Abstract
The early diagnosis of esophageal cancer (EC) is extremely challenging due to a lack of effective diagnostic methods. The study presented herein aims to assess whether serum volatile organic compounds (VOCs) could be utilised as emerging diagnostic biomarkers for EC. Gas chromatography-ion mobility spectrometry (GC-IMS) was used to detect VOCs in the serum samples of 55 patients with EC, with samples from 84 healthy controls (HCs) patients analysed as a comparison. All machine learning analyses were based on data from serum VOCs obtained by GC-IMS. A total of 33 substance peak heights were detected in all patient serum samples. The ROC analysis revealed that four machine learning models were effective in facilitating the diagnosis of EC. In addition, the random forests model for 5 VOCs had an AUC of 0.951, with sensitivities and specificities of 94.1 and 96.0%, respectively.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is a common tumor of the gastrointestinal tract1. Its discreet onset and lack of specific symptoms in the early stages mean that most patients are at an advanced stage of the disease when they enter the clinic2, resulting in a poor prognosis for EC with a 5-year survival rate of around 15–25%3. Currently, endoscopy combined with histopathology is most commonly used to diagnose and treat patients with EC, however this approach is time-consuming and complex; these methods cannot be used for universal screening of early EC. Analysis of serum tumor markers is a simple and cost-effective diagnostic method that is in addition less invasive and more acceptable to patients. However, the markers currently used in such analysis, including CEA, CA 19–9 and CA 12–5, have low sensitivity and specificity and cannot be used for the early diagnosis of EC4,5.
Volatile organic compounds (VOCs) can be generated by a variety of different biochemical reactions in the human body, such as during the maintenance of cell membrane integrity, energy metabolism, and oxidative stress amongst other basic cellular functions6. There is ample evidence that VOCs can provide useful information about the metabolic state of an organism. Our body's VOCs reflect biochemical reactions caused by biological activities such as cell death, oxidative stress, or inflammation. Disease-associated VOCs may be part of a cascade of reactions occurring during the body's response to injury. Studies have reported that cancer cells and healthy cells can produce distinct VOCs during human metabolic activities7, and the VOCs produced by different tumor cell types are also distinguishable8. As such, it is important to explore the distinct VOCs released by various cancer types to identify representative VOCs for each tumor type that could be utilised as a diagnostic tool9,10. A significant amount of work has been done to find a relationship between the physical characteristics of VOCs and cancer. VOCs associated with cancer can be used to detect several types of cancer at an early stage, thus providing a significantly higher chance of survival11. The diagnostic model described by Wang et al. based on 16 VOCs in perioperative exhaled breath was efficacious in distinguishing between lung cancer patients and healthy people12. Daulton et al. tested the urine metabolomics of 45 pancreatic ductal adenocarcinomas and 33 healthy controls using gas chromatography-ion mobility spectrometry (GC-IMS) and identified significant differences in four VOCs between these two groups13. Serum is a commonly used biofluid in metabolomics, monitoring serum levels of specific metabolites has become an important method of diagnosing early stage disease, and serum is an easily accessible and content-rich matrix, making it ideal for suggesting early stage disease. Because of this, alterations in the concentration of metabolites induced by tumor cells during changes in the pathophysiological environment of cancer can be demonstrated by serum testing. Although the differences in urine and plasma between EC patients and controls have already been studied by a team14,15,16, we used a new device, the GC-IMS, to detect serum VOCs in EC patients, with the intention of exploring a simpler and faster diagnostic method. In addition, although some studies have suggested that non-volatile organic compounds (NOCs) may also be relevant to disease diagnosis, this study focused on the development of diagnostic models for VOCs, as the GC-IMS itself is used for the detection of VOCs.
In this study, we aimed to identify specific VOCs in patients with EC by detecting and analysing serum metabolites. Additional external validation was performed to assess the performance of these VOCs in the diagnosis of EC.
Results
Participant characteristics
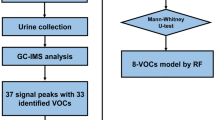
The overall study design is demonstrated in Fig. 1. There were no significant differences between the training and test group populations in terms of age, gender. More detailed clinical characteristics are presented in Table 1. In the training and test sets, the two groups were broadly consistent in terms of the proportion of people who smoked and drank alcohol. A greater proportion of individuals in the EC group compared to the HC group were of advanced age, had a history of smoking and a history of alcohol consumption (Supplementary Table S1).
VOC profile analysis in EC patients and HCs
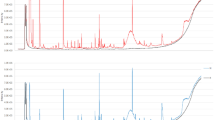
The whole graph (Fig. 2) has a blue background and the red vertical lines are the RIP peaks (Reactive Ion Peaks, normalised). Each point on either side of the RIP peak represents a volatile organic substance. The colours represent the abundance of the substance, with white indicating lower concentrations, red indicating higher concentrations and darker colours indicating higher concentrations. The VOC signal peaks for each serum sample were characterized by retention index, drift time and peak strength (Fig. 2A). The two-dimensional coordinates (retention index × drift time) of the location of the signal peak were retrieved to characterize the compound and to simultaneously obtain peak height data (Fig. 2B). At high concentrations of the analyte to be analysed, two or more molecules can share a proton or electron, leading to the formation of dimers or even multimers, so that one VOCs may simultaneously map multiple peak heights, which makes the qualitative results more accurate. Based on comparisons with standard profiles, a total of 33 VOC signal peaks were initially selected from all training set serum samples. See Supplementary Table S2 for specific peak height values.
Diagnostic performance of serum VOCs with machine learning algorithms
Four types of machine learning (RF, NN, SVM, DT) were applied to the analysis of the peak height data for the 33 VOCs described above. The RF, NN, and SVM models all achieved an AUC of 0.9 or more in the test set study. Table 2 reveals the detailed diagnostic performance of the four models. Among them, the RF approach provided the highest AUC of 0.971, with a sensitivity of 94.1% and specificity of 100%. Note: The F1 value represents the reconciled mean of the check accuracy and the check completeness rates, measuring the balance of precision and recall of the model. F1 is calculated as (2 × Precision x Recall)/(Precision + Recall).
Estimation of importance of the serum VOCs using Random forests analysis
As demonstrated in Fig. 3A, the top eight peak heights of VOCs in the Gini coefficient ranking were obtained from RF model analysis. Figure 3B reveals the differences between the peak heights of the top eight VOCs for EC patients compared to HCs. These included three up-regulated VOCs (3-nonen-2-one, Butanol-2, methyl 3-(methylthio) propanoate) and three down-regulated VOCs ((E)-3-hexen-1-ol-1, (E)-3-hexen-1-ol-2, (E)-3-hexen-1-ol-3). The peak heights of two VOCs (1-Hexanol, Butanol-1) were not statistically different. Overall, the eight peak heights identified correspond to five distinct VOC substances.
Estimation of importance of the serum VOCs using Random forests analysis (A) The top eight peak heights with Gini coefficients by RF. (B) Comparisons of peak height of volatile organic compounds in patients with EC and HC. The peak height of (E)-3-hexen-1-ol-2 (A), 3-nonen-2-one (B), 1-Hexanol (C), (E)-3-hexen-1-ol-1 (D), Butanol-1 (E), (E)-3-hexen-1-ol-3 (F), Butanol-2 (G), and methyl 3-(methylthio)propanoate (H). *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U-test).
The model constructed using RF in combination with the five identified VOCs provided an AUC of 0.951, a sensitivity of 94.1% and a specificity of 96.0% (Fig. 4A). In addition, the 5-VOC model facilitated effective separation of patients in the I-IV stage EC group from HCs (Fig. 4B, Supplementary Table S3).
Serum biomarkers and pathological parameters
Further analysis of the 8-VOCs obtained through the RF algorithmic modelling. As revealed in Supplementary Table S4, levels of (E)-3-hexen-1-ol-2, (E)-3-hexen-1-ol-1 and (E)-3-hexen-1-ol-3 were significantly different in patients with EC stages I-IV versus HCs. In addition, 3-nonen-2-one, 1-Hexanol, Butanol-1, Butanol-2, and methyl 3-(methylthio)propanoate exhibited differences between HCs and EC patients primarily in stages III and IV. Regarding the comparison of VOCs at different cancer stages (Supplementary Fig. S1), there was no significant trend in changes in VOC peak height values between patients at each stage.
Correlation analysis of VOCs in serum
Correlation analysis revealed that VOCs were highly differentiated between the EC group and HCs (Supplementary Fig. S2), suggesting different patterns of correlation between the two groups of VOCs.
Discussion
In this study, serum VOC levels were measured by GC-IMS and serum biomarkers for EC were identified. A total of five VOCs were selected for use in the construction of our early diagnosis model for EC.
Differences in VOCs generated in the exhaled breath or urine of patients with EC and non-cancerous individuals have been previously verified. In a prospective study of exhaled gas VOCs in patients with EC and benign gastrointestinal disorders, Kumar et al. demonstrated that certain VOCs produced by exhalation in patients with EC were significantly different from those in patients with benign gastrointestinal disorders16,17. A multi-centre validation study reported that a 5-VOC model consisting of butyric acid, valeric acid, hexanoic acid, butyraldehyde and decanal had good diagnostic accuracy for esophagogastric cancer18. Moreover, Zou et al. measured exhaled breath samples from 29 patients with EC and 57 healthy individuals by proton transfer reaction mass spectrometry (PTR-MS). In doing so, they identified that compared to the healthy population, the exhaled breath mass spectra of patients with EC exhibited significantly different intensities associated with seven characteristic ions (m/z 136, m/z 34, m/z 63, m/z 27, m/z 95, m/z 107, and m/z 45), and ROC analysis of the diagnostic model constructed from these ions yielded an area under the curve (AUC) of 0.94319. However, exhaled VOCs are collected under demanding conditions and are susceptible to the specific collection environment20, posing a challenge to ensure sample stability from clinical collection to laboratory storage21. Moreover, Huang et al. utilised selected ion flow tube mass spectrometry (SIFT-MS) to analyze urinary VOCs in patients with gastro-esophageal cancer versus non-cancerous controls and obtained seven significantly different VOCs, which could well distinguish the two groups by constructing an ROC model (AUC value of 0.904)15. As a draw-back to these approaches, the effects of drugs can affect changes in the metabolism of VOCs in the urine.
As an important component of human metabolites, VOCs have been demonstrated to possess diagnostic value in a multitude of diseases. In metabolomics studies, serum is the second most commonly used biofluid after urine22. Although research on serum biomarkers has been conducted for decades and analysis of a variety of substance indicators has facilitated the clinical diagnosis of tumors, the diagnostic value of VOCs in the serum of patients with tumors has rarely been explored. For example, Sana et al. used solid-phase microextraction gas chromatography/mass spectrometry to detect VOCs in the serum of gestational diabetes mellitus (GDM) patients and identified that 2-propanol was a potential volatile marker for assessing cognitive impairment in pregnant women with GDM23. The Wazeerud-Din team further assisted in the investigation of unhealthy exposure to microbial emissions by quantifying microbial VOCs in human serum24. While little is known about VOCs in serum compared to other samples, such as urine, exhaled breath, bile, faeces, etc., we understand that endogenous VOCs are mainly produced by human cells during processes such as energy metabolism and oxidative stress, and are excreted via the blood to reach alveolar cells or kidney epithelial cells. This knowledge suggests to us that serum VOCs are more representative of the initial metabolic changes in tumor cells. Serum VOCs arise from direct alterations in cellular metabolism, which may be a gradual process, and concentrations of these VOCs may be higher in later stages of cancer. We therefore analysed the relationship between several VOCs screened and tumor stage and unfortunately did not obtain very satisfactory results, which may be related to the small number of samples, among other reasons (Supplementary Table S4 & Supplementary Fig. S1). Therefore, there is an urgent need to explore the value of serum VOCs in the diagnosis of cancerous tumors.
Efforts to explore the relationship between the characteristics of VOCs and the presence of tumors have been ongoing for some time. In contrast to the continuing research on characteristic differential VOCs in cancer patients, little research has been carried out regarding the mechanisms of production of the cancer- associated VOCs. Aldehydes are produced by several distinct metabolic mechanisms, the main source of which is associated with the activity of cytochrome P450 (CYP450) and the lipid redox peroxidation processes in which it participates25. Cytochrome P450 represents a large family of auto-oxidisable ferrous haemoglobin proteins that belong to a class of monooxygenases. In human physiology, CYP450 facilitates the oxidative metabolism of a variety of endogenous and exogenous compounds26. Studies have demonstrated that CYP450 plays a key role in cancer pathogenesis and is involved in the promotion of tumorigenesis. Excessive production of reactive oxygen species (ROS) has been revealed to be associated with the mechanism by which VOCs are generated. Meanwhile, ROS generation can be mediated by CYP450 overexpression in tumor cells27. Furthermore, the main source of alcohols is the diet, where ingested food and alcohol are absorbed into the bloodstream through the digestive system; alcohol can also be detected in urine, bile and exhaled breath. In the human body, ethanol is catalyzed by alcohol dehydrogenases (ADHs) to acetaldehyde, which in turn is oxidized by aldehyde dehydrogenases (ALDHs) to acetate. Acetate is known to be further involved in processes such as lipid metabolism28. The level of acetaldehyde can be regulated by the balance between its production from alcohol by ADHs and its subsequent conversion to acetate by ALDHs29,30. Alterations in ketone levels may be closely associated with tumor production, and of note, the production of ketones is closely associated with the oxidation of fatty acids. Furthermore, the metabolism of fatty acids results in the production of acetyl coenzyme A, which can act as a precursor to the ketogenic process31,32.
In this study, we eventually identified five VOCs, which were (E)-3-hexen-1-ol, 1-Hexanol, 3-nonen-2-one, Butanol, and methyl 3-(methylthio)propanoate. These compounds can be mainly classified as alcohols and ketones. Other researchers have identified variations in the same classes of compounds in distinct populations. Alterations in (E)-3-hexen-1-ol have been indentified in the exhaled breath of e-cigarette and smoker populations33. Moreover, cyclohexanone is an important cancer-related VOC that is known to be significantly upregulated in the bile of patients with gallbladder cancer34. Additionally, changes in the levels of alcohol VOCs have been observed in a variety of cancers and it has been reported that alcohol may induce cancer through abnormal DNA methylation35. Hasim et al. stated that plasma acetone concentrations were significantly increased in poorly differentiated EC patients compared to well-differentiated EC patients36. We speculate that due to the altered dietary status of most EC patients at the time of disease onset, this may be related to the elevated ketone bodies, and the exact physiological mechanism processes need to be further investigated.
At present, the diagnosis of EC primarily relies on the observation of clinical manifestations, CT and other imaging methods. However, these methods lack specificity and can be subjective dependent on the practitioner. Endoscopy and gastrointestinal imaging are important methods for providing a definitive diagnosis of EC, but unfortunately these methods are time consuming and not widely available for early screening of the population. In the study presented herein, a machine learning approach was used to analyze the heterogeneous signal patterns of serum from patients with EC in order to obtain a higher diagnostic accuracy using a limited data set. Four machine learning algorithms were applied to the construction of a diagnostic model for EC. Overall, a 5-VOC model with an AUC of 0.951 was built based on an RF algorithm, which achieved a sensitivity and specificity of 94.1 and 96.0%, respectively. Therefore, we strongly suggest that serum VOCs are suitable biomarkers for the diagnosis of EC.
Limitations of the study
One limitation of this study is that the specific metabolic generation pathways of VOCs in the findings have not been determined. Additionally, the metabolic relationship between the screened serum biomarkers and EC, as well as their future clinical diagnostic value, require further exploration. The control population included in the study was younger relative to the disease group, and the tumor patients were older at the time of diagnosis, which may have had some impact on the results. Furthermore, more clinical samples are necessary for validation.
Materials and methods
Study population
A total of 55 EC cases were recruited for the study. In addition, 84 HC individuals were recruited for comparison. The EC patients had not undergone any preoperative anti-cancer treatment. The specific inclusion criteria were as follows (1) Patients had no history of other malignancies, radiotherapy or other treatments. (2) Patients were able to provide blood samples and complete medical history. (3) The patients had undergone radical resection and were confirmed to have EC by pathological examination. At the same time, blood samples from healthy people were collected from the Health Check Centre as a control group, and the HC group had normal urine, liver and kidney function indicators and no tumors or other major diseases were found. The study protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University and informed consent was obtained from all participating patients and their families. This study was conducted in accordance with the Declaration of Helsinki. Of the 55 EC samples collected, 38 were selected as the training set and 17 as the test set; of the 84 HCs, 59 were in the training set and 25 were included in the test set.
Sample preparation
All serum samples were obtained early in the morning before meals. Serum samples were collected in inert separating procoagulant tubes. The collected samples were centrifuged at 6000 g and 12,000 g for 10 min at 4 °C. The supernatant was aliquoted and immediately stored at − 80 °C.
Analysis of the VOCs
VOCs in serum were measured with confidence using GC-IMS (“FlavorSpec” brand, Dortmund, Germany). The GC-IMS method effectively separates complex VOC fractions in serum by GC, which is then tandem with IMS, achieving secondary separation based on the mass of the ion to be measured and the one-dimensional collision cross-sectional area. Retention indices from GC and drift times from IMS can be used for two-dimensional characterisation, while signal response intensity can be relied upon for quantification. It is worth noting that the GC column used in this study was the strongly polarised MXT-WAX column. Based on orthogonal experiments, we adhered to the confirmed research proposal. Firstly, 200 μL of serum was placed in each headspace vial and incubated at 60 °C for 10 min. Subsequently, 1000 μL of gas was then extracted from the headspace vial for analysis. Nitrogen was used as the carrier gas. IMS drift gas was always maintained at 150 mL/min with the following carrier gas gradients: 0 min: 2 mL/min; 1 min: 2 mL/min; 8 min: 100 mL/min; 10 min: 150 mL/min; 15 min: 150 mL/min. The additional main parameters were maintained as follows: T1 drift tube temperature at 45 °C; T2 gas chromatography column temperature at 80 °C; T3 inlet temperature at 80 °C; T4 connection line 1 at 80 °C and T5 connection line 2 at 45 °C. Volume of headspace vial is 20 ml. All samples were stored at room temperature for no more than 3 h and freeze thawed no more than twice.
Statistical analysis
Continual variables are presented as means ± standard deviations, while qualitative variables are expressed as percentages. Continuous variables were analyzed using the t-test or Mann Whitney U-test and qualitative variables were analyzed using the Pearson chi-square test or the continuity-corrected chi-square test. The “corrplot” package was utilised for correlation analysis. The above analyses were performed using the R programme (× 64 4.2.0). Furthermore, the Mann–Whitney U test was used to compare the levels of each VOC and the area under the curve (AUC) was calculated for the receiver operating characteristic (ROC) curves using MedCalc 9.3.9.0. Four types of machine learning approaches (random forests (RF), neural network (NN), support vector machines (SVM), decision trees (DT)) were used for diagnostic model building. The Gini coefficient evaluated the RF algorithm. The NN algorithm uses the stochastic gradient descent (SGD) optimisation algorithm, while SVM uses a nonlinear kernel function. The C4.5 algorithm implemented the DT.
Ethics declarations
The studies involving human participants were reviewed and approved by the Ethics Committee of Qilu Hospital of Shandong University. The patients/participants provided their written informed consent to participate in this study.
Institutional review board statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Qilu Hospital of Shandong University. The study was carried out in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA A Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Smyth, E. C. et al. Oesophageal cancer. Nat. Rev. Dis. Primers 3, 17048. https://doi.org/10.1038/nrdp.2017.48 (2017).
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381(9864), 400–412. https://doi.org/10.1016/S0140-6736(12)60643-6 (2013).
Acharya, A., Markar, S. R., Matar, M., Ni, M. & Hanna, G. B. Use of tumor markers in gastrointestinal cancers: Surgeon perceptions and cost-benefit trade-off analysis. Ann. Surg. Oncol. 24(5), 1165–1173. https://doi.org/10.1245/s10434-016-5717-y (2017).
Mealy, K. et al. Tumour marker detection in oesophageal carcinoma. Eur. J. Surg. Oncol. 22(5), 505–507. https://doi.org/10.1016/s0748-7983(96)92998-4 (1996).
Phillips, M. et al. Effect of oxygen on breath markers of oxidative stress. Eur. Respir. J. 21(1), 48–51. https://doi.org/10.1183/09031936.02.00053402 (2003).
Weber, C. M. et al. Evaluation of a gas sensor array and pattern recognition for the identification of bladder cancer from urine headspace. Analyst 136(2), 359–364. https://doi.org/10.1039/c0an00382d (2011).
Dima, A. C., Balaban, D. V. & Dima, A. Diagnostic application of volatile organic compounds as potential biomarkers for detecting digestive neoplasia: A systematic review. Diagnostics (Basel) 11(12), 2317. https://doi.org/10.3390/diagnostics11122317 (2021).
Cicolella, A. Volatile organic compounds (VOC): Definition, classification and properties. Rev. Mal. Respir. 25(2), 155–163. https://doi.org/10.1016/s0761-8425(08)71513-4 (2008).
Wang, L. et al. Volatile organic compounds as a potential screening tool for neoplasm of the digestive system: A meta-analysis. Sci. Rep. 11(1), 23716. https://doi.org/10.1038/s41598-021-02906-8 (2021).
Janfaza, S., Khorsand, B., Nikkhah, M. & Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 4(1), bpz014. https://doi.org/10.1093/biomethods/bpz014 (2019).
Wang, P. et al. Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. EClinicalMedicine 47, 101384. https://doi.org/10.1016/j.eclinm.2022.101384 (2022).
Daulton, E. et al. Volatile organic compounds (VOCs) for the non-invasive detection of pancreatic cancer from urine. Talanta 221, 121604. https://doi.org/10.1016/j.talanta.2020.121604 (2021).
Bhatt, A. et al. Volatile organic compounds in plasma for the diagnosis of esophageal adenocarcinoma: A pilot study. Gastrointest. Endosc. 84(4), 597–603. https://doi.org/10.1016/j.gie.2015.11.031 (2016).
Huang, J. et al. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem. 85(6), 3409–3416. https://doi.org/10.1021/ac4000656 (2013).
Kumar, S. et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann. Surgery 262(6), 981–990. https://doi.org/10.1097/sla.0000000000001101 (2015).
Kumar, S. et al. Selected ion flow tube mass spectrometry analysis of exhaled breath for volatile organic compound profiling of esophago-gastric cancer. Anal. Chem. 85(12), 6121–6128. https://doi.org/10.1021/ac4010309 (2013).
Markar, S. R. et al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol 4(7), 970–976. https://doi.org/10.1001/jamaoncol.2018.0991 (2018).
Zou, X. et al. Exhaled gases online measurements for esophageal cancer patients and healthy people by proton transfer reaction mass spectrometry. J. Gastroenterol. Hepatol. 31(11), 1837–1843. https://doi.org/10.1111/jgh.13380 (2016).
Chen, H. et al. COVID-19 screening using breath-borne volatile organic compounds. J. Breath Res. 15(4), 047104. https://doi.org/10.1088/1752-7163/ac2e57 (2021).
Chin, S. T., Romano, A., Doran, S. L. F. & Hanna, G. B. Cross-platform mass spectrometry annotation in breathomics of oesophageal-gastric cancer. Sci. Rep. 8(1), 5139. https://doi.org/10.1038/s41598-018-22890-w (2018).
Kiseleva, O., Kurbatov, I., Ilgisonis, E. & Poverennaya, E. Defining Blood Plasma and Serum Metabolome by GC-MS. Metabolites 12(1), 15. https://doi.org/10.3390/metabo12010015 (2021).
Sana, S. R., Chen, G. M., Lv, Y., Guo, L. & Li, E. Y. Metabonomics fingerprint of volatile organic compounds in serum and urine of pregnant women with gestational diabetes mellitus. World J. Diabetes 13(10), 888–899. https://doi.org/10.4239/wjd.v13.i10.888 (2022).
Wazeerud-Din, I. J. et al. Quantification of seven microbial volatile organic compounds in human serum by solid-phase microextraction gas chromatography-tandem mass spectrometry. Chemosphere 266, 128970. https://doi.org/10.1016/j.chemosphere.2020.128970 (2021).
Pryor, W. A., Das, B. & Church, D. F. The ozonation of unsaturated fatty acids: Aldehydes and hydrogen peroxide as products and possible mediators of ozone toxicity. Chem. Res. Toxicol. 4(3), 341–348. https://doi.org/10.1021/tx00021a014 (1991).
Coon, M. J. Cytochrome P450: Nature’s most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 45, 1–25. https://doi.org/10.1146/annurev.pharmtox.45.120403.100030 (2005).
Gonzalez, F. J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. 569(1–2), 101–110. https://doi.org/10.1016/j.mrfmmm.2004.04.021 (2005).
Matejcic, M., Gunter, M. J. & Ferrari, P. Alcohol metabolism and oesophageal cancer: A systematic review of the evidence. Carcinogenesis 38(9), 859–872. https://doi.org/10.1093/carcin/bgx067 (2017).
Crabb, D. W., Matsumoto, M., Chang, D. & You, M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. 63(1), 49–63. https://doi.org/10.1079/pns2003327 (2004).
Ma, S. et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol. Cancer Res. 6(7), 1146–1153. https://doi.org/10.1158/1541-7786.MCR-08-0035 (2008).
Erhart, S. et al. 3-Heptanone as a potential new marker for valproic acid therapy. J. Breath Res. 3(1), 016004. https://doi.org/10.1088/1752-7155/3/1/016004 (2009).
Hakim, M. et al. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 112(11), 5949–5966. https://doi.org/10.1021/cr300174a (2012).
Papaefstathiou, E., Stylianou, M., Andreou, C. & Agapiou, A. Breath analysis of smokers, non-smokers, and e-cigarette users. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1160, 122349. https://doi.org/10.1016/j.jchromb.2020.122349 (2020).
Zhang, X. et al. A panel of bile volatile organic compounds servers as a potential diagnostic biomarker for gallbladder cancer. Front. Oncol. 12, 858639. https://doi.org/10.3389/fonc.2022.858639 (2022).
Varela-Rey, M., Woodhoo, A., Martinez-Chantar, M. L., Mato, J. M. & Lu, S. C. Alcohol, DNA methylation, and cancer. Alcohol Res. 35(1), 25–35 (2013).
Hasim, A. et al. Revealing the metabonomic variation of EC using 1H-NMR spectroscopy and its association with the clinicopathological characteristics. Mol. Biol. Rep. 39(9), 8955–8964. https://doi.org/10.1007/s11033-012-1764-z (2012).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81972005), Major Scientific and Technological Innovation Project of Shandong Province (2021CXGC010603, 2021CXGC011105).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.Z. and Yi.Z.; Methodology, Q.L. and S.L.; Software, M.M.; Validation, M.M., X.G. and Y.L.Z.; Formal Analysis, Q.L.; Investigation, Y.X.Z. and L.Y.; Resources, Yi.Z.; Data Curation, Q.L. and S.L.; Writing—Original Draft Preparation, Q.L.; Writing—Review & Editing, S.L.; Visualization, Y.X.Z. and L.Y.; Supervision, Y.L. Zhang; Project Administration, Y.L.Z. and X.Z.; Funding Acquisition, Yi.Z. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Q., Li, S., Mao, M. et al. Serum-volatile organic compounds in the diagnostics of esophageal cancer. Sci Rep 14, 17722 (2024). https://doi.org/10.1038/s41598-024-67818-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67818-9
- Springer Nature Limited