Abstract
The development of flexible electronic devices has been a primary focus in various fields, and silver nanowire (Ag NW) networks show significant promise due to their unique electrical and mechanical properties. However, achieving well-defined and stable nanowire coatings on polymer substrates remains challenging. This work presents a novel and simple approach for directly coating Ag NWs on cyclic olefin copolymer (COC) substrates utilizing ultraviolet/ozone (UVO) treatment, a method not previously demonstrated for this specific material system up to our knowledge. The compatibility of this approach with COC eliminates the need for complex pre- and post-treatment processes, making it a more straightforward and environmentally friendly way to improve adhesion between Ag NWs and COC. The Ag NWs/COC electrodes exhibited excellent optoelectrical performance, with a high optical transmittance of 84% and a low sheet resistance of 13 Ω/sq—metrics that compare favorably to industry standards for transparent conductive films. Additionally, the Ag NWs/COC electrodes displayed excellent mechanical stability, showing no changes in sheet resistance after both tape adhesion and film bending tests. The novelty of the presented Ag NW-COC system, combined with the simplicity and environmental benefits of the UVO coating approach, as well as the demonstrated performance and stability of the resulting electrodes, make this work a significant advancement towards realizing the commercial potential of flexible electronics for biocompatible and wearable device applications.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The development of transparent and flexible electronics has drawn immense attention across various fields, including optoelectronics, sensor technologies, energy storage systems, and the creation of wearable devices with integrated sensing functionalities1,2,3,4,5. Still, Indium tin oxide (ITO) is commonly employed as a transparent conductive electrode in most electronic devices6. However, the fabrication process of ITO films is costly and complex, requiring high vacuum sputtering and high-temperature processing (typically between 300 and 400 °C) to achieve optimal metal oxide crystallization7,8,9. These processing requirements tend to restrict the use of ITO with polymer-based substrates and limit its application in flexible devices.
Moreover, the inherent brittleness of ITO makes it incompatible with the flexible configurations demanded by various applications. Consequently, researchers have explored alternative transparent conductive materials as viable replacements for ITO. These potential replacements include metal nanowires10,11,12,13,14, conductive polymers15,16, metal grids17,18, and carbon-based materials like graphene19, carbon nanotubes20,21, and reduced graphene oxide22.
Among the alternatives being investigated, silver nanowires (Ag NWs) have proven to be one of the most promising alternatives to ITO, as their properties meet the increasing requirements of the next generation of electronic devices23. Ag NWs offer comparable sheet resistance and transparency to ITO and are considered a sustainable and cost-effective option24,25. Interestingly, Ag NWs have been explored on various polymer-based substrates as viable options for next-generation flexible electronics26,27,28,29. Polymer substrates offer several advantages, including lower cost, inherent flexibility, lightweight and wearable portability, and compatibility with various fabrication techniques. Among the various polymer substrates available, PET (polyethylene terephthalate), polyethylene naphthalene (PEN), PI (polyimide)-containing aromatic structures, PDMS (polydimethylsiloxane), and thermoplastic polyurethane (TPU) are the most widely reported substrates30,31,32,33,34. Due to its favorable characteristics, another polymer that holds promise for optoelectronic devices is cyclic olefin copolymer (COC)35,36,37,38,39. COC is a polymer material consisting of ethylene and norbornene units, is known for its excellent biocompatibility, minimal water absorption, low absorption losses at terahertz (THz) frequencies, transparent optical properties, robust mechanical stability, resistance to polar solvents and acids, and cost-effectiveness. Furthermore, COC can be processed into planar films with thicknesses as low as 13 µm, creating compact and lightweight devices with enhanced flexibility, bendability, and optical properties40,41. Several studies have explored using COC as a substrate for flexible transparent electrodes, taking advantage of its material properties42,43,44,45. These efforts have demonstrated novel electrode structures, such as metal meshes embedded within COC42,43, as well as the use of 2D materials, such as Mxene, as flexible and transparent electrodes on a COC substrate, for high-performance and cost-effective, flexible electronics45.
One of the primary challenges encountered in the utilization of Ag NW films as transparent electrodes is the poor adhesion between the Ag NW network and the polymer substrates46,47.
This is due to the different surface properties of Ag NWs and polymers, which create a substantial barrier to achieve strong adhesion when depositing silver nanowires onto a substrate. Various techniques have been explored to overcome this issue, such as post-substrate surface treatments, incorporation of interfacial layers or binders, and the creation of hybrid coatings11,48,49,50.
Sintering, as a post-treatment method, is a widely applied method for overcoming the adhesion challenges between Ag NWs and polymer substrates11,51,52,53,54,55. Song et al.11 successfully employed high-intensity pulsed light (HIPL) for sintering Ag NWs onto different polymer substrates, including PET, PI, and PEN. The sintering process with HIPL has demonstrated excellent adhesion, as evidenced by embedded conductive networks that exhibit strong peel resistance against different tests. Nevertheless, the sintering method is unsuitable for heat-sensitive plastic substrates and is often complex and costly, hindering their commercial viability in manufacturing transparent conductive electrodes (TCEs).
An alternative, simpler method to enhance the adherence of Ag NWs to polymer substrates is the use of silane coupling agents48. Nam et al.48 showed that for Ag NWs that have been modified with (3-aminopropyl)trimethoxysilane (APTES), the adhesion of the nanowires on a Polyester (PE) substrate was greatly increased. Even after undergoing 2500 bending cycles, the electrical conductivity of the APTES-Ag NWs/PE electrodes remained consistently stable. This excellent adhesion was further confirmed by the tape test (peel-off test), which demonstrated consistent electrical conductivity of the film without any variations.
Hybrid coating formation was also explored to improve the adherence and the stability of Ag NWs to the polymer substrates. Nair et al.49 reported an ink formulation consisting of Ag NWs and PEDOT: PSS, which demonstrated good adhesion to PET substrates. The resulting transparent conductive electrodes printed with this composite ink exhibited excellent mechanical stability, with less than a 20% resistance variation after subjecting them to 10,000 bending cycles.
In another study by Chen et al.50, the authors developed highly flexible, transparent, and conductive PET films by applying ZnO layer to cover the Ag NW networks. The results showed that the ZnO layer effectively prevented Ag NW oxidation and served as an adhesion promotion layer that fixed the Ag NW network to the substrate. Bending tests confirmed the mechanical stability of the films, with no significant changes observed in sheet resistance (9 Ω/sq) or transmittance (92% in the visible range) even after 1000 bending cycles.
Another promising method to address the adhesion of Ag NWs to polymer substrates is the use of pre-substrate surface treatment techniques, focusing on modifying the surface chemistry of the substrate before Ag NWs deposition. Previous studies have utilized various surface activation methods, such as plasma and UVO treatments35,56,57,58,59 and chemical treatments26,60,61, to improve the adhesion of Ag NWs to polymer substrates. However, these surface activation methods alone have not achieved strong bonding between the coated Ag NWs and the polymer substrates, requiring further surface modifications to enable strong and durable adhesion26,59,62,63,64.
In this work, we explored the feasibility of using COC substrate for flexible, transparent Ag NW-based electrodes, which has not been investigated to the best of our knowledge. A simple and straightforward method is introduced for fabricating the electrodes using UVO pre-treatment followed by spin coating. The UVO pre-treatment significantly enhances the surface wettability of the COC substrate, resulting in a homogeneous coating of the Ag NWs. The Ag NWs become well-integrated and embedded into the COC surface through the UVO-induced interfacial interactions, increasing the coated film's stability. The findings of this research can contribute to the development of novel substrates and strategies for improving the performance of optoelectronic devices.
Materials and methods
Materials
D+ glucose, Polyvinylpyrrolidone (PVP) with an average molecular weight of 30,000 (30 k), isopropanol, and ethanol (absolute, > 99.8%) were obtained from Sigma-Aldrich. Silver nitrate (AgNO3) (≥ 99.9%) was obtained from Fisher Scientific, and Sodium Chloride (NaCl) was purchased from Techno PharmChem. Microposit S1813 positive photoresist was obtained from micro resist technology. COC polymer foil (thickness: 170 µm, Tg: 140 °C) was purchased from microfluidic ChipShop. A Millipore-Q system produced deionized water (DI).
Ag NWs hydrothermal synthesis
Ag NWs were synthesized using a simple hydrothermal technique described in the previous work65 with some modifications. Briefly, four individual solutions were prepared in DI. During stirring, the glucose solution (1.3 M) was added to the AgNO3 solution (0.4 M). The PVP solution (0.07 M) was then added to the mixture and stirred for an additional 20 min. The NaCl solution (0.16 M) was then slowly injected dropwise into the mixture during stirring, resulting in a turbid hydrosol. This mixture was transferred to a Teflon-lined stainless-steel autoclave and heated in an oven at 160 °C for 22 h. The final product was collected by centrifugation at 2500 rpm for 30 min, followed by three rounds of washing and centrifugation with water and isopropanol (2000 rpm for 2 min each). Finally, the purified product was dispersed in 10 ml of ethanol and sonicated for about 2 min for further coating applications.
Ag NWs coating process
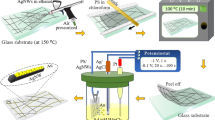
The process of coating Ag NWs on COC films is schematically shown in Fig. 1. First, the COC film was cleaned using isopropanol and kept under sonication for 10 min. Next, the film was treated with a UVO cleaner (Ossila, Model: L2002A3) for 10 min with emitted UV wavelengths of 185 nm and 254 nm, with an intensity of 15 mW·cm−2 at 185 nm. Following the UVO treatment, the Ag NWs dispersion was vortexed for about 2 min. Then, 0.5 ml of Ag NWs dispersion was applied on top of the film during spin coating at 1200 rpm for 60 s. This step was repeated three times, with air drying employed following each deposition. Finally, the coated film was cured at 110 °C for 30 min, cleaned with ethanol, and dried with compressed nitrogen.
Ag NWs/COC electrodes patterning
The reported implemented technique’s feasibility and simplicity were demonstrated by using photolithography to fabricate electrodes and other microfeatures with silver nanowires. Photolithography offers precise control over pattern definition and feature dimensions and is compatible with COC. By incorporating Ag NWs into this process, the aim is to showcase the compatibility of the technique with existing microfabrication methods while also highlighting its potential for applications in flexible electronics technologies. Figure 2 shows a schematic diagram describing the fabrication process of patterning Ag NWs on flexible COC films. Briefly, a COC film is initially cleaned with IPA and then spin-coated with a photoresist (Microposit S1813) (Fig. 2a). Next, the film is heated at 100 °C for 2 min (Fig. 2b) and then patterned using a direct photolithography system (Dilase 650, Kloe) as schematically shown in Fig. 2c. The film was then developed (Fig. 2d), and then treated with UVO (Fig. 2e), spin-coated with the Ag NWs solution (Fig. 2f), and heated (Fig. 2g). Finally, the photoresist layer is stripped off using ethanol, and the film is dried with compressed nitrogen gas revealing the Ag NW electrodes (Fig. 2h).
Schematic diagram for the fabrication process of Ag NW-based electrodes on a COC film. (a) Spin-coating of positive photoresist layer; (b) heating the COC film at 100 °C for 2 min; (c) patterning the photoresist layer via photolithography; (d) developing the photoresist; (e) patterned COC film UVO treatment; (f) spin-coating Ag NWs; (g) heating at 110 °C for 30 min; (h) stripping the photoresist with ethanol and drying with N2 gas.
Characterization
The surface topography of the Ag NWs-coated samples was investigated using AC imaging mode on an ASYLUM MFP-3D atomic force microscopy (AFM). Imaging was conducted using high-frequency silicon tips with a diameter of 40 nm and a resonance frequency of 300 kHz. Samples were scanned across a 20 µm × 20 µm area at an optimized scan frequency of 0.75 Hz. The images were processed and analyzed using Gwyddion (v2.64) software to determine the Root Mean Square (RMS) surface roughness. The microstructure and coverage of the Ag NWs on the COC films were examined and assessed using scanning electron microscopy (JEOL JSM-6710FFEG-SEM). The crystalline structure of the coated samples was characterized using XRD analysis. The measurements were conducted on a Bruker D2 Phaser instrument using a scan range of 5° to 90° (2θ) with an increment of 0.015°. The optical properties of Ag NWs/COC electrodes were measured using a UV–Visible spectrophotometer (PerkinElmer, Model: Lambda 1050). The haze percentage was determined in accordance with the methodology outlined in Ref.66. The sheet resistance of the samples coated with Ag NWs was measured using a four-point probe instrument (Ossila, Model: T2001A4). For each sample, sheet resistance was measured at different locations on the substrate, and the results were averaged. The wettability of the Ag NWs/COC surfaces was evaluated using a goniometer (Ossila, Model: L2004A1). A 5 µL droplet of deionized water was deposited on each coated sample, and the resulting contact angle was measured using the tangent method with Ossila Contact Angle (v4.1.4) software. Contact angles were measured at various locations for each sample using a minimum of ten droplets, and the average contact angle was calculated. All surface characterization procedures were conducted immediately following the respective treatment methods.
Results and discussions
Structural characterization of COC coated with Ag NWs
The structural characteristics of the Ag NW films on the COC substrate were analyzed through various techniques to investigate the morphology, crystal structure, surface roughness of the Ag NW films, and the adhesion between the nanowires with the COC substrate. Figure 3a shows the scanning electron microscopy (SEM) images of the prepared Ag NWs/COC electrodes. The SEM images clearly show that the Ag NWs coated on the COC surface are densely packed, with interconnection between the nanowires. The average length of the Ag NWs was measured to be between 200 and 400 μm, while the average diameter was found to be between 40 and 60 nm, confirming the high aspect ratio of the synthesized Ag NWs. The coated Ag NWs form a homogeneously distributed network, attributed to the improved wetting on COC resulting from the formation of polar species during the UVO treatment67,68,69,70.
Structural and surface characterization of Ag NWs/COC electrodes. (a) SEM images showing the morphology and the high density of the coated Ag NWs on the COC substrate, (b) XRD analysis showing the diffraction peaks of Ag NWs, (c,d) AFM topography image and corresponding line-height profile, revealing the surface roughness and the relatively high number of junctions formed by the coated Ag NWs on the COC substrate.
Figure 3b demonstrates the X-ray diffraction (XRD) pattern of coated Ag NWs on COC substrate, which shows peaks at 2θ angles of 38° and 45° that corresponded to the characteristic peaks of Ag NWs, which are in agreement with the previous studies65,71,72. The high intense peak observed at 2θ angle of 17° corresponds to the characteristic peak of amorphous COC copolymer73.
Furthermore, a crucial requirement for transparent conducting electrodes is to possess a smooth surface topography. Atomic force microscopy (AFM) was employed to investigate coated Ag NWs onto COC films, confirming reduced surface roughness with a root mean square roughness value of 13.7 nm (Fig. 3c). These results suggest a compatible interface between the Ag NWs coating and the UVO-treated COC films. In addition, the respective line-height profile in Fig. 3d revealed a relatively high number of junctions formed by the coated Ag NWs, attributed to the dense network of Ag NWs in the COC films, as supported by the SEM results (Fig. 3a).
The wettability of the samples was evaluated using contact angle measurements, with the results presented in Table 1. The water contact angle measurements provide valuable insights into the surface wettability of the different samples. The untreated COC exhibited a high contact angle of 95 ± 3°, indicating a hydrophobic nature and poor wetting characteristics. However, after UVO treatment, the contact angle significantly decreased to 51 ± 1°, suggesting enhanced surface hydrophilicity. This improvement can be attributed to the alteration of surface properties, such as the introduction of oxygen functional groups. When Ag NWs were coated onto the COC surface, the contact angle increased to 77 ± 2° attributed to the specific characteristics of the Ag NWs coating, such as morphology and thickness of the Ag NWs coating, as well as the interactions at the interface between Ag NWs and COC74. Particularly, the standard deviations associated with the contact angle measurements of the coated COC samples were very small. This minimal deviation suggests a high degree of uniformity across the coated surfaces. The UVO treatment likely plays an important role in achieving this uniformity by promoting the homogeneous distribution of Ag NWs on the films.
Notably, an attempt was made to deposit Ag NWs onto the COC surface without prior activation through UVO treatment. However, it was observed that the solution immediately split off from the surface, indicating a lack of adhesion between the Ag NWs and the substrate surface. This behavior can be attributed to COC’s natural hydrophobicity and low surface energy. It has been shown by several studies that UVO treatment of polymer materials can be used to increase surface energy and, consequently the wettability of the surface67,68,69,70. A significant decrease in contact angle was reported after exposing COC to UVO radiation for 20 min68. XPS results showed that oxygen functionality was added to the surface. This is because the radiation breaks bonds on the COC chain, creating radicals that can then react with ozone and oxygen in the air to form oxygen-containing species68,75. In the current study, the generation of these oxygen functionalities increases the surface free energy, thereby facilitating strong bonding between Ag NWs and the COC film.
Electrical, optical, mechanical, and adhesion properties of Ag NWs/COC electrodes
The electrical conductivity of the COC films coated with Ag NWs was evaluated using four-point probe measurements (Ossila, Model: T2001A4). The average sheet resistance and conductivity of Ag NWs/COC electrodes were measured to be 12.9 ± 2.34 Ω/sq and 490.4 ± 51 S/m, respectively. The observed excellent conductivity can be attributed to the densely interconnected network of Ag NWs coated on the COC film.
The optical properties of Ag NWs/COC electrodes were investigated through the transmission spectra of the fabricated Ag NW electrodes compared to a commercially available ITO electrode (Fig. 4a). The Ag NWs/COC electrodes exhibit a transparency of approximately 84% across the visible light range. Despite exhibiting a marginally reduced transparency in the visible spectrum compared to ITO electrodes (approximately 89% transparency), the Ag NWs electrodes demonstrated adequate optical properties to be suitable for applications in optoelectronic devices.
Optical and mechanical performance evaluation of the Ag NWs/COC electrodes. (a) Optical transmittance spectra comparing the COC film, Ag NWs/COC electrode, and a reference ITO/glass electrode. (b) The haze percentage of the fabricated Ag NWs/COC electrode was measured in the visible range (380–780 nm). (c) Relative resistance variations of the Ag NWs/COC electrode under repeated bending cycles with minimal changes in their values up to 1000 bending cycles. (d) Sheet resistance changes of the Ag NWs/COC electrode during the tape peeling test with minimal changes in their values even after three peel-off cycles.
When compared to other flexible transparent electrodes reported in the literature, the transparency of our Ag NWs/COC electrodes is quite competitive. For example, Ag NWs electrodes on PET substrates have been reported to have transparencies ranging from 80 to 85%26,27,76,77. Similarly, Ag NWs networks deposited on flexible PDMS substrates have demonstrated transparencies up to 90%28,29,78. Graphene-based flexible transparent electrodes have also been demonstrated with typical transparencies of 80–90%22,79,80,81. Conductive polymer electrodes, like PEDOT: PSS on PET and PES, were reported with transparency levels of 80–95%82,83.
Additionally, haze, which measures the scattering of transmitted light, is an important property for optoelectronic device applications66. The Ag NWs/COC electrode exhibited a low haze value of around 6% across the measured wavelength range (Fig. 4b), attributed to the inherent optical properties of the Ag NWs network embedded in the COC. This low haze is a desirable optical property for many transparent electrode applications, such as display applications.
The exceptional mechanical flexibility of Ag NW network-based electrodes is a key advantage, particularly for applications in wearable devices, stretchable displays, and generally in the field of soft electronics. Bending tests on Ag NWs/COC electrodes were conducted to assess the mechanical flexibility of the prepared electrodes. The electrodes were bent repeatedly around a Mayer rod with a radius of 9.5 mm for 100 to 1000 bending cycles. The resulting variations in the electrodes’ sheet resistance are presented in Fig. 4c. The initial sheet resistance prior to bending is denoted as Ro, while R represents the measured sheet resistance after each bending cycle. Remarkably, the change in sheet resistance was minimal, with an average increase of only 1% after up to 1000 bending cycles. These findings provide evidence of the outstanding flexibility of Ag NWs/COC electrodes due to the robust adhesion of the Ag NWs on the COC substrate and the inherent characteristics of the high flexibility of the prepared Ag NWs.
To further investigate the adhesion strength between the Ag NWs films and the COC substrate, the adhesion tape test on the Ag NWs/COC electrodes is employed, as shown in Fig. 4d. During the test, a strip of commercial Scotch tape was firmly pressed onto the electrode surface to ensure complete contact. Subsequently, the tape was peeled off rapidly, and the sheet resistance was consequently measured before and after the peeling process. Interestingly, the results demonstrated that the average sheet resistance of the electrodes remained unchanged even after being subjected to the adhesive tape test up to three times. Additionally, no visible traces of Ag NWs were observed adhering to the removed tape. As shown in Fig. S1, the SEM images of the Ag NW/COC electrodes reveal no changes in the morphology and distribution of the Ag NWs even after three cycles of the peel-off test. The Ag NW network remained intact and uniformly distributed on the COC surface, with no observable delamination or detachment of the nanowires. This suggests that the Ag NWs formed a strong and reliable bond on the surface of COC, ensuring their mechanical stability on the COC substrate.
Application of Ag NWs/COC as a transparent electrode in LED devices
The excellent sheet resistance, adhesion, stability, and optical transparency of the Ag NWs coating on COC films led to the fabrication of Ag NWs transparent conductive electrodes (TCE) for flexible polymer-based wearable LED devices via photolithography using a simple lift-off method, provides a more straightforward patterning approach compared to traditional transparent conductive materials like ITO. Figure 5a shows the assembled device with the Ag NW electrodes serving as interconnections for an LED circuit. As shown in the figure, the electrodes maintained electrical connectivity even under bending conditions, allowing the LED circuit to operate without damage. Moreover, complex Ag NW patterns on a COC film (Fig. 5b) were fabricated to showcase the versatility of the proposed fabrication approach in micro-scale applications. Optical microscopy images of the patterned Ag NW electrodes (Figs. S2, S3) revealed excellent edge definition, with sharp and well-defined boundaries between the coated and uncoated regions, demonstrating this approach’s potential for high-resolution patterning. Additionally, the Ag NW coating uniformity across the patterned areas was excellent, with no visible defects or discontinuities.
Demonstration of the patterned Ag NWs/COC flexible transparent electrodes. (a) An LED-based electronic device integrated with the Ag NWs/COC electrode. (b) Different electrode patterns fabricated on the flexible Ag NWs/COC platform showcase the design flexibility. This includes a rectangular grid with a width of 300 µm and concentric circles with thicknesses of 500 µm.
While the Ag NW/COC system has shown promising results, some challenges may arise. The COC combined with Ag NWs is a new material system for this application, and its long-term stability, particularly under environmental stresses like temperature, humidity, and UV exposure, requires further investigation. Additionally, COC may deform or degrade at higher temperatures (above the glass transition temperature), limiting the range of thermal treatments applicable during fabrication and applications. Moreover, the scalability of the fabrication approach for large-area or high-volume production would also need to be further evaluated.
Conclusion
Developing flexible optoelectronic devices requires innovative transparent conductive electrodes that address the limitations of brittle materials like ITO. These electrodes should possess specific properties: cost-effectiveness, processability through solution-based methods, high transparency, high electrical conductivity, a smooth surface, and compatibility with low-cost patterning techniques. While silver nanowires have shown promise, achieving well-defined and stable nanowire coatings on various polymer substrates remains challenging. In this work, for the first time, we explored the application of UVO treatment to directly coat Ag NWs on COC substrates. This simple and environmentally friendly method provides a practical solution, eliminating the need for complex pre- and post-treatment processes required for other polymer/nanowire systems. The strong adhesion between the Ag NWs and the COC substrate is a key advantage, highlighting COC’s potential as an enabling material for robust coatings and films. The resulting Ag NWs/COC electrodes demonstrated excellent properties, including high optical transmittance, low sheet resistance, a smooth surface, and high mechanical stability. Importantly, the electrodes maintained their performance even after tape-peeling and bending tests, emphasizing their suitability for flexible electronic applications. As a proof-of-concept, we also successfully fabricated an LED circuit using the Ag NW/COC electrodes as interconnections showing the same light intensity with bending. The novelty of the Ag NW-COC system, combined with the simplicity and environmental benefits of the UVO coating approach and the demonstrated high performance of the electrodes, make this work a significant advancement towards realizing the commercial potential of flexible electronics. Future research directions could explore the scalability of this method and investigate the integration of these electrodes into a wider range of flexible optoelectronic devices and systems. Moreover, further research is needed to investigate the long-term stability and durability of the AgNW-COC system under realistic operating conditions and environmental factors.
Data availability
All data generated or analyzed during this study are included in this present article and are available from the corresponding author upon reasonable request.
References
Hecht, D. S., Hu, L. & Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 23(13), 1482–1513 (2011).
Azani, M. R., Hassanpour, A. & Torres, T. Benefits, problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 10(48), 2002536 (2020).
Jung, E. et al. Roll-to-roll preparation of silver-nanowire transparent electrode and its application to large-area organic light-emitting diodes. Org. Electron. 41, 190–197 (2017).
Gao, W., Ota, H., Kiriya, D., Takei, K. & Javey, A. Flexible electronics toward wearable sensing. Acc. Chem. Res. 52(3), 523–533 (2019).
Wen, D. et al. Inkjet printing transparent and conductive MXene (Ti3C2Tx) films: A strategy for flexible energy storage devices. ACS Appl. Mater. Interfaces 13(15), 17766–17780 (2021).
Kim, J. et al. Transparent electrodes based on spray coated fluorine-doped tin oxide with enhanced optical, electrical and mechanical properties. J. Mater. Chem. C 8(41), 14531–14539 (2020).
Gaynor, W., Burkhard, G. F., McGehee, M. D. & Peumans, P. Smooth nanowire/polymer composite transparent electrodes. Adv. Mater. 23(26), 2905–2910 (2011).
Paine, D. C. et al. A study of low temperature crystallization of amorphous thin film indium–tin–oxide. J. Appl. Phys. 85(12), 8445–8450 (1999).
Lewis, B. G. & Paine, D. C. Applications and processing of transparent conducting oxides. MRS Bull. 25(8), 22–27 (2000).
Ding, S. et al. One-step fabrication of stretchable copper nanowire conductors by a fast photonic sintering technique and its application in wearable devices. ACS Appl. Mater. Interfaces 8(9), 6190–6199 (2016).
Song, C.-H., Han, C. J., Ju, B.-K. & Kim, J.-W. Photoenhanced patterning of metal nanowire networks for fabrication of ultraflexible transparent devices. ACS Appl. Mater. Interfaces 8(1), 480–489 (2016).
Park, J. H. et al. Flash-induced self-limited plasmonic welding of silver nanowire network for transparent flexible energy harvester. Adv. Mater. 29(5), 1603473 (2017).
Li, X., Wang, Y., Yin, C. & Yin, Z. Copper nanowires in recent electronic applications: Progress and perspectives. J. Mater. Chem. C 8(3), 849–872 (2020).
Kim, D. et al. Biocompatible cost-effective electrophysiological monitoring with oxidation-free Cu–Au core–shell nanowire. Adv. Mater. Technol. 5(12), 2000661 (2020).
Vosgueritchian, M., Lipomi, D. J. & Bao, Z. Highly conductive and transparent PEDOT: PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 22(2), 421–428 (2012).
Li, P., Sun, K. & Ouyang, J. Stretchable and conductive polymer films prepared by solution blending. ACS Appl. Mater. Interfaces 7(33), 18415–18423 (2015).
Zhu, Y., Sun, Z., Yan, Z., Jin, Z. & Tour, J. M. Rational design of hybrid graphene films for high-performance transparent electrodes. ACS Nano 5(8), 6472–6479 (2011).
Wu, W., Tassi, N. G., Walls, D. J., Zhang, L. & Willner, B. High performance transparent conductor of graphene wrapped copper/nickel microgrids. Appl. Phys. Lett. 105, 22 (2014).
Wu, J. et al. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 4(1), 43–48 (2010).
Ng, M. A., Hartadi, L. T., Tan, H. & Poa, C. P. Efficient coating of transparent and conductive carbon nanotube thin films on plastic substrates. Nanotechnology 19(20), 205703 (2008).
Tran, N.-H., Nguyen, V. C., Lee, J.-H., Song, J.-I. & Kim, H.-C. High-performance flexible smart window based on copper nanowire/multi-walled carbon nanotube transparent conducting film. J. Mater. Sci. 58(13), 5678–5692 (2023).
Becerril, H. A. et al. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2(3), 463–470 (2008).
Zhang, L. et al. Recent progress for silver nanowires conducting film for flexible electronics. J. Nanostruct. Chem. 11(3), 323–341 (2021).
Jin, Y. et al. Cohesively enhanced conductivity and adhesion of flexible silver nanowire networks by biocompatible polymer sol–gel transition. Adv. Funct. Mater. 25(10), 1581–1587 (2015).
Duan, S., Zhang, L., Wang, Z. & Li, C. One-step rod coating of high-performance silver nanowire–PEDOT: PSS flexible electrodes with enhanced adhesion after sulfuric acid post-treatment. RSC Adv. 5(115), 95280–95286 (2015).
Lian, L., Dong, D., Feng, D. & He, G. Low roughness silver nanowire flexible transparent electrode by low temperature solution-processing for organic light emitting diodes. Org. Electron. 49, 9–18 (2017).
Li, D., Han, T., Zhang, L., Zhang, H. & Chen, H. Flexible transparent electrodes based on silver nanowires synthesized via a simple method. R. Soc. Open Sci. 4, 170756 (2017).
Cui, N. et al. Stretchable transparent electrodes for conformable wearable organic photovoltaic devices. Flex. Electron. 5, 31 (2021).
Yan, X. et al. Ultra-thin foldable transparent electrodes composed of stacked silver nanowires embedded in polydimethylsiloxane. Mater. Res. Express 9, 015006 (2022).
Spechler, J. A., Koh, T. W., Herb, J. T., Rand, B. P. & Arnold, C. B. A transparent, smooth, thermally robust, conductive polyimide for flexible electronics. Adv. Funct. Mater. 25(48), 7428–7434 (2015).
Cho, D.-Y., Shin, Y.-H., Kim, H.-K. & Technology, C. Highly transparent Si-doped In2O3 films prepared on PET substrate using roll-to-roll sputtering. Surf. Coat. Technol. 259, 109–112 (2014).
Dong, X. et al. Flexible nonfullerene organic solar cells based on embedded silver nanowires with an efficiency up to 11.6%. J. Mater. Chem. A. 7(5), 1989–1995 (2019).
Kang, S.-B., Kim, H.-J., Noh, Y.-J., Na, S.-I. & Kim, H.-K. Face-to-face transferred multicrystalline ITO films on colorless polyimide substrates for flexible organic solar cells. Nano Energy 11, 179–188 (2015).
Weerasinghe, H. C., Sirimanne, P. M., Simon, G. P. & Cheng, Y. B. Cold isostatic pressing technique for producing highly efficient flexible dye-sensitised solar cells on plastic substrates. Res. Appl. 20(3), 321–332 (2012).
Dawaymeh, F., Abbas, Y., Khaleel, M., Alazzam, A. & Alamoodi, N. J. P. Tuning the surface wettability of cyclic olefin copolymer by plasma treatment and graphene oxide deposition and reduction. Polymers 13(14), 2305 (2021).
Agha, A., Dawaymeh, F., Alamoodi, N. & Alazzam, A. Enhancing fabrication of hybrid microfluidic devices through silane-based bonding: A focus on PDMS-COC and PDMS-LiNbO3. Appl. Res. 1, e202300116 (2024).
Dawaymeh, F., Ayoub, E., Alazzam, A., Khaleel, M. & Alamoodi, N. Destabilization of Enhanced Oil Recovery Nanofluid Emulsions Using Graphene Oxide-Patterned Microchannels. Available at SSRN 4736028.
Agha, A. et al. A review of cyclic olefin copolymer applications in microfluidics and microdevices. Macromol. Mater. Eng. 307(8), 2200053 (2022).
Agha, A., Dawaymeh, F., Alamoodi, N., Abu-Nada, E. & Alazzam, A. Microfluidic fabrication using cyclic olefin copolymer and hydrocarbon solvents. Eur. Polym. J. 196, 112329 (2023).
Donati, F., Pucci, A., Boggioni, L., Tritto, I. & Giacomo, R. New cyclic olefin copolymer for the preparation of thermally responsive luminescent films. Macromol. Chem. Phys. 210, 728–735 (2009).
Khanarian, G. & Celanese, H. Optical properties of cyclic olefin copolymers. Opt. Eng. 40, 1024–1029 (2001).
Khan, A. et al. High-performance flexible transparent electrode with an embedded metal mesh fabricated by cost-effective solution process. Small 12(22), 3021–3030 (2016).
Khan, A. et al. Template-electrodeposited and imprint-transferred microscale metal-mesh transparent electrodes for flexible and stretchable electronics. Adv. Eng. Mater. 21(12), 1900723 (2019).
Yu, H. H., Hwang, S.-J. & Hwang, K.-C. Preparation and characterization of a novel flexible substrate for OLED. Opt. Commun. 248(1), 51–57 (2005).
Waheed, W., Anwer, S., Khan, M. U., Sajjad, M. & Alazzam, A. 2D Ti3C2Tx-MXene nanosheets and graphene oxide based highly sensitive humidity sensor for wearable and flexible electronics. Chem. Eng. J. 480, 147981 (2024).
Kim, D.-G., Kim, J., Jung, S.-B., Kim, Y.-S. & Kim, J.-W. Electrically and mechanically enhanced Ag nanowires-colorless polyimide composite electrode for flexible capacitive sensor. Appl. Surf. Sci. 380, 223–228 (2016).
Lee, J.-C. et al. Operation range-optimized silver nanowire through junction treatment. Electron. Mater. Lett. 16, 491–497 (2020).
Nam, S., Lee, S.-M., Kim, J., Oh, I.-H. & Chang, S.-T. (3-Aminopropyl)triethoxysilane-modified silver nanowire network with strong adhesion to coating substrates for highly transparent electrodes. Coatings 11(5), 499 (2021).
Nair, N. M. et al. Printable silver nanowire and PEDOT:PSS nanocomposite ink for flexible transparent conducting applications. ACS Appl. Electron. Mater. 2(4), 1000–1010 (2020).
Chen, Y. et al. Highly flexible, transparent, conductive and antibacterial films made of spin-coated silver nanowires and a protective ZnO layer. Phys. E Low Dimens. Syst. Nanostruct. 76, 88–94 (2016).
Lee, D. J., Oh, Y., Hong, J.-M., Park, Y. W. & Ju, B.-K. Light sintering of ultra-smooth and robust silver nanowire networks embedded in poly(vinyl-butyral) for flexible OLED. Sci. Rep. 8(1), 14170 (2018).
Jiu, J. et al. High-intensity pulse light sintering of silver nanowire transparent films on polymer substrates: The effect of the thermal properties of substrates on the performance of silver films. Nanoscale 5, 11820 (2013).
Hwang, H.-J. et al. Rapid pulsed light sintering of silver nanowires on woven polyester for personal thermal management with enhanced performance, durability and cost-effectiveness. Sci. Rep. 8(1), 17159 (2018).
Jiu, J. et al. Strongly adhesive and flexible transparent silver nanowire conductive films fabricated with a high-intensity pulsed light technique. J. Mater. Chem. A 22(44), 23561–23567 (2012).
Kaikanov, M. et al. Electrical conductivity enhancement of transparent silver nanowire films on temperature-sensitive flexible substrates using intense pulsed ion beam. Nanotechnology 32(14), 145706 (2021).
Shenton, M., Lovell-Hoare, M. & Stevens, G. J. Adhesion enhancement of polymer surfaces by atmospheric plasma treatment. J. Phys. D Appl. Phys. 34(18), 2754 (2001).
Sundriyal, P., Pandey, M. & Bhattacharya, S. J. Plasma-assisted surface alteration of industrial polymers for improved adhesive bonding. Int. J. Adhes. Adhes. 101, 102626 (2020).
Ho, X., Tey, J. N., Cheng, C. K. & Wei, J. Highly flexible transparent conductors based on 2D silver nanowire network. In 2015 IEEE 65th Electronic Components and Technology Conference (ECTC) 1749–1752 (IEEE, 2015).
Sadeghianlemraski, M., Lee, B. Y., Davidson-Hall, T., Leonenko, Z. & Aziz, H. J. Enhanced photo-stability of inverted organic solar cells via using polyethylenimine in the electron extraction layers. Org. Electron. 73, 26–35 (2019).
Lai, Z. et al. Directly electroplated metallization on flexible substrates based on silver nanowire conductive composite for wearable electronics. ACS Appl. Nano Mater. 4(11), 12098–12107 (2021).
Wang, J., Jin, Y., Wang, K., Wang, X. & Xiao, F. J. Facile transfer of a transparent silver nanowire pattern to a soft substrate using graphene oxide as a double-sided adhesion-tuning layer. ACS Appl. Mater. Interfaces 15(4), 5709–5719 (2023).
Song, J. Y., Oh, J. H., Choi, D. & Park, S. M. Highly efficient patterning technique for silver nanowire electrodes by electrospray deposition and its application to self-powered triboelectric tactile sensor. Sci. Rep. 11(1), 21437 (2021).
An, E. Y. et al. Self-patterned stretchable electrode based on silver nanowire bundle mesh developed by liquid bridge evaporation. Nanomaterials 11(11), 2865 (2021).
Lee, C. et al. Flash-induced nanowelding of silver nanowire networks for transparent stretchable electrochromic devices. Sci. Rep. 8(1), 2763 (2018).
Bari, B. et al. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 4(29), 11365–11371 (2016).
Galeotti, F., Mróz, W., Catellani, M., Kutrzeba-Kotowska, B. & Kozma, E. Tailorable perylene-loaded fluorescent nanostructures: A multifaceted approach enabling their application in white hybrid LEDs. J. Mater. Chem. C 4(23), 5407–5415 (2016).
Delplanque, A. et al. UV/ozone surface treatment increases hydrophilicity and enhances functionality of SU-8 photoresist polymer. Appl. Surf. Sci. 314, 280–285 (2014).
Lin, T.-Y., Pfeiffer, T. T. & Lillehoj, P. B. Stability of UV/ozone-treated thermoplastics under different storage conditions for microfluidic analytical devices. RSC Adv. 7(59), 37374–37379 (2017).
Sham, M. L., Li, J., Ma, P. C. & Kim, J.-K. Cleaning and functionalization of polymer surfaces and nanoscale carbon fillers by UV/ozone treatment: A review. J. Compos. Mater. 43(14), 1537–1564 (2009).
Bae, G., Park, T. & Song, I.-H. Surface modification of polymethylmethacrylate (PMMA) by ultraviolet (UV) irradiation and IPA rinsing. Micromachines 13(11), 1952 (2022).
Ding, H. et al. Large scale preparation of silver nanowires with different diameters by a one-pot method and their application in transparent conducting films. RSC Adv. 6(10), 8096–8102 (2016).
Li, W., Meredov, A. & Shamim, A. Coat-and-print patterning of silver nanowires for flexible and transparent electronics. NPJ Flexible Electron. 3(1), 19 (2019).
Nazir, F., Iqbal, M., Khan, A., Mazhar, M. & Hussain, Z. Fabrication of robust poly L-lactic acid/cyclic olefinic copolymer (PLLA/COC) blends: Study of physical properties, structure, and cytocompatibility for bone tissue engineering. J. Mater. Res. Technol. 13, 1732–1751 (2021).
Yu, K. & He, T. Silver-nanowire-based elastic conductors: Preparation processes and substrate adhesion. Polymers 15(6), 1545 (2023).
Roy, S. et al. Surface analysis, hydrophilic enhancement, ageing behavior and flow in plasma modified cyclic olefin copolymer (COC)-based microfluidic devices. Sens. Actuators B Chem. 150(2), 537–549 (2010).
Park, Y. et al. Large-scale transfer of Ag nanowires from PET to PC film using a roll-to-roll UV lamination process for a capacitive touch sensor. RSC Adv. 13, 1551–1557 (2023).
Ma, Y. et al. Stability of silver-nanowire-based flexible transparent electrodes under mechanical stress. Appl. Sci. 14(1), 420 (2024).
Hao, T. et al. A stretchable, transparent, and mechanically robust silver nanowire–polydimethylsiloxane electrode for electrochromic devices. Polymers 15(12), 2640 (2023).
De, S. et al. Flexible, transparent, conducting films of randomly stacked graphene from surfactant-stabilized, oxide-free graphene dispersions. Small 6(3), 458–464 (2010).
Tung, V. C. et al. Low-temperature solution processing of graphene–carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 9(5), 1949–1955 (2009).
Li, X. et al. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat. Nanotechnol. 3(9), 538–542 (2008).
Khasim, S. et al. Post treated PEDOT-PSS films with excellent conductivity and optical properties as multifunctional flexible electrodes for possible optoelectronic and energy storage applications. Opt. Mater. 125, 112109 (2022).
Nie, S. et al. High conductivity, semiconducting, and metallic PEDOT:PSS electrode for all-plastic solar cells. Molecules 28(6), 2836 (2023).
Acknowledgements
F.D. and M. A. acknowledge Khalifa University of Science and Technology for financial support under the startup fund (FSU-2023-002) No. 8474000455.
Author information
Authors and Affiliations
Contributions
Fadi Dawaymeh: conceptualization, writing, data curation, work design, investigation, validation, editing, and review. Marwa Abd-Ellah: conceptualization, project administration, resources, writing, validation, editing and review, and funding acquisition. Anas Alazzam: resources, validation, editing, and review. Abdulrahman Agha: Investigation, validation, and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dawaymeh, F., Agha, A., Alazzam, A. et al. Exploring cyclic olefin copolymer (COC) for flexible silver nanowire electrode. Sci Rep 14, 16989 (2024). https://doi.org/10.1038/s41598-024-68019-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68019-0
- Springer Nature Limited