era, M. A. et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 112(12), 2664–2670 (2008).
Sheridan, T. B., Carter, H. B., Wang, W., Landis, P. B. & Epstein, J. I. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J. Urol. 179(3), 901–904 (2008).
Lin, K., Szabo, Z., Chin, B. B. & Civelek, A. C. The value of a baseline bone scan in patients with newly diagnosed prostate cancer. Clin. Nucl. Med. 24(8), 579–582 (1999).
Hurley, P. J. et al. Secreted protein, acidic and rich in cysteine-like 1 (SPARCL1) is down regulated in aggressive prostate cancers and is prognostic for poor clinical outcome. Proc. Natl. Acad. Sci. U. S. A. 109(37), 14977–14982 (2012).
Xiang, Y. et al. SPARCL1 suppresses metastasis in prostate cancer. Mol. Oncol. 7(6), 1019–1030 (2013).
Sekikawa, S. et al. Underexpression of alpha-1-microglobulin/bikunin precursor predicts a poor prognosis in oral squamous cell carcinoma. Int. J. Oncol. 53(6), 2605–2614 (2018).
Huang, H. et al. High level of serum AMBP is associated with poor response to paclitaxel-capecitabine chemotherapy in advanced gastric cancer patients. Med. Oncol. 30(4), 748 (2013).
Fujita, K. et al. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci. Rep. 7, 42961 (2017).
Hamm, A. et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 8, 25 (2008).
Lima, T. et al. Application of proteogenomics to urine analysis towards the identification of novel biomarkers of prostate cancer: An exploratory study. Cancers (Basel) 14(8), 2001 (2022).
Trojan, L. et al. Lymph and blood vessel architecture in benign and malignant prostatic tissue: Lack of lymphangiogenesis in prostate carcinoma assessed with novel lymphatic marker lymphatic vessel endothelial hyaluronan receptor (LYVE-1). J. Urol. 172(1), 103–107 (2004).
Jiang, W. G. et al. The potential lymphangiogenic effects of hepatocyte growth factor/scatter factor in vitro and in vivo. Int. J. Mol. Med. 16(4), 723–728 (2005).
Latil, A. et al. Gene expression profiling in clinically localized prostate cancer: A four-gene expression model predicts clinical behavior. Clin. Cancer Res. 9(15), 5477–5485 (2003).
Sakai, Y. et al. Bcl-2 mediated modulation of vascularization in prostate cancer xenografts. Prostate 69(5), 459–470 (2009).
Vermassen, T. et al. Urinary prostate protein glycosylation profiling as a diagnostic biomarker for prostate cancer. Prostate 75(3), 314–322 (2015).
Vermassen, T. et al. Diagnostic accuracy of urinary prostate protein glycosylation profiling in prostatitis diagnosis. Biochem. Med. (Zagreb) 25(3), 439–449 (2015).
Lih, T. M., Dong, M., Mangold, L., Partin, A. & Zhang, H. Urinary marker panels for aggressive prostate cancer detection. Sci. Rep. 12(1), 14837 (2022).
Occhipinti, S. et al. Low levels of urinary PSA better identify prostate cancer patients. Cancers (Basel) 13(14), 3570 (2021).
Lever, J., Krzywinski, M. & Altman, N. Points of significance: Model selection and overfitting. Nat. Methods 13(9), 703–704 (2016).
Eskra, J. N., Rabizadeh, D., Pavlovich, C. P., Catalona, W. J. & Luo, J. Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis. 22(3), 362–381 (2019).
Acknowledgements
The authors would like to thank the SNSF and the Innosuisse programme for their support and Alexandra Veloudios for the collection of urine samples and the assistance in providing patient data. We are obliged to all patients for their dedicated collaboration.
Funding
This work was supported by the “Innosuisse-Swiss Innovation Agency” (40242.1 IP-LS), the “UZH Entrepreneur Fellowship” (University of Zurich, MEDEF 20018), and the “BRIDGE Proof of Concept programme” (Swiss National Science Foundation and Innosuisse, 40B1-0_203684/1).
Author information
Authors and Affiliations
Contributions
B.P. and K.B. acquired data. B.P. prepared the urine samples for mass spectrometry and ELISA measurements. B.P. performed the ELISA experiments. K.B. and N.J.R reviewed and evaluated immunohistochemistry. The collection of samples and clinical data were performed by A.R., F.D., M.V., K.S., F.D., P.M., A.G., C.P., T.H., P.O., and V.C. Substantial contributions to the conception, design, and intellectual content of the paper was made by B.P., K.B., A.H., N.J.R., L.D., M.V., D.E., and I.B. The paper was written by B.P. and I.B and significantly reviewed by K.B, A.H., C.P., N.J.R., M.V.,L.D. and D.E. All authors have read and agreed to the published version of the manuscript. All authors agree to publish this manuscript.
Corresponding author
Ethics declarations
Competing interests
Patents: This study was submitted for patent application (applicant: University of Zürich; inventors: I. Banzola, N. Alijaj, B. Pavlovic, D. Eberli). The patent application was submitted to the European patent office (application number: PCT/EP2022/086491). Conflicts of interest: N.J.R. discloses an advisory board function and receipt of honoraria from F. Hoffmann-La Roche AG. This study was submitted for patent application (applicant: University of Zürich; inventors: I. Banzola, N. Alijaj, B. Pavlovic, D. Eberli). The patent application was submitted to the European patent office (PCT/EP2022/086491). I. Banzola is founder and CEO of ONtrack Biomedical AG, a spin-off company of the University of Zurich and University Hospital Zurich, dedicated to developing PCa diagnostics. D. Eberli is also a founder of ONtrack Biomedical AG. Other authors do not have any conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pavlovic, B., Bräutigam, K., Dartiguenave, F. et al. Urine biomarkers can predict prostate cancer and PI-RADS score prior to biopsy. Sci Rep 14, 18148 (2024). https://doi.org/10.1038/s41598-024-68026-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68026-1
- Springer Nature Limited