Abstract
Covid-19 disease is implicated in increased mortality among immunocompromised patients. The JAK inhibitor, baricitinib (bar), or the IL-6 inhibitor, tocilizumab (toc), demonstrated a survival benefit in patients with severe disease.However, evidence supporting their use in immunocompromised patients with severe Covid-19 is scarce.We aimed to assess clinical outcomes of bar/toc treatment in immunocompromised patients. A multi-center registry of consecutive immunocompromised patients hospitalized due to severe Covid-19 during the Omicron variant dominance period. After excluding patients who did not require high oxygen supply, patients treated with bar/toc were compared to patients treated by standard of care (SOC). Primary outcome was in hospital mortality. Secondary outcomes were 30 and 60 day mortality, super-infection and thromboembolic events. Among an overall 228 immunocompromised patients hospitalized in six Israeli hospitals with severe Covid-19, 112 patients required high oxygen support, of whom 48 (43%) were treated with bar/toc. In-hospital mortality rates were exceptionally high and did not significantly differ between bar/toc and SOC treated patients (62.5% vs. 64.1%, p = 1.0). A logistic regression analysis revealed that advanced age and incomplete vaccination were predictors of in-hospital mortality. Patients treated with bar/toc had no excess of suspected super-infection (62.8% vs. 60.7%, p = 0.84) or thromboembolic events (8.3% vs 3.1%, p = 0.39). In immunocompromised patients with severe Covid-19 and a high oxygen demand, bar/toc therapy was not associated with reduced mortality or with a higher rate of associated complications, compared to SOC. Larger prospective studies should better address efficacy and safety.
Similar content being viewed by others
Introduction
SARS-CoV-2 infection is associated with a hyperinflammatory state that contributes to disease severity and increased mortality rate1. Several studies have shown that the IL-1–IL-6 axis plays a pivotal role in the Covid-19-related hyperinflammatory reaction2,3, laying the rationale for using agents that suppress their release and/or action as a therapeutic modality for patients with severe Covid-19.
Several large randomized controlled trials evaluated the use of baricitinib (bar) as the leading JAK-inhibitor agent under research4,5,6. The RECOVERY meta-analysis found a 20% reduction in mortality among hospitalized patients receiving bar compared to control4.
Tocilizumab (toc) is a monoclonal antibody that inhibits binding of the proinflammatory cytokine IL-6 to its receptor. In a meta-analysis of over 10,000 patients hospitalized with Covid-19, it has been demonstrated that the addition of toc was associated with a 17% reduction in mortality7.
Data is evolving on the clinical outcomes of Covid-19 in immunocompromised patients. Analyses have found a higher risk of hospitalization or death from Covid-19 in those with a variety of conditions causing immunosuppression, including hematological malignancies, solid organ transplant recipients and HIV8,9,10. Therefore, better therapeutic modalities for severe Covid-19, especially in this group of patients, is a priority.
Most Covid-19 treatment guidelines are structured according to disease severity, but not according to host factors including host immunity11. Accordingly, the use of bar/ toc in immunocompromised patients is largely based on expert opinion12,13 while guidelines omit specific recommendations for treatment in this patient population11,14. This is a consequence of a knowledge gap regarding the efficacy of immunomodulatory drugs in these patients, due to their underrepresentation or exclusion from previous trials. Moreover, the safety of further immune modulation in patients who are already immunocompromised is uncertain.
This study is an attempt to evaluate the safety and efficacy of either bar or toc as an add-on treatment for immunocompromised patients hospitalized with severe Covid-19.
Methods
Patient population and study design
This is a retrospective study including adult immunocompromised patients who were hospitalized due to severe Covid-19 in six Israeli hospitals (Rabin medical center, Tel Aviv Sourasky medical center, Hadassah medical center, Soroka University medical center, Rambam medical center and Lady Davis Carmel medical center) between November 20th 2021-April 30th 2022 (during a surge of Covid-19 dominated by the omicron variant).
The study was approved by the local IRBs in each institute according to local regulations and the Helsinki declaration (0333-22-RMC; 0504-22-TLV; 0366-22-HMO; 0154-22-SOR; 0200-20-RMB; 0114-22-CMC).
Immunocompromised patients were defined, based on published data as those previously diagnosed with hematological malignancies, solid organ transplant recipients, primary hypogammaglobulinemia patients, people living with HIV and have CD4 T lymphocyte cell counts ≤ 200 cells/mm^3, patients treated with a B-cell depleting agent during the past 12 months and patients on a high-dose long-term corticosteroid treatment15. (A full list of immunocompromising status eligible in the study are detailed in Table S1).
Patients were defined as having severe Covid-19 infection, based on clinical guidelines, if they had a positive PCR for SARS-CoV-2 and one of the following criteria: Oxygen saturation of ≤ 93% on ambient air, respiratory rate of > 30/min or PAO2/FIO2 ≤ 30016. Patients < 18 years old or previously treated with baricitinib or tocilizumab were excluded.
Data regarding all patients with severe Covid-19 hospitalized during this time period, in whom an underlying immunosuppressive condition exists, was collected. Data was collected from electronic medical records (EMR) and included demographic information, disease characteristics and outcomes. Oxygen requirement during hospitalization was encoded based on need of low-flow nasal cannula, high-flow nasal cannula (HFNC), nonrebreather face mask, bilevel positive airway pressure (BIPAP) or invasive mechanical ventilation (IMV) at two time points: on admission and at the peak of disease (i.e., maximum oxygen support during hospitalization). The Chameleon EMR is the EMR used in all participating centers and it is automatically updated by the Ministry of Health for mortality cases, thus allowing long-term survival follow up.
In the final analysis, only patients with high oxygen requirements during hospitalization (i.e., use of HFNC, nonrebreather face mask, BIPAP or IMV) were included, in concordance with NIH guidelines recommending immunomodulation in patients with escalating oxygen requirement. This also represents our common practice to consider immunomodulation in a subset of severe Covid-19 patients in whom disease progresses rapidly.
Data collected included laboratory studies, Covid-19 directed therapies, in-hospital mortality, mortality during follow up, secondary infections and thromboembolic events.
Patients’ vaccination status was recorded based on number of vaccine doses given and their timing related to hospitalization date. With the Pfizer-BioNTech Covid-19 Vaccine being predominantly used in Israel. Up-to-date vaccination status was defined as having at least two vaccine doses with the last one given within four months prior to hospitalization.
Patients were classified into two groups based on whether they received bar/toc treatment in addition to standard of care (SOC) or SOC only. Bar treatment was defined if a patient received at least one dose of baricitinib (4 mg or dose adjusted to kidney function). Toc treatment was defined if a patient received at least one dose of tocilizumab (8 mg/kg; 800 mg maximum). SOC was defined by the respective hospital during the study period in alignment to NIH guidelines which included dexamethasone, remdesivir and low molecular weight heparin (LMWH). A small percentage of patients received convalescent plasma as an add on to the SOC. While guidelines were non-conclusive regarding clinical benefit of convalescent plasma, a decision was made individually per patient by the treating physician.
Primary outcome was defined as all-cause mortality during hospital admission, which was extracted from patients’ electronic medical records. Secondary outcomes were 30- and 60 days mortality, suspected secondary infection rate and thromboembolic events. Mortality cases at 30 and 60 days were extracted from the patients’ electronic records. A suspected secondary infection was defined by the initiation of antibiotic therapy during the admission based on the decision of the treating physician. Thromboembolic event was defined as radiological evidence of deep vein thrombosis or pulmonary embolism.
Statistical analyses
Demographic and clinical characteristic were compared using Fisher’s exact test for Categorical variables and Mann–Whitney test for continuous variables. Kaplan-Meir model was applied to compare 30 days and 60 days mortality between groups. A logistic regression was used to evaluate the effect of treatment with baricitinib and tocilizumab on in-hospital mortality, with age, gender, obesity, CKD, underlying immunosuppression, and up-to-date vaccination status as covariates.
Ethics approval
The study was approved by the local IRBs in each institute according to local regulations and the Helsinki declaration. The IRB’s at Rabin Medical Center, Rambam Medical Center, Soroka University Medical Center, Hadassah Hebrew University Medical Center, Tel Aviv Sourasky Medical Center, and Carmel Medical Center granted a waiver for written informed consent, due to the retrospective nature of this study.
Results
Patients’ characteristics and disease severity
A total of 228 immunocompromised patients, hospitalized in six major medical centers in Israel due to severe Covid-19 during the study period, were identified.
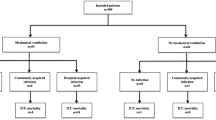
Of these 228 patients, 116 (50.8%) required low-flow oxygen support (nasal cannula) and 112 (49.1%) were dependent on high-flow oxygen support (either high-flow nasal cannula, non-rebreather face mask, BIPAP or IMV) during hospitalization. Only these 112 patients were included in our final analysis.
Of these 112 patients, 48 (42%) were treated with bar/toc (36 with bar and 12 with toc) in addition to SOC (SOC + bar/toc group), and the remaining 64/112 (57%) were treated with SOC only (Fig. 1).
Patients’ and disease characteristics among the study population, according to whether bar/toc treatment was given or not, are presented in Table 1 and Table 2, respectively. The median patients’ age was 69.1 years (95% CI 63.0–77.0) with no significant difference in age between the groups (p = 0.784). Patients in the treatment group had a lower percentage of female patients as compared to the control group (25% vs. 46.9%, p = 0.019).
Patients in the SOC + bar/toc group had a higher representation of hematological malignancies as compared to the SOC group (70.8% vs. 40.6%, p = 0.002), as well as a lower representation of solid organ transplant recipients (12.5% vs. 45%, p < 0.001). Overall, 88 (78.6%) of the patients had an up-to-date vaccination status, with no significant difference between the two groups (85.4% vs. 73.4%, p = 0.164).
On admission, the patients in the control group required higher levels of oxygen support than the patients treated with bar/toc: low-flow nasal cannula, high-flow nasal cannula and invasive mechanical ventilation (IMV) were used in 60.4, 22.9 and 0% in SOC + bar/toc group vs. 43.8, 37.5, and 14.1% in the SOC group (p = 0.005). At peak of disease, high- flow nasal cannula and IMV were used in 54.2 and 39.6% vs. 45.3% and 53.1% in SOC + bar/toc and the SOC groups, respectively (p = 0.183).
Values of laboratory markers for disease severity were not significantly different between the groups. Concurrent medications, commonly used for severe Covid-19 according to international guidelines, were administered to a high proportion of patients in both groups.
Median time from hospitalization to baricitinib administration was 3 days (IQR 1,6 range 0–31 days). Data regarding days from hospitalization to tocilizumab treatment were missing.
Primary endpoint
In-hospital mortality in immunocompromised patients with severe Covid-19 on high-flow oxygen support was 63.4% (71/112 patients) and did not significantly differ between the SOC + bar/toc and the SOC groups (30/48 (62.5%) vs. 41/64 (64.1%) of patients, respectively, p = 1.0).
Secondary endpoints
The Kaplan-Meyer comparing the survival curves of the two groups at 30- and 60days following hospitalization are presented in Fig. 2.
A logistic regression analysis revealed that older age and a non-up-to-date vaccination status were predictors of in-hospital mortality (CI 1.003-1.098 for age and 0.067-0.907 for up-to-date vaccination) whereas bar/toc treatment, hematologic malignancy, prior solid organ transplantation, gender or overweight were not (Fig. 3).
There was a high rate of suspected secondary infections with no significant difference between the two groups (60.7% and 62.8% in SOC and SOC + bar/toc group respectively, p = 0.841). Septic shock or bacteremia occurred in three patients treated with bar/toc vs. five patients treated with SOC only. One patient treated with bar had pulmonary aspergillosis. A total of two patients, one from each group, had CMV reactivation (i.e. detectable plasma CMV DNA). Two cases of thromboembolic events (PE or DVT) occurred in the SOC group and four events occurred in patients treated with SOC + bar/toc (p = 0.417).
Discussion
In this multi-center study, we attempted to retrospectively evaluate the efficacy and safety of bar and toc as immune-modulation therapy in immunocompromised patients with severe Covid-19, who require high-oxygen support, during the Omicron predominance. Our findings do not suggest any survival benefit for treatment with bar or toc compared to SOC. At the same time, no significant increase in the rate of treatment-related adverse events were observed among bar/toc treated patients as compared to SOC treated patients, despite theoretical concerns regarding the use of immunomodulatory agents in patients who are already immunocompromised.
The JAK inhibitor baricitinib and the IL-6 inhibitor tocilizumab are recommended treatments for patients with severe Covid-19 disease, based on data demonstrating survival benefit4,7. The well-known hyperinflammatory state associated with severe Covid-19, involving the overproduction of pro-inflammatory cytokines, has dictated the recommendation for adding a second immunomodulator to dexamethasone in hospitalized patients who require HFNC oxygen or NIV, while bar or toc are accepted as therapy of choice15. Regarding patients who are already immunosuppressed, adding these treatments may be of a concern because they can further compromise patients’ ability to achieve viral clearance. Indeed, in immunocompromised patients, the benefit is still elusive, and their use in this susceptible population is based on expert opinion rather than on solid data11,12,13,14,17. This is a consequence of almost total exclusion of immunocompromised patients from clinical trials, and the presence of only scarce retrospective data. Currently, few retrospective studies of toc in solid organ transplant recipients with Covid-19 have been published, showing no survival benefit18,19,20. To the best of our knowledge, there are no published studies evaluating bar use in solid organ transplant recipients or studies on the use of either bar or toc in any other immunocompromised population with severe Covid-19. As a result, treatment algorithms for immunocompromised patients with severe Covid-19 are largely extrapolated from results of trials performed on immunocompetent patients. Interpretation of the data derived from studies of immunocompetent patients is imprecise when patients with weakened immune systems are treated by immunomodulators.
The patients included in our registry were generally treated according to the above data and following NIH treatment guidelines11, and therefore immune-modulation therapy was considered upon escalating oxygen requirements (generally, beyond low flow oxygen support). Accordingly, in this current study, immunomodulation was almost exclusively given to patients with high oxygen demand- only 10 patients with low flow oxygen support were treated with immunomodulation, as opposed to 48 patients requiring high flow oxygen support. This led to narrowing the initial cohort of 228 severe Covid-19 immunocompromised patients only to those 112 patients with high oxygen demand.
The underlying immunosuppression of patients in our cohort was derived from various etiologies but is representative of major etiologies commonly seen in the medical departments; 53% of patients had hematological malignancies and 32% were solid organ transplant recipients.
Our results show a strikingly high in-hospital mortality rate of more than 60%. This rate is higher than previously published for Omicron-variant infected patients admitted to the ICU21,22 and probably reflects the underlying immunosuppression of the patients in our cohort. Indeed, in ICU patients infected with the SARS-COV-2 Omicron variant, previous study have showed almost twice as high mortality rate for immunocompromised compared to immunocompetent patients23, whereas pre-omicron data points to around 60% mortality rate in cancer patients with Covid-19 admitted to the ICU24.
A major limitation of our study is its retrospective design, and therefore, no strict criteria for initiation of immunomodulation therapy was set. Indeed, the initiation of bar/toc treatment was a discretionary decision made by the treating physicians. Accordingly, out of the 112 patients with a very severe disease, only 48 (42%) were treated with bar/toc immunomodulation. We believe that this low number of treated patients reflects the clinical dilemma of physicians reluctant to use drugs with a potential increased risk of infectious complication in this group of susceptible patients, and the absence of solid data to support decision making.
As a result of its retrospective nature, patients in this study who were treated with bar/toc had different characteristics compared of those treated with SOC only, and therefore our results are amenable to selection bias. Importantly, there was a higher rate of oxygen requirement as well as higher rates of IMV use on admission in the SOC group, suggesting that the patients in this group presented with a more severe disease. Respiratory status is the strongest predictor for mortality in Covid-19 patients, and since the potential bias is in favor of the bar/toc group, it may strengthen the finding of no survival benefit for immunomodulation therapy.
To minimize the risk of bias , we applied a logistic regression analysis to evaluate the effect of bar/toc treatment on in-hospital mortality adjusted to pivotal variables. Importantly, older age and lack of up-to-date vaccination were the only independent predictors for in-hospital mortality. This well correlates with previous reports25, and further strengthens our findings.
Another important limitation of this study is the missing data regarding the level of oxygen requirement at the time of immunomodulation administration. Although majority of patients required only low-flow oxygen support on admission, 47% of them were eventually mechanically ventilated, reflecting the unpredictable clinical course of their disease. Both, the RECOVERY and REMAP CAP trials26,27, reported survival benefit for toc treatment when initiated early during the course of disease. This suggests that toc may be more beneficial when given early in patients who progress rapidly. This hypothesis was further substantiated by a large retrospective study by Singh et al.28, showing that toc reduced mortality from Covid-19 only when given before the time patients required non-rebreather, high-flow nasal cannula (HFNC) or a ventilator support, raising the concept of an early “therapeutic window” for immune modulation therapy. It is therefore plausible that the lack of benefit for bar/toc in our study is derived from the hesitancy in treating immunocompromised patients with immunomodulation, resulting in treatment delay, and missing the “therapeutic window” for treatment initiation.
An alternative explanation for our results can arise from the unique phenotype of disease seen in immunocompromised patients. Numerous studies have found prolonged viral replication among immunocompromised patients resulting in a prolonged, persistent infection29. Although bar has been found to have a negative effect on viral replication30, treating with immunomodulation agents while viral replication is highly active, might have a net negative effect due to active viral replication. Due to its retrospective nature, we were not able to collect information regarding the kinetics of the viral load in patients included in this study, which is a major limitation. Future studies, using the threshold cycle (Ct) value as a surrogate to current viral load, may be necessary to properly determine the optimal timing for initiation of immunomodulation therapy to ensure an adequate anti-viral effect in addition to immune modulation.
In this study, we also analyzed possible adverse events of immunomodulation in immunocompromised patients with severe Covid-19. One of the major potential risks for immunomodulation in the setting of severe Covid-19, particularly in immunocompromised patients, is secondary bacterial infections31. Indeed, we have found a high rate of suspected secondary bacterial infections in both groups (62.8% vs. 60.7% in the SOC + toc/bar and in the SOC groups, respectively), with no significant difference in the rates of overall infections, septic shock, or bacteremia events. Of note, there was one case of aspergillosis in a patient treated with bar.
Thromboembolic events occurred in two patients in the SOC group vs. four patients in SOC + bar/toc group, with no statistically significant difference. For both outcomes, sample size was small and cannot exclude a potential difference given a larger sample size.
Overall, 8/12 of patients receiving toc and 22/36 of patients receiving bar succumbed to their disease. Unfortunately, we could not address any differences in this outcome between these groups due to the small sample size of the cohort.
In conclusion, our results do not support any survival benefit for bar/toc treatment in immunocompromised patients with severe Covid-19 disease requiring high oxygen support. Considering an epidemiologic shift, where immunization against Covid-19 is granting a good protection to immunocompetent individuals but have a lower efficacy in the immunocompromised population32,33,34, the proportion of immunocompromised patients among the severely ill patients is, and expected to remain high23. Our study adds an important piece of data, but it is prudent that larger prospective studies should be conducted to further evaluate the therapeutic role of immunomodulation agents in immunocompromised patients with severe Covid-19 disease.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Gustine, J. N. & Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 191(1), 4–17 (2021).
Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27(6), 992-1000.e3 (2020).
van de Veerdonk, F. L. et al. A guide to immunotherapy for COVID-19. Nat. Med. 28(1), 39–50 (2022).
Abani, O. et al. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. The Lancet 400(10349), 359–368 (2022).
Kalil, A. C. et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med 384(9), 795–807 (2021).
Marconi, V. C. et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 9(12), 1407–1418 (2021).
Shankar-Hari, M. et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A Meta-analysis. Jama 326(6), 499–518 (2021).
Ao, G. et al. The association between severe or death COVID-19 and solid organ transplantation: A systematic review and meta-analysis. Transpl. Rev. (Orlando) 35(3), 100628 (2021).
Vijenthira, A. et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 136(25), 2881–2892 (2020).
Wang, Y. et al. An updated meta-analysis on the association between HIV infection and COVID-19 mortality. Aids 35(11), 1875–1878 (2021).
Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda (MD): National Institutes of Health (US), 2021.
Cesaro S, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 2022; 36(6): 1467–80.
El Chaer, F., Auletta, J. J. & Chemaly, R. F. How I treat and prevent COVID-19 in patients with hematologic malignancies and recipients of cellular therapies. Blood 140(7), 673–684 (2022).
Bhimraj, A. et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciac724 (2022).
Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. (2022).
National Institutes of Health. COVID-19 treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/.
Trøseid, M. et al. Immunocompromised patients have been neglected in Covid- 19 trials: A call for action. Clin. Microbiol. Infect. 28(9), 1182–1183 (2022).
Avery, R. K. Update on COVID-19 therapeutics for Solid organ transplant recipients Including Omicron Surge. Transplantation 106(8), 1528–1537 (2022).
Bodro, M. et al. Use of anti-cytokine therapy in kidney transplant recipients with COVID-19. J. Clin. Med. 10, 8 (2021).
Pereira, M. R. et al. Tocilizumab for severe COVID-19 in solid organ transplant recipients: A matched cohort study. Am. J. Transpl. 20(11), 3198–3205 (2020).
Corriero, A. et al. COVID-19 variants in critically ill patients: A comparison of the Delta and Omicron variant profiles. Infect. Dis. Rep. 14(3), 492–500 (2022).
Tandon, P. et al. The fourth wave: Vaccination status and intensive care unit mortality at a large hospital system in New York City. Acute Crit. Care 37(3), 339–346 (2022).
de Prost, N. et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat. Commun. 13(1), 6025 (2022).
Nadkarni, A. R., Vijayakumaran, S. C., Gupta, S. & Divatia, J. V. Mortality in cancer patients with COVID-19 who are admitted to an ICU or who have severe COVID-19: A systematic review and meta-analysis. JCO Glob. Oncol. 7, 1286–1305 (2021).
Britton, A. et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalizations among immunocompromised adults during SARS-CoV-2 Omicron Predominance—VISION network, 10 states, December 2021-August 2022. MMWR Morb. Mortal Wkly Rep. 71(42), 1335–1342 (2022).
Gordon, A. C. et al. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. N. Engl. J. Med. 384(16), 1491–1502 (2021).
Horby, P. W. et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. The Lancet 397(10285), 1637–1645 (2021).
Singh, A. K. et al. Impact of timing of Tocilizumab use in hospitalized patients with SARS-CoV-2 infection. Respir. Care 66(12), 1805–1814 (2021).
Corey, L. et al. SARS-CoV-2 variants in patients with immunosuppression. N. Engl. J. Med. 385(6), 562–566 (2021).
Spinelli, F. R., Conti, F. & Gadina, M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci. Immunol. 5, 47 (2020).
Shafran, N. et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 11(1), 12703 (2021).
Hall, V. G. et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 385(13), 1244–1246 (2021).
Lee, A. R. Y. B. et al. Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 376, e068632 (2022).
Shostak, Y. et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir. Med. 9(6), e52–e53 (2021).
Author information
Authors and Affiliations
Contributions
A.S, M.L and A.G initiated and designed the study. A.G, Y.D, K.H, T.S, D.G, A.W, S.I, M.E and R.N.D.-collected and analyzed the data. A.N, J.S, R.B, J.B, A.S and M.L contributed to the interpretation of the results. M.L drafted the manuscript with input from all authors. A.S supervised the project. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goldstein, A., Neuberger, A., Darawsha, Y.Q. et al. Clinical outcomes of immunomodulation therapy in immunocompromised patients with severe Covid-19 and high oxygen requirement. Sci Rep 14, 16985 (2024). https://doi.org/10.1038/s41598-024-68013-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68013-6
- Springer Nature Limited