Abstract
Understanding the prevalence of abnormal lung function and its associated factors among patients recovering from COVID-19 is crucial for enhancing post-COVID care strategies. This study primarily aimed to determine the prevalence and types of spirometry abnormalities among post-COVID-19 patients in Malaysia, with a secondary objective of identifying its associated factors. Conducted at the COVID-19 Research Clinic, Faculty of Medicine, University Technology MARA, from March 2021 to December 2022, this study included patients at least three months post-discharge from hospitals following moderate-to-critical COVID-19. Of 408 patients studied, abnormal spirometry was found in 46.8%, with 28.4% exhibiting a restrictive pattern, 17.4% showing preserved ratio impaired spirometry (PRISm), and 1.0% displaying an obstructive pattern. Factors independently associated with abnormal spirometry included consolidation on chest X-ray (OR 8.1, 95% CI 1.75–37.42, p = 0.008), underlying cardiovascular disease (OR 3.5, 95% CI 1.19–10.47, p = 0.023), ground-glass opacity on chest X-ray (OR 2.6, 95% CI 1.52–4.30, p < 0.001), and oxygen desaturation during the 6-min walk test (OR 1.9, 95% CI 1.20–3.06, p = 0.007). This study highlights that patients recovering from moderate-to-critical COVID-19 often exhibit abnormal spirometry, notably a restrictive pattern and PRISm. Routine spirometry screening for high-risk patients is recommended.
Similar content being viewed by others
Introduction
The Coronavirus 2019 (COVID-19) pandemic is the worst-ever global health emergency, resulting in substantial human casualties and economic downturn. As of 6th March 2024, global COVID-19 infections have reached 704 million, leading to over seven million fatalities1. Even though the World Health Organization (WHO) no longer considers COVID-19 a public health emergency of international concern2, the continual emergence of new virus variants poses a persistent threat to the end of the pandemic.
Viruses responsible for Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and COVID-19 primarily target the lower respiratory tract, leading to acute lung injuries like pneumonia and acute respiratory distress syndrome (ARDS)3. Survivors of SARS and MERS demonstrated abnormal lung function, reduced effort tolerance, and impaired quality of life months or even years after the illness4,5,6. Recent studies conducted in China highlighted that abnormal lung function was observed in 47.2% of hospitalized COVID-19 patients upon discharge7, 75.4% after a month8, and 25.5% after three months9. A study of previously hospitalized COVID-19 survivors in China reported dyspnea rates of 26.1% at six months and 14.1% at two years, with impaired functional status observed in 14.1% at six months and 8.4% at two years10. Nearly a quarter experienced impaired health-related quality of life (HRQOL) due to somatic symptoms or psychiatric symptoms at six months, with somatic symptoms remaining constant while psychiatric symptoms halved by two years10. In various respiratory diseases, lung function, dyspnea symptoms, and functional status are often negatively correlated with HRQOL11.
Malaysia has reported 5.27 million COVID-19 cases to date, with a recovery rate of 98.9%1. Routine assessment of lung function in patients recovering from COVID-19, however, remains a major challenge here due to a few constraints. First, conducting widespread lung function tests is time-consuming, costly, and manpower intensive. Second, equipment (such as spirometer and body plethysmograph) and expertise required to perform lung function tests are only available in selected tertiary healthcare centres. Third, non-respiratory clinicians often have difficulty interpreting the results of lung function tests. Fourth, the management strategies for abnormal lung function following COVID-19 remain unclear, particularly lacking standardized guidelines. Thus, only a very small proportion of patients recovering from COVID-19 were offered lung function tests.
Research looking into the prevalence of abnormal lung function and its associated factors among patients recovering from COVID-19 in Malaysia is essential to help healthcare authorities develop follow-up strategies to enhance post-COVID care. This study focuses on evaluating lung function in patients with COVID-19 at least three months after their hospital discharge, aiming to determine the prevalence and types of abnormal spirometry results as primary objectives. The secondary objective is to identify the factors associated with spirometry abnormalities.
Methods
Study design and patients
This is a cross-sectional study of patients attending the COVID-19 Research Clinic at the Faculty of Medicine, University Technology MARA (UITM) in Malaysia, from March 2021 to December 2022. The inclusion criteria were Malaysians aged eighteen years and above, with confirmed COVID-19 via validated reverse transcription-polymerase chain reaction method, who had moderate-to-critical illness according to the WHO classification12, and were at least three months post-discharge from either the Sungai Buloh Hospital or the UITM Medical Centre13. Patients with pre-existing chronic lung diseases before COVID-19, including bronchial asthma, as well as individuals who were pregnant, completely immobilized, had uncontrolled psychiatric illness, or were contraindicated for spirometry were excluded. The exclusion criteria were determined based on patients’ electronic records or interviews.
A minimum sample size of 386 subjects was determined using the formula for a cross-sectional study—sample size = Z1-α2p(1—p)/d2 14. Z represented the confidence interval at 95%, d denoted the margin of error at 5%, and p referred to the proportion of abnormal lung function (52.7%) among SARS survivors in a previous study5. All patients provided written informed consent before participating in the study. The study received ethics approval from the Medical Research Ethics Committee of the Ministry of Health Malaysia (NMRR-20-2011-56330 (IIR) and the respective hospitals, and it was conducted in adherence to the Declaration of Helsinki.
Procedure and outcomes
Eligible patients were consecutively identified from the COVID-19 registry of Sungai Buloh Hospital and UITM Medical Centre. Those meeting all inclusion criteria and having none of the exclusion criteria were scheduled for early physical appointments at the COVID-19 Research Clinic.
-
1.
Demographic, clinical, and hospitalization data:
Demographic, clinical, and hospitalization data were gathered through face-to-face interviews and the electronic records. Demographic information included age, gender, and ethnicity, while clinical details included smoking status and the presence of underlying chronic diseases. Hospitalization data included the duration of illness before admission, length of hospital stays, COVID-19 severity at presentation, the most severe COVID-19 episode during hospitalization, pharmacotherapy administered, respiratory support provided, the occurrence of respiratory complications, and details regarding intensive care unit (ICU) admission, including its length of stay.
The severity of COVID-19 was defined according to the WHO classification as: asymptomatic, mild (symptomatic without pneumonia), moderate (pneumonia without hypoxia), severe (pneumonia with hypoxia requiring oxygen supplementation), and critical (critically ill, such as ARDS, sepsis, or septic shock)12. Available treatments for COVID-19 during the study period included corticosteroids, hydroxychloroquine, immunomodulators (tocilizumab, interferon beta, and interferon alpha), and antivirals (favipiravir, lopinavir-ritonavir, ritonavir, and atazanavir)15. Respiratory support was categorized into oxygen supplementation by nasal cannulae, venti-mask, or high-flow mask, non-invasive mechanical ventilation (NIV) or nasal high flow (NHF), and invasive mechanical ventilation (IMV)16,17. Common respiratory complications of COVID-19 that were recorded included ARDS, pulmonary embolism, pneumothorax, and pleural effusion18,19.
-
2.
Patients reported outcomes (PROs):
Patients were instructed to independently complete the modified Medical Research Council (mMRC) dyspnea scale and the post-COVID-19 Functional Status (PCFS) scale with minimal assistance from investigators. The mMRC and PCFS were interpreted as per the original validation of the questionnaire20,21. A higher score indicates a greater degree of symptom severity and impairment, respectively.
-
3.
Lung function tests:
Spirometry was conducted using SpiroUSB™ (Vyaire Medical, Chicago, IL) to obtain dynamic lung volumes, including the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). The cut-off value of ≥ 80% of the predicted was deemed normal for both parameters. Spirometry results were categorized into four groups: normal spirometry—normal FEV1, normal FVC, and FEV1/FVC > 0.7; restrictive pattern—reduced or normal FEV1, reduced FVC, and FEV1/FVC > 0.7; obstructive pattern—reduced FEV1, reduced or normal FVC, and FEV1/FVC < 0.7; and preserved ratio impaired spirometry (PRISm)—reduced FEV1, normal FVC¸ and FEV1/FVC > 0.722,23. For patients with an obstructive pattern, post-bronchodilator spirometry was performed to identify reversible airflow obstruction. Those with a restrictive pattern were scheduled for static lung volumes and diffusion capacity measurement within two weeks using PFT Vyntus Bodybox™ (Vyaire Medical, Chicago, IL). The parameters measured included residual volume (RV), total lung capacity (TLC), diffusion capacity for carbon monoxide (DLCO), and carbon monoxide transfer coefficient (DLCO/Va). All lung function tests were conducted by certified respiratory technicians following the American Thoracic Society (ATS) and European Respiratory Society guidelines24,25.
-
4.
Cardiopulmonary functional tests:
Patients underwent a 6-min walk test (6MWT) under the guidance of a certified respiratory physiotherapist, following the ATS guideline26. Their pulses and oxygen saturation were continuously monitored using the Nonin® WristOx2 ™ 3150 Bluetooth Pulse Oximeter. A 1-min sit-to-stand test (1MSTS) guided by the same respiratory physiotherapist followed and in accordance with the procedure outlined in a previous study27. Both assessments utilized a digital stopwatch for time measurement, and the Borg scale was employed to assess the severity of dyspnea and fatigue. Both tests were sensitive for respiratory diseases but not specific for abnormal spirometry26,27.
-
5.
Radio-imaging:
All patients underwent a standard posterior-anterior chest X-ray examination. Only those demonstrating a restrictive pattern in spirometry were scheduled for high-resolution computed tomography (HRCT) of the lungs within one month. Two radiologists, blinded to patients’ information, independently reviewed the chest X-ray images to identify consolidation, ground-glass opacity (GGO), and lung parenchymal reticulation which were commonly reported in previous COVID-19 literature28,29,30,31. On a chest X-ray, consolidation was defined as a homogeneous opacification that obscures airway walls and blood vessels; GGO was defined as a hazy lung radiopacity with indistinct pulmonary vessel edges; and reticulation was defined as a collection of numerous small linear opacities, according to the Fleischner Society Glossary32. HRCT images, when available, were also reviewed to detect these findings, as well as organizing pneumonia (OP) and other relevant abnormalities such as lung nodules, atelectasis, pleural effusion or thickening, diaphragmatic elevation, cardiomegaly, and fractures, if present. To prevent cross-referencing, all chest X-ray images were reported before any HRCT evaluations were conducted. The two radiologists did not refer to the chest X-ray results when reporting the HRCT images, and vice versa. The reporting of HRCT scans was done randomly, so the radiologists who reported the HRCT might not be the same one who evaluated the chest X-ray. Lung involvement severity was assessed using the CT-score method developed by Kunhua Li et al33. Each lobe received a score ranging from 0 to 5 based on its level of involvement: 0 (0%), 1 (< 5%), 2 (5–25%), 3 (26–49%), 4 (50–75%), and 5 (> 75%). The total score, representing cumulative involvement across all lobes, ranged from 0 to 25 points.
Statistical analyses
Categorical variables are presented as percentages, while continuous variables are presented as mean ± standard deviation (SD). Patients were categorized into those with normal versus those with abnormal spirometry for two-group comparisons, as well as normal versus restrictive pattern or PRISm/obstructive pattern spirometry for three-group comparisons. Between-group differences were assessed using an independent t-test for continuous variables and a Chi-Square test for categorical variables. A two-sided p-value of less than 0.05 was considered statistically significant.
For multivariate analyses, variables exhibiting significant two-sided p-values in the univariate analyses were included as covariates in binary logistic regression and multinomial logistic regression. The latter analysis excluded variables showing multicollinearity (variance inflation factor > 5). The analysis aimed to derive odds ratios (OR), 95% confidence intervals (95% CI), and two-sided p-values. Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS for Windows version 25.0, SPSS Inc, Chicago, IL, USA).
Results
Sociodemographic and clinical characteristics
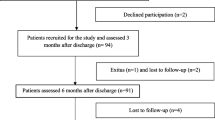
A total of 408 patients were included in the study (Fig. 1). The sociodemographic and clinical characteristics of these patients are presented in Table 1. The mean age of the patients was 51.6 ± 13.32 years. The majority were male (59.8%), of Malay ethnicity (71.8%), and had underlying chronic diseases (63.7%). The most common disease was hypertension (43.4%), followed by diabetes mellitus (30.6%), obesity (17.9%), cardiovascular disease (4.9%), chronic kidney disease (1.5%), chronic liver disease (0.5%), and cerebrovascular disease (0.2%). Only 23.3% of the patients were current or ex-smokers.
Hospitalization and management of the patients
The patients were admitted to the hospital after a mean duration of 8.7 ± 5.32 days from symptom onset and the mean hospitalization duration was 13.0 ± 10.62 days. Most of the patients had severe illness (61.3%) on admission (Table 2). Subsequently, 28.9%, 55.6%, and 15.5% developed critical, severe, and moderate illness during their hospital stay, respectively. Corticosteroids (83.1%) were the most frequently administered medication, followed by antivirals (37.0%), immunomodulators (14.2%), and hydroxychloroquine (13.7%). Among patients requiring respiratory support (78.4%), 47.8% received supplemental oxygen only, 13.2% had NIV or HFNO, and 17.4% underwent IMV. Respiratory complications occurred in 26.5% of patients, with pulmonary embolisms accounting for 24.3% of these cases. For the 43.4% of patients who were admitted to the ICU, the mean duration of ICU stay was 10.6 ± 16.32 days.
PROs, cardiopulmonary functional tests, and chest X-ray findings at follow-up
The patients were assessed at a mean duration of 162.6 ± 113.97 days post-hospital discharge (Table 3). They reported a mean mMRC score of 0.9 ± 0.95 and a mean PCFS score of 0.4 ± 0.74. Of 404 patients who completed the 6MWT, 31.4% experienced oxygen desaturation. Among the 402 patients who completed 1MSTS, 29.1% experienced oxygen desaturation. Chest X-ray revealed abnormalities in 33.6% of patients which included GGO (26.0%), lung parenchymal reticulation (10.1%), and consolidation (4.2%).
Spirometry and factors associated with abnormal results
Abnormal spirometry was detected in 46.8% of the patients, with 28.4% having a restrictive pattern, 17.4% having PRISm, and 1.0% having an obstructive pattern. The mean values of their FEV1, FVC, and FEV1/FVC are presented in Fig. 2.
Factors associated with abnormal spirometry results included patients’ age (p < 0.001), hypertension (p < 0.001), cardiovascular disease (p = 0.010), corticosteroids treatment (p = 0.006), IMV support (p = 0.042), ARDS (p = 0.035), pulmonary embolism (p = 0.007), ICU admission (p = 0.001), duration from discharge to follow-up (p = 0.001), oxygen desaturation with 6MWT (p = 0.002), oxygen desaturation with 1MSTS (p = 0.003), as well as the presence of consolidation (p < 0.001), GGO (p < 0.001), and parenchymal reticulation on chest X-ray (p = 0.004) (Table 4).
Multivariate analyses using binary logistic regression showed that patients with underlying cardiovascular disease (OR 3.5, 95% CI 1.19–10.47, p = 0.023), those with oxygen desaturation during 6MWT (OR 1.9, 95% CI 1.20–3.06, p = 0.007), and those with consolidation (OR 8.1, 95% CI 1.75–37.42, p = 0.008) or GGO appearance (OR 2.6, 95% CI 1.52–4.30, p < 0.001) on chest X-ray were significantly more likely to have abnormal spirometry.
Restrictive pattern, obstructive pattern, and PRISm
Multinomial logistic regression, using normal spirometry as a reference, showed that female patients (OR 2.1, 95% CI 1.13 -3.94, p = 0.019), those treated with immunomodulators (OR 2.4, 95% CI 1.07–5.28, p = 0.034), those with consolidation (OR 10.1, 95% CI 1.88–54.83, p = 0.007) or GGO appearance (OR 2.2, 95% CI 1.16–4.26, p = 0.016) on chest X-ray were significantly more likely to show a restrictive pattern spirometry. Conversely, patients treated with hydroxychloroquine (OR 4.4, 95% CI 1.15–16.97, p = 0.030), those who developed pulmonary embolism (OR 2.3, 95% CI 1.07–5.13, p = 0.033), those with oxygen desaturation in the 6MWT (OR 2.4, 95% CI 1.20–4.75, p = 0.014), those with consolidation (OR 8.6, 95% CI 1.41–52.57, p = 0.020) or GGO appearance (OR 2.6, 95% CI 1.3–5.3, p = 0.009) on chest X-ray were significantly more likely to show PRISm and obstructive pattern spirometry.
Findings on body plethysmography, diffusion capacity, and HRCT of the lungs
Eighty-nine patients underwent body plethysmography and diffusion capacity assessment, revealing a mean RV of 57.8 ± 39.08% predicted, a mean TLC of 65.1 ± 13.25% predicted, a mean DLCO of 62.5 ± 13.94% predicted, and a mean DLCO/Va of 103.6 ± 17.52 (Table 5). Of 80 patients who underwent HRCT of the lungs, 81.3% had GGO, 52.5% had OP, 85.0% had parenchymal reticulation, and 33.8% showed other findings. The mean CT score for these patients was 9.8 ± 5.96.
Discussion
The current study highlights that nearly half of the patients hospitalized for moderate-to-critical COVID-19 continue to show abnormal spirometry even after an average of five months after discharge. Approximately one-third of them displayed a restrictive pattern, while another one-fifth surprisingly manifested PRISm. This study identifies chest X-ray as a reliable tool for predicting abnormal spirometry and its subtypes, particularly when consolidation and GGO are present. Furthermore, the 6MWT could be a valuable tool for predicting abnormal spirometry. Although certain clinical data were also found to be useful, the 1MSTS and PROs do not add additional value to the prediction of spirometry abnormalities.
A meta-analysis of seven studies, primarily conducted in China, revealed that 22.9% of patients hospitalized for COVID-19 demonstrated abnormal spirometry within three months post-discharge34. Among these, 15.0% exhibited a restrictive pattern while 7.9% showed an obstructive pattern34. A separate study in Thailand reported abnormal spirometry in 17.2% of patients hospitalized for mild-to-severe COVID-19 at sixty days post-discharge, with 9.2% having an obstructive pattern and 8.0% having a restrictive pattern35. In Spain and Belgium, studies reported solely a restrictive pattern among hospitalized COVID-19 patients. In the Spanish study, 14.3% of patients requiring oxygen supplementation for pneumonia exhibited this pattern at two months, 9.3% at six months, and 6.7% at twelve months36. In the Belgian study, 55% of patients admitted to the ICU for ARDS demonstrated a restrictive pattern at three months37. Compared to these other studies, our study showed a high prevalence of abnormal spirometry potentially attributed to the predominance of severe and critical COVID-19 cases among our cohort. The observation that the restrictive pattern was the most common spirometry abnormality aligns with findings in China34, France38, Spain36, and Belgium37. The increased proportion of patients with an obstructive pattern in the Thailand study, however, could be due to the non-exclusion of individuals with pre-existing lung diseases, including bronchial asthma and chronic obstructive pulmonary disease (COPD)35.
The majority of existing studies have focused on investigating the lung function of patients recovering from COVID-19 based on severity of illness. These studies have consistently shown that individuals with more severe illness tend to exhibit significantly lower static lung volumes and diffusion capacity, while their dynamic lung volumes in spirometry often remain preserved8,38,39,40,41. To date, only a study in Thailand and China have respectively reported significantly lower dynamic lung volumes in patients with more severe illness35,42, while another study in the Netherlands found only FVC to be significantly lower in such cases43. Additional studies have shown that for individuals post-COVID-19, spirometry indices were not significantly different from the healthy population44, those with other viral upper respiratory tract infections45, or the same cohort of patients one year before the infection46. A review by Thomas et al. further concluded that spirometry indices are often well-preserved in COVID-19, without being significantly affected by illness severity47. As far as we know, our study is the first to demonstrate no significant differences in spirometry patterns between patients with varying severity of COVID-19.
Our study identifies several factors associated with abnormal spirometry in patients recovering from COVID-19, notably abnormal chest X-ray and 6MWT during follow-up, as well as underlying cardiovascular disease. In Thailand, individuals with abnormal chest X-ray after COVID-19 had significantly lower dynamic lung volumes35. Additionally, chest CT abnormalities after COVID-19 were correlated with lower dynamic lung volumes and diffusion capacity among those in Austria48, Netherlands40, and China49, although not in France38. Oxygen desaturation during the 6MWT was associated with diffusion capacity impairment among post-COVID patients in the Netherlands40, but not in Thailand or Germany35,50. The relationship between lung function and mMRC scores or 6MWT in individuals post-COVID has not been extensively explored in previous studies, where these measures were assessed but not specifically analyzed for their association or correlation36,41,51,52. To date, only one study from Austria has reported a negative correlation between lung function and mMRC scores48, while another study from the Netherlands reported an association between poor lung function and oxygen desaturation in the 6MWT40. Additionally, two other studies, one from China and another from Belgium, found concurrent abnormalities in lung function, radio-imaging, 6MWT, and mMRC in the same cohort of post-COVID patients, suggesting a potential relationship between these factors8,37. Other factors associated with impaired lung function in previous studies included older age36,46, female gender36, lower body mass index36, underlying chronic lung disease46, higher inflammatory markers at presentation36,49, previous ARDS37, and shorter discharge-to-follow-up interval48,51. Corticosteroid treatment was linked to better lung function recovery37,51, while this was not observed with other treatment modalities43. Overall, our study findings are consistent with most of other studies.
Our study is the first to report PRISm in post-COVID patients. PRISm, previously known as pre-COPD, restrictive, or non-specific pattern, has a prevalence of 4.7–22.3% in the general population53. Recent studies indicate that it primarily affects the small airways and vessels while sparing lung parenchyma53,54. Two studies have shown that 25.1% and 32.6% of individuals with PRISm, respectively progress to spirometry-defined COPD in five years55,56. Conversely, improvement of spirometry from obstructive pattern to PRISm over time has also been observed57. Therefore, individuals with PRISm in this study could either indicate an improvement from airflow obstruction or an early sign of deterioration to COPD after COVID-19. Additionally, the possibility that this reflects population prevalence rather than being directly attributed to COVID-19 cannot be discounted. Future studies that prospectively following up on this patient cohort could provide a definitive answer. The high prevalence of the restrictive pattern among our patients can be explained by the aberrant wound healing typically following diffuse alveolar damage by SARS-CoV-2, leading to severe scarring and fibrosis58. Respiratory muscle weakness following SARS-CoV-2 infection could also be another possibility59.
The findings from this study have several clinical implications. First, spirometry should be routinely performed in patients post moderate-to-critical COVID-19 due to the high prevalence of abnormality. Second, when universal spirometry screening is not feasible among them, a targeted risk stratification approach considering chest X-ray, 6MWT, and specific clinical characteristics is recommended. Third, chest X-ray proves to be the most reliable screening tool for abnormal spirometry, with the additional benefits of being readily available and cost-effective. Treating clinicians and radiologists should focus on detecting consolidation and GGO features. Fourth, the 6MWT also emerges as a valuable screening tool for abnormal spirometry. Fifth, 1MSTS and PROs may not add significant value to the screening and should not be prioritized during follow-up. Sixth, this study suggests the potential development of a scoring system that combines these factors, providing a practical tool for clinicians to efficiently select patients for lung function tests.
The large sample size of this study allows for the generalizability of the result. It is one of the few studies in the Southeast Asia, where outcomes may differ from other parts of the world due to variations in genetic, environmental, and lifestyle factors. The study focused on patients hospitalized with moderate-to-critical COVID-19 who were more susceptible to long-term lung injuries. The comprehensive study outcomes include objective assessments like lung function, radio-imaging, and cardiopulmonary functional evaluations, alongside subjective assessments such as PROs. However, this study was conducted during the peak of the pandemic. Travel restrictions, public reluctance to visit hospitals, and constrained healthcare resources could lead to several weaknesses. First, the convenience sampling method may introduce bias. Second, not every patient can undergo examination with body plethysmography, for diffusion capacity, and with HRCT. Third, some patients who were offered these investigations defaulted. Fourth, due to practical and logistic reasons the follow-up assessments could not be conducted at a fixed interval, such as three months, six months, or twelve months post-discharge. Fifth, no spirometry was done before the COVID-19 infection to demonstrate baseline normality. Sixth, factors associated with specific abnormal spirometry patterns should be interpreted with caution, as the sample size may not be powerful enough to accurately reflect these secondary outcomes. Seventh, although patients’ age and discharge-to-follow-up interval may show statistical significance in multivariate analysis, an OR of 1.0 indicates a lack of clinical significance. Eighth, the OR for ARDS could not be generated using logistic regression due to the complete separation phenomenon and the events were extremely low resulting in a lack of statistical power for the analysis. Ninth, multidimensional assessment of PROs such as HRQOL was not performed. Lastly, lung function tests were not conducted as a follow-up after the study to observe potential changes in patterns.
Conclusions
Patients recovering from moderate-to-critical COVID-19 often demonstrated abnormal spirometry, particularly manifesting a restrictive pattern and PRISm. Therefore, spirometry should be routinely offered to those at higher risk of abnormalities, such as individuals with abnormal chest X-ray and 6MWT during follow-up, as well as those with underlying cardiovascular disease. PRISm represents a novel finding among post-COVID patients, warranting further follow-up to elucidate the underlying mechanism of this lung function abnormality.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Worldometer. COVID - Coronavirus Statistics. https://www.worldometers.info/coronavirus/? (2024).
Wise, J. Covid-19: WHO declares end of global health emergency. BMJ 381, p1041. https://doi.org/10.1136/bmj.p1041 (2023).
Vos, L. M. et al. Lower respiratory tract infection in the community: Associations between viral aetiology and illness course. Clin. Microbiol. Infect. 27, 96–104. https://doi.org/10.1016/j.cmi.2020.03.023 (2021).
Hui, D. S. et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60, 401–409. https://doi.org/10.1136/thx.2004.030205 (2005).
Ngai, J. C. et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 15, 543–550. https://doi.org/10.1111/j.1440-1843.2010.01720.x (2010).
Park, W. B. et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J. Korean Med. Sci. 33, e169. https://doi.org/10.3346/jkms.2018.33.e169 (2018).
Mo, X. et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Resp. J. https://doi.org/10.1183/13993003.01217-2020 (2020).
Huang, Y. et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Resp. Res. 21, 163. https://doi.org/10.1186/s12931-020-01429-6 (2020).
Zhao, Y.-M. et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine https://doi.org/10.1016/j.eclinm.2020.100463 (2020).
Huang, L. et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Resp. Med. 10, 863–876. https://doi.org/10.1016/S2213-2600(22)00126-6 (2022).
Shavro, S. A., Ezhilarasu, P., Augustine, J., Bechtel, J. J. & Christopher, D. J. Correlation of health-related quality of life with other disease severity indices in Indian chronic obstructive pulmonary disease patients. Int. J. Chron. Obstruct. Pulmon. Dis. 7, 291–296. https://doi.org/10.2147/copd.S26405 (2012).
World Health Organisation. Clinical management of COVID-19: Interim guidance, 27 May 2020. https://reliefweb.int/report/world/clinical-management-covid-19-interim-guidance-may-2020 (2020).
British Thoracic Society. Guidance on respiratory follow up of patients with a clinico-radiological diagnosis of covid-19 pneumonia., 2020. https://www.brit-thoracic.org.uk/quality-improvement/covid-19/covid-19-information-for-the-respiratory-community/ (2020).
Charan, J. & Biswas, T. How to calculate sample size for different study designs in medical research?. Indian J. Psychol. Med. 35, 121–126. https://doi.org/10.4103/0253-7176.116232 (2013).
Sim, B. L. H. et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study. Lancet Reg. Health West Pac. 4, 100055. https://doi.org/10.1016/j.lanwpc.2020.100055 (2020).
Luján, M., Sayas, J., Mediano, O. & Egea, C. Non-invasive respiratory support in COVID-19: A narrative review. Front. Med. https://doi.org/10.3389/fmed.2021.788190 (2022).
Ouyang, L., Yu, M., Zhu, Y. & Gong, J. Respiratory supports of COVID-19 patients in intensive care unit: A systematic review. Heliyon 7, e06813. https://doi.org/10.1016/j.heliyon.2021.e06813 (2021).
Chan, W. Y. et al. Chest radiograph (cxr) manifestations of the novel coronavirus disease 2019 (COVID-19): A Mini-review. Curr. Med. Imaging 17, 677–685. https://doi.org/10.2174/1573405616666201231103312 (2021).
Ekanem, E. et al. Spontaneous pneumothorax: An emerging complication of COVID-19 pneumonia. Heart Lung 50, 437–440. https://doi.org/10.1016/j.hrtlng.2021.01.020 (2021).
Chai, C.-S. et al. Clinical phenotypes of COPD and health-related quality of life: A cross-sectional study. Int. J. Chron. Obstruct. Pulmon. Dis. 14, 565–573. https://doi.org/10.2147/COPD.S196109 (2019).
Klok, F. A. et al. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. https://doi.org/10.1183/13993003.01494-2020 (2020).
Moore, V. C. Spirometry: Step by step. Breathe 8, 232–240. https://doi.org/10.1183/20734735.0021711 (2012).
Wan, E. S. et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Resp. Res. 15, 89. https://doi.org/10.1186/s12931-014-0089-y (2014).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338. https://doi.org/10.1183/09031936.05.00034805 (2005).
Graham, B. L. et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 49, 1600016. https://doi.org/10.1183/13993003.00016-2016 (2017).
American Thoracic Society statement. guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117. https://doi.org/10.1164/ajrccm.166.1.at1102 (2002).
Ozalevli, S., Ozden, A., Itil, O. & Akkoclu, A. Comparison of the Sit-to-Stand Test with 6min walk test in patients with chronic obstructive pulmonary disease. Respir. Med. 101, 286–293. https://doi.org/10.1016/j.rmed.2006.05.007 (2007).
Martínez Chamorro, E., Díez Tascón, A., Ibáñez Sanz, L., Ossaba Vélez, S. & Borruel Nacenta, S. Radiologic diagnosis of patients with COVID-19. Radiologia. 63(1), 56–73. https://doi.org/10.1016/j.rxeng.2020.11.001 (2021).
Kaufman, A. E. et al. Review of radiographic findings in COVID-19. World J. Radiol. 12, 142–155. https://doi.org/10.4329/wjr.v12.i8.142 (2020).
Rousan, L. A., Elobeid, E., Karrar, M. & Khader, Y. Chest x-ray findings and temporal lung changes in patients with COVID-19 pneumonia. BMC Pulmonary Med. 20, 245. https://doi.org/10.1186/s12890-020-01286-5 (2020).
Abougazia, A. et al. Chest X-ray findings in COVID-19 patients presenting to primary care during the peak of the first wave of the pandemic in qatar: Their association with clinical and laboratory findings. Pulm. Med. 2021, 4496488. https://doi.org/10.1155/2021/4496488 (2021).
Hansell, D. M. et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 246, 697–722. https://doi.org/10.1148/radiol.2462070712 (2008).
Li, K. et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 55, 327–331. https://doi.org/10.1097/rli.0000000000000672 (2020).
Torres-Castro, R. et al. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 27, 328–337. https://doi.org/10.1016/j.pulmoe.2020.10.013 (2021).
Eksombatchai, D. et al. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: A prospective cohort study. PLoS One 16, e0257040. https://doi.org/10.1371/journal.pone.0257040 (2021).
Tarraso, J. et al. Lung function and radiological findings 1 year after COVID-19: A prospective follow-up. Respir. Res. 23, 242. https://doi.org/10.1186/s12931-022-02166-8 (2022).
Truffaut, L. et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir. Res. 22, 29. https://doi.org/10.1186/s12931-021-01625-y (2021).
Frija-Masson, J. et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. https://doi.org/10.1183/13993003.01754-2020 (2020).
Mo, X. et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. https://doi.org/10.1183/13993003.01217-2020 (2020).
van den Borst, B. et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 73, e1089–e1098. https://doi.org/10.1093/cid/ciaa1750 (2021).
Lerum, T. V. et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. https://doi.org/10.1183/13993003.03448-2020 (2021).
Lv, D. et al. Pulmonary function of patients with 2019 novel coronavirus induced-pneumonia: A retrospective cohort study. Ann. Palliat. Med. 9, 3447–3452. https://doi.org/10.21037/apm-20-1688 (2020).
Krueger, T. et al. Pulmonary function three to five months after hospital discharge for COVID-19: A single centre cohort study. Sci. Rep. 13, 681. https://doi.org/10.1038/s41598-023-27879-8 (2023).
Iversen, K. K. et al. Lung function decline in relation to COVID-19 in the general population: A matched cohort study with prepandemic assessment of lung function. J. Infect. Dis. 225, 1308–1316. https://doi.org/10.1093/infdis/jiab636 (2022).
Tamminen, P. et al. Lung function during and after acute respiratory infection in COVID-19 positive and negative outpatients. Eur. Respir. J. https://doi.org/10.1183/13993003.02837-2021 (2022).
Lewis, K. L. et al. COVID-19 and the effects on pulmonary function following infection: A retrospective analysis. eClinicalMedicine https://doi.org/10.1016/j.eclinm.2021.101079 (2021).
Thomas, M., Price, O. J. & Hull, J. H. Pulmonary function and COVID-19. Curr. Opin. Physiol. 21, 29–35. https://doi.org/10.1016/j.cophys.2021.03.005 (2021).
Sonnweber, T. et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. https://doi.org/10.1183/13993003.03481-2020 (2021).
Zhao, Y. M. et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 25, 100463. https://doi.org/10.1016/j.eclinm.2020.100463 (2020).
Daher, A. et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 174, 106197. https://doi.org/10.1016/j.rmed.2020.106197 (2020).
Zhang, H. et al. Lung-function trajectories in COVID-19 survivors after discharge: A two-year longitudinal cohort study. EClinicalMedicine 54, 101668. https://doi.org/10.1016/j.eclinm.2022.101668 (2022).
Anastasio, F. et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir. J. https://doi.org/10.1183/13993003.04015-2020 (2021).
Zhao, N. et al. Preserved ratio impaired spirometry is associated with small airway dysfunction and reduced total lung capacity. Respir. Res. 23, 298. https://doi.org/10.1186/s12931-022-02216-1 (2022).
Lu, J. et al. Subtyping preserved ratio impaired spirometry (PRISm) by using quantitative HRCT imaging characteristics. Respir. Res. 23, 309. https://doi.org/10.1186/s12931-022-02113-7 (2022).
Wan, E. S. et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am. J. Respir. Crit. Care Med. 198, 1397–1405. https://doi.org/10.1164/rccm.201804-0663OC (2018).
Wijnant, S. R. A. et al. Trajectory and mortality of preserved ratio impaired spirometry: The Rotterdam Study. Eur. Respir. J. https://doi.org/10.1183/13993003.01217-2019 (2020).
Fortis, S. et al. Combined forced expiratory volume in 1 second and forced vital capacity bronchodilator response, exacerbations, and mortality in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 16, 826–835. https://doi.org/10.1513/AnnalsATS.201809-601OC (2019).
Mason, R. J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. https://doi.org/10.1183/13993003.00607-2020 (2020).
Severin, R. et al. The effects of COVID-19 on respiratory muscle performance: Making the case for respiratory muscle testing and training. Eur. Respir. Rev. https://doi.org/10.1183/16000617.0006-2022 (2022).
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We want to express our gratitude to all the patients who participated in the study.
Funding
Open Access funding provided by Universiti Malaysia Sarawak. This study was supported by research grants from University Malaysia Sarawak (F05/RISE/2089/2021), Selangor State Government (100 – 3/2/3JLD25), Lung Foundation of Malaysia (GI/F05/LFM/2021), Persatuan Pendidikan Kesihatan Paru-Paru (IRG/F05/PPKP/85316/2022), Compass Medical Sdn Bhd (GI/F05/CMSB/2021), and Aliran Pasifik (M) Sdn Bhd (IRG/F05/APMSB/85317/2022). The funding bodies only financially supported this study and did not take part in the design of the study; or collection, analysis, and interpretation of the data; or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the conception and design of the study, data acquisition, data analysis and interpretation, drafting of the article, and critically revising it. All authors made final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chai, CS., Bin Ibrahim, M.A., Binti Azhar, N.A. et al. Post-discharge spirometry evaluation in patients recovering from moderate-to-critical COVID-19: a cross-sectional study. Sci Rep 14, 16413 (2024). https://doi.org/10.1038/s41598-024-67536-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67536-2
- Springer Nature Limited