Abstract
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease. However, the relationship between the systemic immune-inflammation index (SII) and the prognosis of RA patients remains unclear. This study aimed to investigate the association between inflammatory biomarker SII and all-cause and cardiovascular mortality in RA patients. A retrospective analysis was conducted using data from the National Health and Nutrition Examination Survey database spanning from 1999 to March 2020. We assessed the association between the SII and all-cause as well as cardiovascular mortality in RA patients employing multivariable Cox proportional hazards regression analysis and restricted cubic spline plots. Receiver operating characteristic curves were employed to evaluate the prognostic capacity of SII in predicting outcomes in both the RA patients and the general population, alongside its predictive performance compared to other markers. This study comprised 2247 RA patients and a control cohort of 29,177 individuals from the general population. Over a 20-year follow-up period, 738 all-cause deaths and 215 deaths attributable to cardiovascular disease were documented in RA patients. We observed a nonlinear positive correlation between the SII and both all-cause and cardiovascular mortality in RA patients. Of significance, at an SII level of 529.7, the hazard ratio reached 1, signifying a transition from low to high mortality risk. Moreover, subgroup analysis did not reveal any potential interactions. Our study findings indicate a nonlinear positive correlation between the inflammatory biomarker SII and both all-cause and cardiovascular mortality in patients with RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease characterized by polyarticular involvement and synovial inflammation1,2, often leading to severe symptoms and signs such as joint pain, cartilage destruction, bone erosion, and joint deformity3. These manifestations significantly impact patients' medical expenses and quality of life. Global statistics indicate that approximately 1% of the population is affected by RA, with an estimated increase to 31.7 million cases by 20504,5. The consequences of RA extend beyond joint disability, contributing to increased long-term mortality and reduced life expectancy, with cardiovascular disease being a major factor6,7.
Despite the complex pathogenesis of RA and its lack of specific etiology, previous research has demonstrated a close association between cardiovascular risk or other adverse outcomes in RA patients, and systemic inflammatory responses7,8. Furthermore, the SII has been extensively investigated as an indicator that reflects the level of systemic inflammation and immune response9,10. The SII is determined through the formula: (platelet count × neutrophil count)/lymphocyte count. Therefore, utilizing the biomarker SII, which represents the level of systemic immune-inflammation, to assess the risk of all-cause or cardiovascular mortality in RA patients has become a feasible approach. This inflammation biomarker SII offers the advantages of being easy to collect, providing rapid results, low cost, and high efficiency, obtainable through routine blood tests.
This study selected the hematological inflammatory biomarker SII, widely recognized to reflect the level of systemic immune-inflammation, to explore its potential association with mortality in RA patients. The aim is to provide clinicians with a convenient and reliable new tool and insights for prognostic identification of RA patients. Understanding the risk factors influencing mortality in RA patients is crucial for effective management and early intervention to improve patient survival, especially for RA patients at risk of premature cardiovascular-related mortality.
The NHANES is a nationally representative cross-sectional survey aimed at assessing the health and nutritional status of the U.S. population11. Leveraging the large-scale national sample information from the NHANES database, this study evaluated the association between the novel inflammatory biomarker SII and all-cause mortality and cardiovascular mortality in RA patients. Therefore, the hypothesis posited is that SII could serve as a prognostic biomarker for RA patients.
Methods
Study design and data collection
This retrospective cohort study utilized data extracted from the 1999–2020 NHANES in the United States. NHANES, accessible at http://www.cdc.gov/nchs/nhanes, is a comprehensive survey that represents the U.S. population. It employs a complex, multi-stage, stratified design, incorporating household interviews and examinations conducted in Mobile Examination Centers (MEC). The database is managed and maintained by the National Center for Health Statistics (NCHS). All participants provided written informed consent, and the study obtained approval from the NCHS Institutional Review Board (IRB), with detailed information available at https://www.cdc.gov/nchs/nhanes/irba98.htm. Since NHANES is a publicly available database containing anonymous individual information, no additional ethical approval or informed consent was necessary. Moreover, the methods employed in this study strictly adhered to pertinent guidelines and regulations.
Study population
The study population consisted of adult participants diagnosed with RA alongside individuals from the general population. Exclusions were made based on specific criteria: (1) age below 20 years; (2) presence of pre-existing cancer or cardiovascular disease; (3) diagnosis of alternative forms of arthritis; (4) absence of essential hematologic laboratory test results or covariate data; (5) unavailability or undisclosed mortality data; and (6) inability to acquire sample data weights.
Identification of RA patients and calculation of SII
Diagnosis of RA was ascertained through a questionnaire including questions such as "Has a doctor ever told you that you have arthritis?" (MCQ160a) with response choices "Yes" or "No" and "What type of arthritis was it?" (MCQ195; MCQ191; MCQ190) offering options like Rheumatoid arthritis, Osteoarthritis, Psoriatic arthritis, Other. This approach has been widely employed in various NHANES studies related to RA12,13.
Hematologic laboratory indicators required for this study were sourced from NHANES laboratory data. Whole blood cell counts were determined utilizing the Beckman Coulter method. Additionally, white blood cell differential counts were conducted employing VCS technology. For comprehensive procedural details, readers are directed to Chapter 7 of the NHANES Laboratory Procedures Manual. The SII was calculated using the formula: SII = (platelet count × neutrophil count)/lymphocyte count.
Ascertainment of mortality
To determine the mortality status of the follow-up population, we utilized the National Death Index (NDI) public-use linked mortality file as of December 31, 2019, linked with the NHANES data through a probabilistic matching algorithm. Additionally, causes of death were ascertained using the International Classification of Diseases, 10th Revision (ICD-10)14.
Covariates
This study encompassed covariates based on independent risk factors for RA identified in previous research. Covariates included age, gender, race, income-to-poverty ratio, education level, body mass index (BMI), diabetes, hypertension, triglycerides, total cholesterol, smoking, and alcohol consumption to control for confounding bias. Trained interviewers conducted household and sampled population surveys using the Computer Assisted Personal Interview (CAPI) system. These surveys collected information such as age, gender, race, income-to-poverty ratio, and education level. Body measurement data were collected by health technicians at MEC, with BMI calculated as weight (in kilograms) divided by height (in meters) squared. BMI categories included normal weight (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25–29.9 kg/m2), obese (BMI: 30–39.9 kg/m2), and morbidly obese (BMI ≥ 40 kg/m2). NHANES employs automated analyzers to enzymatically measure plasma triglyceride and total cholesterol levels from fasting blood samples collected from individuals who fasted for a minimum of 8 h but less than 24 h.
Smoking status was classified into never smokers, former smokers, and current smokers based on responses to survey questions SMQ020 (having ever smoked 100 cigarettes) and SMQ040 (current smoking status). Never smokers were individuals who had not smoked 100 cigarettes in their lifetime and were not currently smoking. Current smokers were those who had smoked over 100 cigarettes and were currently smoking. Former smokers were individuals who had smoked over 100 cigarettes but were not currently smoking. Alcohol consumption status was categorized as heavy (self-reported consumption of ≥ 4 or 5 drinks per day), moderate (self-reported ≤ 3 drinks per day), light (reported past drinking but not in the last year or fewer than 12 occasions in total), and never drinkers (self-reported never drank). Diabetes and hypertension patients were identified through various methods to ensure accuracy. Diabetes identification included questions such as "Has a doctor ever told you that you have diabetes?", "Are you taking insulin?", "Are you taking oral hypoglycemic drugs?", laboratory data with fasting blood glucose concentration ≥ 7.0 mmol/L or HbA1c ≥ 6.5%, or oral glucose tolerance test (OGTT) blood glucose level ≥ 11.1 mmol/L. Similarly, hypertension was determined based on blood pressure readings of ≥ 130/80 mmHg on three or more occasions, or self-reported multiple doctor diagnoses of hypertension.
Statistical analysis
To ensure national representation of the sample and address factors like oversampling and non-response, this study applied MEC weights provided in the NHANES weighting guidelines (https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx). Continuous variables in the baseline demographic table were presented as weighted means ± standard errors, while categorical variables were shown as frequencies and weighted proportions. Differences between SII quartiles (Q1–Q4) groups were assessed using chi-square tests (for categorical variables) or Kruskal–Wallis tests (for continuous variables). Multivariable Cox proportional hazards regression analysis was employed to evaluate the association between SII and mortality, with models adjusted for demographic characteristics (model 2) and all covariates (model 3). Hazard Ratios (HR) and 95% confidence intervals (CI) quantified these associations.
The restricted cubic spline plots were used to visualize the potential nonlinear association between SII and mortality. ROC curves were employed to assess the prognostic utility of SII within distinct cohorts: RA patients and the general population. Additionally, ROC analysis facilitated comparisons of SII's predictive performance against alternative biomarkers. Threshold effect analysis was performed to detail the association between SII and mortality and determine risk thresholds. Subgroup analysis were conducted to evaluate potential interactions between SII and other variables, validating the robustness of the conclusions. All P-values were two-sided, and statistical significance was set at P < 0.05. All statistical analyses were performed using IBM SPSS Statistics 25.0 for Windows (IBM Corporation, Armonk, NY, USA) and R version 4.3.1 for Windows (R Foundation for Statistical Computing, Boston, MA, USA).
Ethics approval
All participants provided written informed consent before undergoing the NHANES survey, and the survey received approval from the NCHS Institutional Review Board (IRB), as detailed at https://www.cdc.gov/nchs/nhanes/irba98.htm. As NHANES is a publicly available database with anonymized personal information, no additional ethical approval or informed consent was necessary.
Results
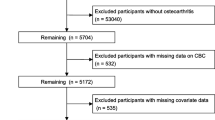
From 1999 to March 2020, a total of 126,130 participants were involved in the NHANES survey. Following the exclusion of individuals who did not meet the research criteria or had missing data, the final cohort comprised 2247 patients with RA. A detailed description of the selection process can be found in Fig. 1. The average age of the participants was 61 ± 14 years, with 41.2% male and 58.8% female. Table 1 presents baseline characteristics, indicating significant differences (p < 0.05) in age, race, BMI, smoking, triglycerides, alcohol consumption, and diabetes among the groups stratified by SII quartiles. Over the 20-year follow-up period, 738 deaths from all causes and 215 deaths related to cardiovascular issues were documented.
The multivariable Cox proportional hazards regression analysis unveiled a nonlinear positive association between SII levels and mortality, which remained consistent even after appropriate or thorough adjustments to the model (models 2 and 3). Specifically, for every increase of 100 units in SII level, the risks of all-cause mortality and cardiovascular mortality rose by 6% (95% CI 1.04–1.07) and 6% (95% CI 1.04–1.09), respectively. Furthermore, compared to the lowest quartile (Q1), patients in the highest quartile (Q4) of SII faced significantly heightened risks of experiencing fatal outcomes (all-cause or cardiovascular mortality) (HR = 2.63, 95% CI 2.06–3.35; HR = 2.86, 95% CI 1.82–4.51). Thus, our study confirms that the positive correlation between SII levels and mortality holds true, whether assessed as a continuous or categorical variable, with detailed effect sizes presented in Table 2.
Restricted cubic spline plots vividly illustrate the nonlinear relationship between the SII and both all-cause and cardiovascular mortality (nonlinear P < 0.05, Fig. 2), while adjusting for all covariates considered in this study. Notably, at an SII level of 529.7, the HR for all-cause and cardiovascular mortality reaches 1, indicating a critical threshold beyond which the prognosis for RA patients deteriorates. Consequently, this threshold serves as a pivotal determinant for distinguishing between high and low mortality risk among RA patients. Subsequent threshold effect analysis, utilizing this delineating threshold with a simplified two-segment linear model, reveals that in the high-risk RA subgroup (SII > 529.7), each increment of 100 units in SII corresponds to a 3% increase in the risks of all-cause and cardiovascular mortality (Table 3). In the subgroup analysis, categorizing patients into high-risk and low-risk groups further highlights significant differences. Compared to low-risk RA patients, those with high-risk RA face a 2.23-fold and 2.52-fold higher risk of all-cause and cardiovascular mortality, respectively (Fig. 3, overall). Additionally, subgroup analysis did not unveil significant variations in the association between SII levels and mortality risk across different populations (Fig. 3).
Association between SII with all-cause (A) and cardiovascular mortality (B) in participants with RA, adjusted for sex, age, race, education, poverty-to-income ratio, hypertension, diabetes, BMI, triglycerides, total cholesterol, alcohol use, and smoking. The shaded areas represent the 95% confidence intervals.
The ROC curve analysis delineates the prognostic efficacy of SII across distinct populations, comprising both RA patients and the general population, underscoring its better predictive capacity for mortality risk in RA patients. Moreover, our ROC analysis conclusively establishes the heightened superiority of the inflammatory biomarker SII over the established biomarker neutrophil-to-lymphocyte ratio (NLR) in forecasting the prognosis of RA patients. In essence, SII not only demonstrates elevated specificity in predicting mortality risk among RA patients but also surpasses the discernible prognostic value offered by other known markers (Fig. 4).
ROC curves used to assess the prognostic capability of SII in predicting outcomes for both RA patients and the general population, in addition to its predictive performance compared to the NLR marker. (A) all-cause mortality and (B) cardiovascular mortality; AUC, area under the curve; SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio.
Discussion
Our study elucidates a nonlinear positive correlation between SII, an inflammatory biomarker, and both all-cause and cardiovascular mortality rates among RA patients. Through statistical analyses, we pinpointed a critical threshold (SII = 529.7) beyond which heightened mortality risks are evident, urging clinicians to exercise increased vigilance when encountering RA patients surpassing this threshold.
The persistent activation of the immune system and chronic inflammation are pivotal elements in the progression of RA15. The construction of the inflammatory biomarker SII principally involves neutrophils and platelets. Prior to this, researchers Dessein et al. identified a noteworthy correlation between neutrophil count and atherosclerosis in RA patients16,17. Neutrophils play a pivotal role in the systemic immune-inflammatory response in RA through mechanisms such as neutrophil extracellular traps (NETs)18,19,20. Initially identified for their pathogen capture and elimination capabilities21, NETs, comprising histones and DNA fibers (release of intracellular components), have been implicated in triggering autoimmune chronic inflammatory responses and subsequent tissue damage19,22. Recent research underscores the direct contribution of NETs to synovial inflammation, cartilage damage, and bone erosion associated with RA18,23. NETs play a role in activating fibroblast-like synoviocytes (FLS) in the synovium, crucial in mediating cartilage damage23,24. Furthermore, NETs are involved in the induction of autoantigens19. Citrullinated proteins within NETs are believed to serve as a source of citrullinated autoantigens, culminating in the production of pathogenic anti-citrullinated protein antibodies (ACPA)25. Studies by Khandpur et al.26 have demonstrated the specific binding of citrullinated fibrinogen in NETs to ACPAs in RA patients, indirectly confirming the potential origin of ACPAs.
In addition to their pathogenic role via NETs, neutrophils contribute to RA development through their cytotoxic and pro-inflammatory properties27,28. As the predominant cell type in the synovial fluid of RA patients29, activated neutrophils perpetuate immune response regulation by secreting cytokines like B cell-activating factor (BAFF) and receptor activator of nuclear factor kappa-B ligand (RANKL)30,31. BAFF stimulates B cells to produce autoantibodies32, while RANKL mediates osteoclast differentiation and is an instrumental factor in RA-related bone erosion33. Neutrophil cytotoxicity involves the release of various cytoplasmic granules post-immune stimulation28, with neutrophil elastase directly contributing to cartilage damage by breaking down collagen, elastin, lubricin, and other substances34. Studies by Fernandes and Odobasic have highlighted a substantial increase in myeloperoxidase (MPO) levels in the plasma, synovial fluid, and tissues of RA patients35,36. MPO, the most abundant cytotoxic enzyme in neutrophil azurophilic granules, alongside reactive oxygen species (ROS) production, exacerbates the inflammatory response, intensifying the cytotoxic process37.
Olumuyiwa and Steven propose platelets as innate immune cells propelling RA pathogenesis38,39. Mounting evidence supports increased platelet and platelet microparticle (PMP) levels in the synovial fluid of RA patients40. During active RA, platelets and PMPs migrate to joints alongside leukocytes40,41. Subsequent activation of PMPs and leukocytes prompts the secretion of diverse pro-inflammatory chemokines and cytokines, signaling to FLS within the synovial cavity42,43. Excessive proliferation of FLS leads to pannus formation, invading bone and cartilage, laying the pathological foundation of RA44.
Bryant R. England explains the elevated cardiovascular mortality in RA patients via shared immune-inflammatory responses, heightened oxidative stress, and endothelial dysfunction mechanisms45. These excessive immune-inflammatory responses not only expedite RA progression but also inflict irreversible cardiovascular endothelial damage, culminating in increased mortality and adverse cardiovascular events46. Therefore, addressing the hyperactive immune-inflammatory response could potentially decelerate RA progression and mitigate adverse outcomes.
To the best of our knowledge, this is the pioneering study scrutinizing the relationship between the inflammatory biomarker SII and All-cause mortality as well as Cardiovascular mortality in RA patients, based on an extensive sample sourced from the American NHANES database. Our study aims to pinpoint indicators predictive of high all-cause and cardiovascular mortality risk in RA patients, equipping clinicians with a novel approach to early identification of high-risk RA patients. Furthermore, these emerging biomarkers derived from peripheral blood offer accessibility, cost-effectiveness, and broad applicability. Therefore, the innovative inflammatory biomarker SII expounded in this study holds substantial clinical significance in prognosticating outcomes for RA patients.
Limitations
Several limitations of our study warrant thorough acknowledgment. Firstly, the reliance on cross-sectional laboratory tests may limit our ability to capture longitudinal changes or responses to interventions accurately. Dynamic monitoring of SII levels would offer a more nuanced understanding of fluctuations in the body's immune-inflammatory status over time. Secondly, the data sourced from the NHANES database hinged on household interviews and questionnaire surveys, potentially introducing inaccuracies in reporting or recall biases. Despite stringent validation procedures by authoritative organizations like NCHS, the inherent risk of such limitations persists. Thirdly, while we meticulously accounted for numerous covariates, the presence of unidentified confounding factors could still impact the validity of our conclusions, particularly indicators reflecting RA disease activity. Finally, this study did not include a comparison of the inflammatory biomarker SII with certain RA-specific cardiovascular risk scores. Further investigation is warranted to determine whether the SII offer additional value beyond the currently employed standards.
Conclusions
Our study has unveiled a non-linear positive correlation between the inflammatory biomarker SII and both all-cause mortality and cardiovascular mortality in RA patients. Notably, when SII levels surpass the threshold of 529.7, it signals the need for clinicians' vigilance towards adverse outcomes. This finding equips clinicians with a novel approach to promptly identify RA patients at heightened risk of mortality. Consequently, our research underscores the clinical utility of the novel inflammatory biomarker SII in prognosticating outcomes for RA patients.
Data availability
The data supporting the conclusions of this article can be accessed at NHANES (https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics and https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory ).
Abbreviations
- RA:
-
Rheumatoid arthritis
- NHANES:
-
National Health and Nutrition Examination Survey
- SII:
-
Systemic immune-inflammation index
- BMI:
-
Body mass index
- NCHS:
-
National center for health statistics
References
Weyand, C. M. & Goronzy, J. J. The immunology of rheumatoid arthritis. Nat. Immunol. 22, 10–18. https://doi.org/10.1038/s41590-020-00816-x (2021).
Weyand, C. M. & Goronzy, J. J. Immunometabolism in the development of rheumatoid arthritis. Immunol. Rev. 294, 177–187. https://doi.org/10.1111/imr.12838 (2020).
Babaahmadi, M. et al. Rheumatoid arthritis: The old issue, the new therapeutic approach. Stem Cell Res. Ther. 14, 268. https://doi.org/10.1186/s13287-023-03473-7 (2023).
van der Woude, D. & van der Helm-van Mil, A. H. M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 32, 174–187. https://doi.org/10.1016/j.berh.2018.10.005 (2018).
Black, R. J. et al. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 5, e594–e610. https://doi.org/10.1016/s2665-9913(23)00211-4 (2023).
Schattner, A. The cardiovascular burden of rheumatoid arthritis—implications for treatment. Am. J. Med. 136, 1143–1146. https://doi.org/10.1016/j.amjmed.2023.09.004 (2023).
Hansildaar, R. et al. Cardiovascular risk in inflammatory arthritis: Rheumatoid arthritis and gout. Lancet Rheumatol. 3, e58–e70. https://doi.org/10.1016/s2665-9913(20)30221-6 (2021).
DeMizio, D. J. & Geraldino-Pardilla, L. B. Autoimmunity and inflammation link to cardiovascular disease risk in rheumatoid arthritis. Rheumatol. Ther. 7, 19–33. https://doi.org/10.1007/s40744-019-00189-0 (2020).
Zhao, E., Cheng, Y., Yu, C., Li, H. & Fan, X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann. Med. 55, 2197652. https://doi.org/10.1080/07853890.2023.2197652 (2023).
Cao, Y. et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front. Immunol. 14, 1087345. https://doi.org/10.3389/fimmu.2023.1087345 (2023).
Hartwell, M. L., Khojasteh, J., Wetherill, M. S., Croff, J. M. & Wheeler, D. Using structural equation modeling to examine the influence of social, behavioral, and nutritional variables on health outcomes based on NHANES data: Addressing complex design, nonnormally distributed variables, and missing information. Curr. Dev. Nutr. 3, nzz010. https://doi.org/10.1093/cdn/nzz010 (2019).
Liu, B., Wang, J., Li, Y. Y., Li, K. P. & Zhang, Q. The association between systemic immune-inflammation index and rheumatoid arthritis: Evidence from NHANES 1999–2018. Arthritis Res. Ther. 25, 34. https://doi.org/10.1186/s13075-023-03018-6 (2023).
Zhou, E., Wu, J., Zhou, X. & Yin, Y. The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: Results from NHANES 1999–2020. Front. Immunol. 14, 1309835. https://doi.org/10.3389/fimmu.2023.1309835 (2023).
Brämer, G. R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat. Q. Rapp. Trimest. Stat. Sanit. Mond. 41, 32–36 (1988).
Shrivastava, A. K. & Pandey, A. Inflammation and rheumatoid arthritis. J. Physiol. Biochem. 69, 335–347. https://doi.org/10.1007/s13105-012-0216-5 (2013).
Dessein, P. H. et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J. Rheumatol. 32, 435–442 (2005).
Dessein, P. H., Norton, G. R., Woodiwiss, A. J., Joffe, B. I. & Wolfe, F. Influence of nonclassical cardiovascular risk factors on the accuracy of predicting subclinical atherosclerosis in rheumatoid arthritis. J. Rheumatol. 34, 943–951 (2007).
O’Neil, L. J. et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann. Rheum. Dis. 82, 630–638. https://doi.org/10.1136/ard-2022-223568 (2023).
Fresneda Alarcon, M., McLaren, Z. & Wright, H. L. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O. Front. Immunol. 12, 649693. https://doi.org/10.3389/fimmu.2021.649693 (2021).
Zhang, L., Yuan, Y., Xu, Q., Jiang, Z. & Chu, C. Q. Contribution of neutrophils in the pathogenesis of rheumatoid arthritis. J. Biomed. Res. 34, 86–93. https://doi.org/10.7555/jbr.33.20190075 (2019).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science (New York, NY) 303, 1532–1535. https://doi.org/10.1126/science.1092385 (2004).
Apel, F., Zychlinsky, A. & Kenny, E. F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 14, 467–475. https://doi.org/10.1038/s41584-018-0039-z (2018).
Carmona-Rivera, C. et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight https://doi.org/10.1172/jci.insight.139388 (2020).
Tolboom, T. C. et al. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 52, 1999–2002. https://doi.org/10.1002/art.21118 (2005).
Corsiero, E., Pratesi, F., Prediletto, E., Bombardieri, M. & Migliorini, P. NETosis as source of autoantigens in rheumatoid arthritis. Front. Immunol. 7, 485. https://doi.org/10.3389/fimmu.2016.00485 (2016).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra140. https://doi.org/10.1126/scitranslmed.3005580 (2013).
O’Neil, L. J. & Kaplan, M. J. Neutrophils in rheumatoid arthritis: Breaking immune tolerance and fueling disease. Trends Mol. Med. 25, 215–227. https://doi.org/10.1016/j.molmed.2018.12.008 (2019).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 593–601. https://doi.org/10.1038/nrrheum.2014.80 (2014).
Cecchi, I. et al. Neutrophils: Novel key players in rheumatoid arthritis. Current and future therapeutic targets. Autoimmun. Rev. 17, 1138–1149. https://doi.org/10.1016/j.autrev.2018.06.006 (2018).
Assi, L. K. et al. Tumor necrosis factor alpha activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 56, 1776–1786. https://doi.org/10.1002/art.22697 (2007).
Tanaka, S. Emerging anti-osteoclast therapy for rheumatoid arthritis. J. Orthop. Sci. 23, 717–721. https://doi.org/10.1016/j.jos.2018.06.001 (2018).
Wei, F., Chang, Y. & Wei, W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine 76, 537–544. https://doi.org/10.1016/j.cyto.2015.07.014 (2015).
Chakravarti, A., Raquil, M. A., Tessier, P. & Poubelle, P. E. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood 114, 1633–1644. https://doi.org/10.1182/blood-2008-09-178301 (2009).
Baici, A., Salgam, P., Cohen, G., Fehr, K. & Böni, A. Action of collagenase and elastase from human polymorphonuclear leukocytes on human articular cartilage. Rheumatol. Int. 2, 11–16. https://doi.org/10.1007/bf00541264 (1982).
Fernandes, R. M., da Silva, N. P. & Sato, E. I. Increased myeloperoxidase plasma levels in rheumatoid arthritis. Rheumatol. Int. 32, 1605–1609. https://doi.org/10.1007/s00296-011-1810-5 (2012).
Odobasic, D. et al. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol. (Hoboken, NJ) 66, 907–917. https://doi.org/10.1002/art.38299 (2014).
Mutua, V. & Gershwin, L. J. A review of neutrophil extracellular traps (NETs) in disease: Potential anti-NETs therapeutics. Clin. Rev. Allergy Immunol. 61, 194–211. https://doi.org/10.1007/s12016-020-08804-7 (2021).
Olumuyiwa-Akeredolu, O. O., Page, M. J., Soma, P. & Pretorius, E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 15, 237–248. https://doi.org/10.1038/s41584-019-0187-9 (2019).
Jiang, S. Z., To, J. L., Hughes, M. R., McNagny, K. M. & Kim, H. Platelet signaling at the nexus of innate immunity and rheumatoid arthritis. Front. Immunol. 13, 977828. https://doi.org/10.3389/fimmu.2022.977828 (2022).
Farr, M., Wainwright, A., Salmon, M., Hollywell, C. A. & Bacon, P. A. Platelets in the synovial fluid of patients with rheumatoid arthritis. Rheumatol. Int. 4, 13–17. https://doi.org/10.1007/bf00683878 (1984).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science (New York, NY) 327, 580–583. https://doi.org/10.1126/science.1181928 (2010).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255. https://doi.org/10.1111/j.0105-2896.2009.00859.x (2010).
Liu, W. et al. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front. Immunol. 9, 2228. https://doi.org/10.3389/fimmu.2018.02228 (2018).
García-Vicuña, R. et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 50, 3866–3877. https://doi.org/10.1002/art.20615 (2004).
England, B. R., Thiele, G. M., Anderson, D. R. & Mikuls, T. R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ (Clin. Res. Ed.) 361, k1036. https://doi.org/10.1136/bmj.k1036 (2018).
Fragoulis, G. E., Panayotidis, I. & Nikiphorou, E. Cardiovascular risk in rheumatoid arthritis and mechanistic links: From pathophysiology to treatment. Curr. Vasc. Pharmacol. 18, 431–446. https://doi.org/10.2174/1570161117666190619143842 (2020).
Author information
Authors and Affiliations
Contributions
Study concept: D.W.B. and Q.M.L. Study design: All authors. Acquisition, analysis, or interpretation of data: W.W., Y.H.L. Statistical analysis: W.W., W.Y., and W.Y.T. Drafting of the manuscript: W.W. and W.Y. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, W., Yao, W., Tang, W. et al. Nonlinear associations of systemic immune-inflammation index with all-cause and cardiovascular mortality in US adults with rheumatoid arthritis. Sci Rep 14, 16639 (2024). https://doi.org/10.1038/s41598-024-67750-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67750-y
- Springer Nature Limited