Abstract
There is no reliable causal evidence for the effect of statins on diabetic nephropathy (DN) and diabetic retinopathy (DR), and the results of previous observational studies are contradictory. Genetic variants linked to low-density lipoprotein cholesterol (LDL-C) from a UK biobank genome-wide association study and located within a 100kb window around HMGCR were used to proxy statins, comparing with PCSK9 inhibitors (control). DN and DR genome-wide association study summary statistics were obtained from the FinnGen study. Secondary MR analyses and NHANES cross-sectional data were used for validation. Drug-target Mendelian randomization (MR) was applied to investigate the association between the genetically proxied inhibition of HMGCR and PCSK9 with DN and DR, p < 0.0125 was considered significant after Bonferroni Correction. To triangulate the findings, genetic variants of whole blood-derived targets gene expression (cis-eQTL) and plasma-derived protein (cis-pQTL) levels were used to perform secondary MR analyses and data from the National Health and Nutrition Examination Survey were used for cross-sectional analysis. Genetically proxied inhibition of HMGCR was associated with higher risks of DN and DR (DN: OR = 1.79, p = 0.01; DR: OR = 1.41, p = 0.004), while no such association was found for PCSK9. Secondary MR analyses confirmed these associations. Cross-sectional analysis revealed a positive link between statin use and DR incidence (OR = 1.26, p = 0.03) and a significant negative association with glomerular filtration rate (Beta = − 1.9, p = 0.03). This study provides genetic evidence that genetically proxied inhibition of HMGCR is associated with increased risks of DN/DR, and this effect may not be attributed to their LDL-C-lowering properties. For patients with diabetic dyslipidemia, PCSK9 inhibitors may be a preferable alternative.
Similar content being viewed by others
Introduction
Statins are currently the most widely prescribed low-density lipoprotein cholesterol (LDL-C) lowering drugs for the primary and secondary prevention of cardiovascular diseases (CVD)1. This holds for individuals with diabetes as well, since diabetes is a leading risk factor for CVD2 and individuals with diabetes are prone to dyslipidemia, which almost always leads to serious destruction of lipid dynamics3. A study from Europe indicates that the utilization rates of statins among patients with type 1 and type 2 diabetes are 19.3% and 45.7%, respectively4.
However, prior studies on the effects of statins on diabetic nephropathy (DN) and diabetic retinopathy (DR) have been subject to significant controversy. In a large observational study with a median follow-up of 2.7 years involving 15,679 individuals with diabetes in Denmark, it was observed that individuals using statins prior to diabetes diagnosis had a lower incidence of DR, but there was no significant difference in the incidence of DN compared to non-statin users5. Similar results were reported in some small-scale randomized controlled trials (RCTs)6,7,8. Additionally, a multicenter cohort study from China suggested that the initiation of statin therapy in the diabetic population was associated with a lower incidence of DN and a reduced risk of renal function decline9. In contrast, a recent retrospective cohort study, which included 81,146 pairs of statin users and controls with an average follow-up of 4.8 years, indicated that statin use in individuals with diabetes was moderately associated with an increased risk of kidney disease progression, ocular complications, and neurological symptoms10. A large-scale RCT has demonstrated that lowering LDL-C did not slow down the progression of various chronic kidney diseases within 5 years of treatment11. Furthermore, statin use may elevate the risk of severe renal failure12 and may also increase blood glucose levels and the risk of ketoacidosis in adolescents with type 1 diabetes13.
Therefore, the evidence regarding the impact of statins on DN and DR remains contentious. Most studies have focused on a follow-up period within 5 years, with limited exploration of the long-term effects of statins. It is noteworthy that a large-scale cohort study with a 12-year follow-up has indicated a higher likelihood of statin users initiating insulin therapy, developing obvious hyperglycemia and acute glycemic complications, and being prescribed a greater variety of antidiabetic medications14. Furthermore, the latest animal experiment suggests that prolonged use of statins exacerbates insulin resistance and renal damage in diabetic mice. The latter is characterized by reduced estimated glomerular filtration rate (eGFR), increased renal fibrosis, intensified renal inflammation, and lipid deposition.15. Based on the current evidence, we postulate that statins may exert multifaceted effects on DN and DR, manifesting distinct impacts at various stages of medication use, but increasing the risk of DN and DR in the long term, which is independent of their LDL-C lowering properties (statins reduce LDL-C levels by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase [HMGCR]16).
With the increasing accessibility of genome-wide association study (GWAS) data, Mendelian randomization (MR) emerges as an effective causal inference method based on genetic variations17. It is less susceptible to confounding factors18 and its results were typically interpreted as lifetime exposure rather than a specific period19,20, thereby enabling a reflection of the long-term impacts of the exposure to some extent. In this study, we selected effective genetic variants as proxies of statins for conducting drug-target MR to investigate the association between statins and DN/DR. To assess whether these associations were dominated by their LDL-C lowering properties, we also selected protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors21, which also have LDL-C lowering effects, as controls and investigated the associations of genetically proxied levels of LDL-C on outcomes. To triangulate the positive findings22,23, genetic variants of whole blood-derived gene expression and plasma-derived circulating protein levels for targets were used for secondary analyses, and data from the National Health and Nutrition Examination Survey (NHANES)24 were used for cross-sectional analysis.
Methods
Study design
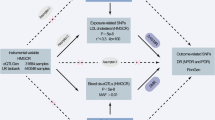
We conducted a series of MR analyses, employing diverse study methods and utilizing data from different sources to triangulate the positive findings. The study design can be found in Fig. 1. This study adhered to the STROBE-MR guidelines (S1 Table).
Study design. HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase; PCSK9 = proprotein convertase subtilisin/kexin type 9; LDL-C = low-density lipoprotein cholesterol; SNP = single nucleotide polymorphism; GWAS = genome-wide association study; cis-eQTL = cis-acting expression quantitative trait loci; cis-pQTL = cis-acting protein quantitative trait loci; NHANES = National Health and Nutrition Examination Survey; UACR = urinary albumin creatinine ratio; eGFR = estimated glomerular filtration rate.
Data sources
We initially obtained the latest summary statistics on LDL-C levels from GWAS which used data from UK Biobank25. Genetic instrumental variables for statins and PCSK9 inhibitors targets were genetic variants significantly associated with LDL-C levels (p < 5.0 × 10−8) and located within a 100kb window around HMGCR (Gene ID: 3156, Chromosome: 5) and PCSK9 (Gene ID: 255,738, Chromosome: (1). All selected single nucleotide polymorphisms (SNPs) allowed for weak linkage disequilibrium (LD clumping r2 < 0.3) and should have a minor allele frequency (MAF) greater than 1% with a F-statistic greater than 10 to minimize bias. In addition, summary statistics on LDL-C from the Global Lipids Genetics Consortium (GLGC) (https://csg.sph.umich.edu/willer/public/glgc-lipids2021/) were obtained for replication analyses.
For genetic variants associated with levels of drug targets gene expression and protein levels, we extracted cis-acting expression quantitative trait loci (cis-eQTL) that significantly associated with HMGCR and PCSK9 (p < 5.0 × 10−8) in whole blood and had an MAF greater than 1% from the eQTLGen Consortium (https://www.eqtlgen.org/cis-eqtls.html) and GTEx v8 (https://www.gtexportal.org/home/datasets), respectively, and obtained cis-acting protein quantitative trait loci (cis-pQTL) in plasma with a F-statistics greater than 10 from the deCODE genetics26.
Outcomes of this study were DN and DR, GWAS summary statistics were all obtained from the 2021 FinnGen study (DN: males and females with 3283 cases and 181,704 controls; DR: males and females with 14,584 cases and 176,010 controls), which have no overlap with the samples of exposure. In addition, the GWAS summary statistics for the outcome of coronary heart disease (CHD) used for positive control analysis were obtained from the CARDIoGRAMplusC4D Consortium (n = 184,305 males and females with 60,801 cases and 123,504 controls)27.
Except that individuals involved in CHD GWAS analyses are mixed populations, all other individuals involved in GWAS analyses are of European ancestry. All GWAS data sources are summarized in the S2 Table. The cross-sectional analysis data were sourced from NHANES 1999-March 2020, spanning ten survey cycles over 21.2 years24.
Statistical analysis
Mendelian randomization
To ensure effective causal inference, MR analysis requires the following conditions to be met: Instrumental variables should associate with the known downstream biomarker (relevance), should share no unmeasured common cause with the outcome (independence), and should affect the outcome only through the exposure (exclusion restriction)28.
In the primary analysis, drug-target MR was employed to investigate the associations of genetically proxied statins and PCSK9 inhibitors with DN and DR, and the random-effects inverse-variance weighted (IVW) model, which were adjusted for LD matrices (r2 < 0.3), was applied to estimate the effects. The weighted mode model, weighted median model, simple mode model, and MR Egger model were applied as sensitivity analyses. To assess the robustness of MR analyses, we used the Cochran Q statistic to detect heterogeneity, where p < 0.05 indicated heterogeneity. To ensure the validity of the exclusion restriction assumption in MR analysis, we employed several methods to detect and address potential horizontal pleiotropy. Specifically, we used the MR-Egger regression method to test for directional pleiotropy, where a non-zero intercept indicates the presence of pleiotropy. Additionally, we applied the MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test to identify and correct for outliers that may influence the results, with p < 0.05 indicating horizontal pleiotropy.
To validate the results of drug-target MR, two replication analyses were conducted. First, a stricter LD threshold (r2 < 0.1) was established to duplicate the above analysis. Second, genetic instrumental variables for statins and PCSK9 inhibitors were re-obtained from summary statistics on LDL-C levels from GLGC GWAS to perform MR analysis for validation. The effectiveness of instrumental variables in the primary analysis was verified by a positive control analysis, which used CHD, the main indication for lipid-lowering drugs, as an outcome for drug-target MR.
We also conducted a two-sample MR to investigate the associations between LDL-C levels and outcomes to explore whether the effects of statins on DN and DR were attributed to their LDL-C-lowering properties. In this analysis, genome-wide variants associated with LDL-C levels (p < 5 × 10–8) should have a low LD (r2 < 0.001), and the variants strongly associated with outcomes were excluded. GWAS summary statistics from GLGC were also used to perform replication analysis for validation.
In the secondary analysis, cis-eQTL and cis-pQTL of HMGCR and PCSK9 were used to perform two-sample MR to further corroborate the results of drug-target MR. Random-effects IVW method was employed to investigate the associations of genetically proxied targets gene expression in whole blood and circulating protein levels in plasma with DN and DR, along with the same sensitivity analysis methods, heterogeneity, and horizontal pleiotropy testing used in the primary analysis.
Furthermore, considering that one SNP may be related to the expression of multiple genes, leading to the presence of horizontal pleiotropy, we performed summary level data (data from GWAS and eQTL) based MR (SMR)29 analysis to identify adjacent genes and examine the associations of these genes and target genes expression with outcomes. Heterogeneity in dependent instruments (HEIDI) test was applied to test the linkage within the associations obtained from cis-eQTL MR results, with p < 0.01 indicating that associations are probably due to linkage30. The SMR and HEIDI methods can be interpreted as analysis to test if the effect size of an SNP on the phenotype is mediated by gene expression.
To correct for multiple analyses, we applied Bonferroni correction to adjust the significance level threshold in drug-target MR and two-sample MR analysis. SMR and HEIDI analysis were performed on SMR 1.3.1, and other MR analyses were performed on R 4.2.3 using the "TwoSampleMR" package.
Cross-sectional analysis using data from NHANES
To further consolidate the positive findings of MR analysis, we used completely different data sources and study methods to investigate the association between statin use and outcome-related indicators. A cross-sectional study utilizing data from NHANES1999-March2020 was performed to determine the associations between statin use and DR as well as DN-related indicators in diabetic individuals. We included all individuals aged 50–84 who self-reported having diabetes, and participants were categorized into two groups: the statins user group (ST) and the non-statins user group (non-ST). The outcomes of interest were self-reported DR, urinary albumin creatinine ratio (UACR), and eGFR. The formulas for UACR and eGFR are shown in the S3 Table.
All statistical analyses considered the complex survey design. Weighted logistic regression models or weighted linear regression models were used to estimate associations between statin use and outcomes. Stratified analyses were performed based on gender and race/ethnicity, which were self-reported by the participants in the survey questionnaire. All statistical analyses were conducted in R 4.2.3, and statistical significance was defined as two-sided P < 0.05. See Supplementary Text for detailed methods.
Ethics approval and consent to participate
Ethical approval was not required for this study as all data used were obtained from publicly available databases.
Results
Selection of instrumental variables
Of 19 and 33 genetic variants were used to proxy the inhibition of HMGCR and PCSK9, respectively (S4 Table). Details of the whole blood-derived cis-eQTL data and plasma-derived cis-pQTL data can be found in the S5 and S6 Table. For LDL-C levels, 176 and 80 genetic variants from the UK Biobank and the GLGC GWAS were identified (S7 Table).
Mendelian randomization results
In the primary analysis, we applied drug-target MR to investigate the association of the inhibition of HMGCR and PCSK9 with the risks of DN and DR. The results revealed a robust association between genetically proxied inhibition of HMGCR and higher risks of DN and DR (DN: IVW odds ratio [OR] = 1.79, 95% confidence interval [95% CI] = 1.14–2.78, p = 0.01; DR: OR = 1.41, 95% CI = 1.11–1.75, p = 0.004) (Fig. 2 and S8/S9 Table), persisting consistently after adjusting for LD threshold and varying data source (results from replication analyses) (Fig. 2 and S10/S11 Table). However, no such association was observed between the genetically proxied inhibition of PCSK9 and the risks of DN and DR (DN: OR = 1.23, 95% CI = 0.94–1.59, p = 0.13; DR: OR = 0.96, 95% CI = 0.83–1.12, p = 0.64) (Fig. 2 and S8/S9 Table). Two-sample MR results demonstrated no correlation between genetic instruments related to LDL-C levels and the risks of DN and DR (DN: OR = 0.96, 95% CI = 0.83–1.11, p = 0.56; DR: 1.02, 95% CI = 0.94–1.1, p = 0.62), replication analyses using GLGC data yielded consistent results ( S11//S12/S13 Table). These findings suggest that the therapeutic impact of lowering LDL-C may not influence the occurrence of DN and DR. However, statin use may potentially increase the risk of DN and DR through pathways independent of LDL-C-lowering effects. To validate the effectiveness of the genetic instruments for HMGCR and PCSK9, drug-target MR was performed using CHD as a positive control. The results demonstrated that the genetically proxied inhibition of both HMGCR and PCSK9 significantly reduced the risk of CHD (HMGCR inhibition: OR = 0.62, 95% CI = 0.52–0.64, p = 8.29E-8; PCSK9 inhibition: OR = 0.43, 95% CI = 0.37–0.51, p = 7.2E-24) (S14 Table), confirming the effectiveness of the genetic instruments.
Associations of statins on DN and DR: results from drug-target MR, cis-eQTL MR, cis-pQTL MR, and SMR. All results were obtained using the inverse-variance weighted method except SMR, and other methods are detailed in the Supplementary materials. Replication 1 analysis adjusted stricter LD threshold (r2 < 0.1); replication 2 analysis changed the data source to GLGC GWAS summary statistics. Horizontal pleiotropy exists in cis-eQTL analysis of HMGCR, cis-eQTL, and cis-pQTL analysis of PCSK9. After Bonferroni correction, p values < 0.0125 were considered strong evidence, while p values ranging from 0.0125 to < 0.05 were considered suggestive evidence. HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase; PCSK9 = proprotein convertase subtilisin/kexin type 9; cis-eQTL = cis-acting expression quantitative trait loci; cis-pQTL = cis-acting protein quantitative trait loci; MR = Mendelian randomization; DN = diabetic nephropathy; DR = diabetic retinopathy; OR = odds ratio; 95% CI = 95% confidence interval.
To triangulate the primary findings, we performed secondary MR analyses utilizing data associated with targets gene expression (cis-eQTL) and protein (cis-pQTL) levels. The results revealed the inhibition of the HMGCR expression in whole blood and the protein level in plasma was significantly associated with the higher risks of DN (cis-eQTL: OR = 1.54, 95% CI = 1.49–1.59, p < 0.0001; cis-pQTL: OR = 1.56, 95% CI = 1.14–2.13, p = 0.006) and DR (cis-eQTL: OR = 1.23, 95% CI = 1.22–1.25, p < 0.0001; cis-pQTL: OR = 1.22, 95% CI = 1.04–1.43, p = 0.02), and the inhibition of PCSK9 gene expression and protein level was associated with the higher risk of DN, but not DR (Fig. 2 and S15/S16/S17/S18 Table). However, sensitivity analyses indicated horizontal pleiotropy in cis-eQTL analysis for the association between HMGCR inhibition with DR and in cis-eQTL/cis-pQTL analyses for the association between PCSK9 inhibition and DN (MR-Egger: p = 0.034, p < 0.001, p < 0.01, respectively) (S16/S18 Table).
We further conducted SMR analysis and HEIDI test using cis-eQTL data to mitigate the impact of horizontal pleiotropy and linkage and for validation. SMR results similarly identified the significant association of the increased risks of DN and DR with the genetically proxied inhibition of HMGCR expression (DN: OR = 1.61, 95% CI = 1.12–2.94, p = 0.03; DR: OR = 1.28, 95% CI = 1.02–1.69, p = 0.02), but not with the inhibition of PCSK9 expression (DN: OR = 1.33, 95% CI = 1.04–1.85, p = 0.05; DR: OR = 1.04, 95% CI = 0.92–1.19, p = 0.57) (Fig. 2 and S19 Table). We also identified a gene, POC5, with significant genetic variants correlated with the top cis-eQTL of HMGCR. However, subsequent analyses did not reveal any association between the expression of POC5 and the outcomes (DN: p = 0.0533; DR: p = 0.243) (S20 Table). Results from the HEIDI test further suggested that these associations are unlikely to be due to linkage (the p-values are all greater than 0.05) (S19/S20 Table).
The detailed results of all MR analyses, as well as the sensitivity analyses, are presented in the corresponding supplementary materials, including the genetic instrumental variables used in each analysis.
Cross-sectional analysis results
This analysis included 4699 participants aged 50–84 who self-reported having diabetes. The median weighted age was 65 years, with 49.6% of the participants being male and 50.4% female. A total of 2336 participants (weighted percentage of 53%) reported using statins (Table 1). Baseline characteristics stratified by sex and race/ethnicity can be found in the S21/S22 Table.
In the univariate weighted logistic regression model, there was a positive association between statin use and a higher incidence of DR (Model 1: OR = 1.2, 95% CI = 1.01–1.42, p = 0.04) (Fig. 3). This association persisted after adjusting for age, sex, race, education level, and all confounding factors combined (Model 2: OR = 1.27, 95% CI = 1.07–1.51, p = 0.01; Model 3: OR = 1.26, 95% CI = 1.03–1.54, p = 0.03) (Fig. 3). In stratified multivariate models based on sex and race, this association remained significant in the male group and the White people group. At the same time, it was no longer significant in the other groups (Fig. 3). In the weighted univariate linear regression model, it was found that there was a significant negative correlation between statin use and an increase in eGFR (Model 1: Beta = − 5.2, 95% CI = − 7 to − 3.3, p < 0.0001) (Fig. 4). This association persisted after multivariate adjustments (Model 2: Beta = − 2.7, 95% CI = − 4.4 to − 1, p = 0; Model 3: Beta = − 1.9, 95% CI = − 3.7 to − 0.19, p = 0.03) (Fig. 4). Stratified analyses indicated that this association remained in the female group and the Black people group. However, across all analyses, no significant association was observed between statin use and UACR levels (Fig. 4).
Associations between statin use and DR, NHANES1999-March2020. All models use weighted logistic regression; model 1 is a univariate analysis; model 2 is adjusted based on gender, age, race, and education level; model 3 and stratified analysis are adjusted for all confounding factors, including age, gender, race, education level, poverty income ratio, hypoglycemic agent use, insulin use, years of diabetes, hypertension status, smoking history (having smoked 100 cigarettes in a lifetime), alcohol consumption frequency (12 times or more every year), BMI, Total cholesterol, HDL-C, LDL-C, Triglycerides, Glycohemoglobin, and Serum glucose; ST = statin users group; Non-ST = non-statin users group; DR = diabetic retinopathy; OR = odds ratio; 95% CI = 95% confidence interval.
Associations between statin use and eGFR/UACR, NHANES1999-March2020. All models use weighted linear regression; model 1 is a univariate analysis; model 2 is adjusted based on gender, age, race, and education level; model 3 and stratified analysis are adjusted for all confounding factors, including age, gender, race, education level, poverty income ratio, hypoglycemic agent use, insulin use, years of diabetes, hypertension status, smoking history (having smoked 100 cigarettes in a lifetime), alcohol consumption frequency (12 times or more every year), BMI, Total cholesterol, HDL-C, LDL-C, Triglycerides, Glycohemoglobin, and Serum glucose; ST = statin users group; Non-ST = non-statin users group; UACR = urinary Albumin Creatinine Ratio; eGFR = estimated Glomerular Filtration Rate; 95% CI = 95% confidence interval.
Discussion
This study represents the first application of MR to investigate the causal association between genetically proxied statins and the risks of DN and DR. Triangulation was implemented using different data sources and study methodologies. We identified compelling evidence supporting that the inhibition of HMGCR, the target of statins, may increase the risks of DN and DR. The results are further substantiated byreplication analysis. We also further explored the data through whole blood-derived gene expression and plasma-derived circulating protein levels data. However, some eQTL and pQTL results exhibited horizontal pleiotropy, raising concerns about their reliability. Despite this, our main finding remains that HMGCR inhibition significantly impacts DN and DR outcomes. Although the cis-eQTL analysis for the association between HMGCR inhibition with DR and cis-eQTL/cis-pQTL analyses for the association between PCSK9 inhibition and DN showed horizontal pleiotropy, further SMR analysis provided reliable results. Cross-sectional analysis results also suggest a potential association of statin use with higher DR incidence and reduced eGFR. While observational studies cannot establish causal evidence, we employed this approach, with data and potential biases sources unrelated to MR, for comparison against positive findings to enhance the reliability of our primary results. However, no reliable association was found between genetically proxied inhibition of PCSK9 and the risks of DN and DR. Additionally, two-sample MR analysis indicated that genetic variants of circulating LDL-C levels were unrelated to the outcomes. This suggests that the effects of genetically proxied statins on outcomes may occur through mechanisms independent of LDL-C-lowering.
Our findings are inconsistent with some previous research. For instance, an RCT involving 50 patients with a 180-day follow-up period demonstrated that simvastatin significantly delayed the progression of retinopathy in patients with diabetic dyslipidemia (p = 0.009)6. An observational nationwide study conducted by Nielsen SF and Nordestgaard BG5 in Denmark indicated that individuals who used statins before a diabetes diagnosis had a lower cumulative incidence of DR (Hazard Ratio [HR] = 0.60, 95% CI = 0.54–0.66, p < 0.0001), but this protective effect was not observed for DN. A multicenter cohort study conducted by Zhou S and colleagues in China revealed that the initiation of statin therapy in diabetic patients was associated with a lower incidence of DN (HR = 0.72, 95% CI = 0.62–0.83) and a reduced risk of renal function decline (HR = 0.6, 95% CI = 0.44–0.81)9. However, a recent cohort study indicated that statin use was associated with the progression of renal disease in diabetic patients (OR = 1.16, 95% CI = 1.12–1.20) and the incidence of ophthalmic manifestations (OR = 1.35, 95% CI = 1.27–1.44)31. Furthermore, recent findings from animal experiments suggest that long-term use of statins may exacerbate DN in diabetic mice via ectopic fat deposition15.
In the face of these inconsistent findings, conclusive causal evidence remains elusive. We entertain the suspicion that the effects of statins on DN and DR might vary with disease progression or prolonged medication use. Large-scale, long-term follow-up RCTs are still needed to obtain reliable evidence, our study may anticipate the outcomes of such investigations. Given that genetic variations are typically fixed at conception, the results of MR analyses are often interpreted as lifelong exposure19. Therefore, our findings might reflect long-term effects, potentially diverging from the clinical studies that assess the effects of medications within specific treatment periods.
Statins, on the one hand, have been demonstrated to possess lipid-lowering-independent effects that improve endothelial dysfunction32,33, which is a critical feature of vascular complications (including DN, DR, and atherosclerosis) in diabetes34, so it should have a protective effect on DN and DR. On the other hand, multiple studies indicated that statins may elevate blood glucose35 and glycated hemoglobin levels36, worsen glycemic control37, accelerate diabetes progression38, and exacerbate insulin resistance36,39,40. These factors may contribute to or exacerbate DN and DR. Furthermore, the latest animal experiment have found that prolonged use of statins not only activates the insulin-regulated Phosphatidylinositol 3-kinase, protein kinase B, mammalian target of rapamycin signaling pathways, leading to increased renal fatty acid synthesis, but also intensifies lipid uptake and inhibits fatty acid oxidation in the kidneys, resulting in ectopic lipid deposition and aggravation of renal damage in diabetic mice15. Therefore, considering the comprehensive findings of this study, we have grounds to believe that the multifaceted effects of statins, independent of their lipid-lowering action, may ultimately pose harm to certain diabetes complications. Substantial in vivo, and in vitro experiments, and clinical trials are still needed to ascertain effect estimates and mechanisms.
Understanding the potential impact of statins on the risks of diabetic nephropathy (DN) and diabetic retinopathy (DR) allows clinicians to better evaluate the risk–benefit profile of these drugs. For patients at high risk of these complications, it may be necessary to reassess the use of statins, implement additional monitoring measures, or consider alternative lipid-lowering therapies. These findings underscore the importance of personalized medicine, suggesting that genetic screening could help identify patients who are more likely to benefit from certain lipid-lowering treatments while minimizing the risk of adverse effects. By integrating these insights into clinical practice, healthcare providers can optimize therapeutic efficacy and safety, ultimately improving patient outcomes.
There are several limitations to this work. First, the MR analysis focused exclusively on the European population, and the NHANES analysis was limited to the American population. Therefore, the generalizability of our results to other racial or ethnic groups may be limited. Second, we did not analyze other microvascular complications of diabetes due to the unavailability of related GWAS data. Third, our results are based on estimating effects using genetic proxies for drug targets, which do not consider drug dosage, duration, and other environmental variables. MR results are typically interpreted as lifetime exposure19, and the associations may vary throughout the life course20, which may lead to inconsistencies with clinical research findings. Further evidence may be needed through individual-level MR data. Fourth, the sample sizes for cis-eQTL of PCSK9 and cis-pQTL of HMGCR data were limited, and there are currently no available cis-eQTL data related to target genes in liver tissue. Further analysis based on these data might yield more valuable insights. Fifth, our findings do not provide additional evidence for the mechanisms through which statins exert their effects on DN and DR, they can only offer possibilities. Sixth, the individual diagnosis of DN cannot be determined in the NHANES. Instead, we used relevant indicators of DN diagnosis to analyze the potential impact of statins on renal function in diabetic individuals. Last, regardless of the study design, confounding and bias cannot be eliminated. However, we used a triangulation design to strengthen our findings.
In conclusion, our study provides evidence for a causal association between genetically proxied statins and increased risks of DN and DR. Importantly, this relationship does not appear to be mediated through the reduction of LDL-C. For patients with diabetic dyslipidemia, PCSK9 inhibitors may be a preferable alternative. Further evidence through RCTs and multiomics studies or animal experiments is needed to explore the underlying mechanisms.
Data availability
Summary statistics of genome-wide association studies (GWAS) and data from the National Health and Nutrition Examination Survey (NHANES) used in this study are publicly available. All GWAS data sources are summarized in the S2 Table. All statistical codes can be requested from WL Wang or R Zhao. Links: https://pubmed.ncbi.nlm.nih.gov/32203549/ (GWAS of LDLC in UK biobank), https://csg.sph.umich.edu/willer/public/glgc-lipids2021/ (GWAS of LDLC in GLGC), https://storage.googleapis.com/finngen-public-data-r5/summary_stats/ (GWAS of DN and DR), https://pubmed.ncbi.nlm.nih.gov/26343387/ (GWAS of CHD), https://www.eqtlgen.org/cis-eqtls.html (eQTL data of HMGCR), https://www.gtexportal.org/home/datasets (eQTL data of PCSK9), https://pubmed.ncbi.nlm.nih.gov/34857953/ (pQTL data of HMGCR and PCSK9), https://www.cdc.gov/nchs/nhanes/index.htm (NHANES).
Abbreviations
- CVD:
-
Cardiovascular diseases
- DN:
-
Diabetic nephropathy
- DR:
-
Diabetic retinopathy
- RCT:
-
Randomized controlled trial
- MR:
-
Mendelian randomization
- LDL-C:
-
Low-density lipoprotein cholesterol
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
- cis-eQTL:
-
Cis-expression quantitative trait loci
- cis-pQTL:
-
Cis-protein quantitative trait loci
- SMR:
-
Summary data-based Mendelian randomization
- NHANES:
-
The National Health and Nutrition Examination Survey
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- IVs:
-
Instrumental variables
- GWAS:
-
Genome wide association study
- SNP:
-
Single nucleotide polymorphism
- MAF:
-
Minor allele frequency
- LD:
-
Linkage disequilibrium
- IVW:
-
Inverse-variance weighted
- MR-PRESSO:
-
MR pleiotropy residual sum and outlier
- ST:
-
Statin group
- GLGC:
-
Global Lipids Genetics Consortium
- CHD:
-
Coronary heart disease
- UACR:
-
Urinary albumin creatinine ratio
- eGFR:
-
Estimated glomerular filtration rate
- OR:
-
Odds ratio
References
Cholesterol Treatment Trialists’ (CTT) Collaboration et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet Lond. Engl. 385, 1397–405 (2015).
Ross, S., Gerstein, H. & Paré, G. The genetic link between diabetes and atherosclerosis. Can. J. Cardiol. 34, 565–574 (2018).
McGarry, J. D. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51, 7–18 (2002).
Brandts, J. et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in type 1 diabetes and type 2 diabetes: Lipid goal attainment in a large German-Austrian diabetes registry. Diabetes Obes. Metab. 25, 3700–3708 (2023).
Nielsen, S. F. & Nordestgaard, B. G. Statin use before diabetes diagnosis and risk of microvascular disease: A nationwide nested matched study. Lancet Diabetes Endocrinol. 2, 894–900 (2014).
Sen, K., Misra, A., Kumar, A. & Pandey, R. M. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res. Clin. Pract. 56, 1–11 (2002).
Gaede, P., Lund-Andersen, H., Parving, H.-H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 26, 77–82 (2008).
Zhou, S. et al. Statin initiation and risk of incident kidney disease in patients with diabetes. CMAJ Can. Med. Assoc. J. J. Assoc. Med. Can. 195, E729–E738 (2023).
Mansi, I. A. et al. Statins and renal disease progression, ophthalmic manifestations, and neurological manifestations in veterans with diabetes: A retrospective cohort study. PLoS One 17(7), e0269982. https://doi.org/10.1371/journal.pone.0269982 (2022).
Haynes, R. et al. Effects of lowering LDL cholesterol on progression of kidney disease. J. Am. Soc. Nephrol. 25, 1825–1833 (2014).
Chung, Y.-H. et al. Statins of high versus low cholesterol-lowering efficacy and the development of severe renal failure. Pharmacoepidemiol. Drug Saf. 22, 583–592 (2013).
Marcovecchio, M. L. et al. ACE inhibitors and statins in adolescents with type 1 diabetes. N. Engl. J. Med. 377, 1733–1745 (2017).
Mansi, I. A. et al. Association of statin therapy initiation with diabetes progression. JAMA Intern. Med. 181, 1–14 (2021).
Huang, T. et al. Long-term statins administration exacerbates diabetic nephropathy via ectopic fat deposition in diabetic mice. Nat. Commun. 14, 390 (2023).
Zhou, Y. et al. HMG-CoA reductase degrader, SR-12813, counteracts statin-induced upregulation of HMG-CoA reductase and augments the anticancer effect of atorvastatin. Biochem. Biophys. Res. Commun. 677, 13–19 (2023).
Walker, V. M., Davey Smith, G., Davies, N. M. & Martin, R. M. Mendelian randomization: A novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int. J. Epidemiol. 46, 2078–2089 (2017).
Sanderson, E., Richardson, T. G., Morris, T. T., Tilling, K. & Davey, S. G. Estimation of causal effects of a time-varying exposure at multiple time points through multivariable mendelian randomization. PLoS Genet. 18, e1010290 (2022).
Fang, S. et al. Association between genetically proxied PCSK9 inhibition and prostate cancer risk: A Mendelian randomisation study. PLoS Med. 20, e1003988 (2023).
Richardson, T. G., Sanderson, E., Elsworth, B., Tilling, K. & Davey, S. G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ. 369, m1203 (2020).
Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 290, 140–205 (2019).
Lawlor, D. A., Tilling, K. & Davey, S. G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45, 1866–1886 (2016).
Munafò, M. R. & Davey, S. G. Robust research needs many lines of evidence. Nature 553, 399–401 (2018).
NHANES - National Health and Nutrition Examination Survey Homepage [Internet]. 2023 [cited 2023 Oct 19]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm
Richardson, T. G. et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 17, e1003062 (2020).
Willer, C. J. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Nikpay, M. et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130 (2015).
Schmidt, A. F. et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 11, 3255 (2020).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Chauquet, S. et al. Association of antihypertensive drug target genes with psychiatric disorders: a Mendelian randomization study. JAMA Psychiatry. 78, 623–631 (2021).
Mansi, I. A. et al. Statins and renal disease progression, ophthalmic manifestations, and neurological manifestations in veterans with diabetes: A retrospective cohort study. PloS One 17, e0269982 (2022).
Liu, C. et al. Statins improve endothelial function via suppression of epigenetic-driven EndMT. Nat. Cardiovasc. Res. 2, 467–485 (2023).
Lahera, V. et al. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: Beneficial effects of statins. Curr. Med. Chem. 14, 243–8 (2007).
Xu, S. et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 73, 924–967 (2021).
Swerdlow, D. I. et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet Lond. Engl. 385, 351–361 (2015).
Alvarez-Jimenez, L., Morales-Palomo, F., Moreno-Cabañas, A., Ortega, J. F. & Mora-Rodríguez, R. Effects of statin therapy on glycemic control and insulin resistance: A systematic review and meta-analysis. Eur. J. Pharmacol. 947, 175672 (2023).
Liew, S. M. et al. Statins use is associated with poorer glycaemic control in a cohort of hypertensive patients with diabetes and without diabetes. Diabetol. Metab. Syndr. 6, 53 (2014).
Odeleye, V., Masarweh, O., Restrepo, J., Alvarez, C.A. & Mansi, I.A. Association of strict versus lenient cholesterol lowering with cardiac outcomes, diabetes progression and complications, and mortality in patients with diabetes treated with statins: Is less more? Drug Saf. (2023)
Grunwald, S. A. et al. Statins aggravate the risk of insulin resistance in human muscle. Int. J. Mol. Sci. 23, 2398 (2022).
Koh, K. K. et al. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 55, 1209–1216 (2010).
Acknowledgements
Thanks to all institutions or organizations that provided accessible data. We thank Dr. Joshua D. Bundy from Tulane University for providing some statistical codes.
Funding
This study was supported by the China Academy of Chinese Medical Sciences (Award number CI2021A01009) and the National Natural Science Foundation of China (Award number 8227153721). The funders of this study were not involved in any research process.
Author information
Authors and Affiliations
Contributions
R.Z., W.L.W., and W.Z. contributed equally to study design, investigation, methodology, data acquisition, data analysis, validation, data visualization, and manuscript writing. J.P.L. contributed to methodology, supervision, and manuscript writing. Y.L., J.G., L.Y., and Z.D.Z. contributed to method evaluation, data curation, and data analysis. C.H. and G.X.Y contributed to data analysis and validation, as well as manuscript writing. B.W. has contributed to funding acquisition, study design, and supervision. All authors contributed to manuscript reviewing and approved the final version. R.Z. and W.L.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, R., Wang, W., Zhang, W. et al. Effects of genetically proxied statins on diabetic nephropathy and retinopathy: a Mendelian randomization study. Sci Rep 14, 16885 (2024). https://doi.org/10.1038/s41598-024-67800-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67800-5
- Springer Nature Limited