Abstract
The prediction of refractory Mycoplasma pneumoniae pneumonia (RMPP) remains a clinically significant challenge. This study aimed to develop an early predictive model utilizing artificial intelligence (AI)-derived quantitative assessment of lung lesion extent on initial computed tomography (CT) scans and clinical indicators for RMPP in pediatric inpatients. A retrospective cohort study was conducted on patients with M. pneumoniae pneumonia (MP) admitted to the Children’s Hospital of Nanjing Medical University, China from January 2019 to December 2020. An early prediction model was developed by stratifying the patients with Mycoplasma pneumoniae pneumonia (MPP) into two cohorts according to the presence or absence of refractory pneumonia. A retrospective cohort of 126 children diagnosed with Mycoplasma pneumoniae pneumonia (MPP) was utilized as a training set, with 85 cases classified as RMPP. Subsequently, a prospective cohort comprising 54 MPP cases, including 37 instances of RMPP, was assembled as a validation set to assess the performance of the predictive model for RMPP from January to December 2021. We defined a constant Φ which can combine the volume and CT value of pulmonary lesions and be further used to calculate the logarithm of Φ to the base of 2 (Log2Φ). A clinical-imaging prediction model was then constructed utilizing Log2Φ and clinical characteristics. Performance was evaluated by the area under the receiver operating characteristic curve (AUC). The clinical model demonstrated AUC values of 0.810 and 0.782, while the imaging model showed AUC values of 0.764 and 0.769 in the training and test sets, respectively. The clinical-imaging model, incorporating Log2Φ, temperature(T), aspartate aminotransferase (AST), preadmission fever duration (PFD), and preadmission macrolides therapy duration (PMTD), achieved the highest AUC values of 0.897 and 0.895 in the training and test sets, respectively. A prognostic model developed through automated quantification of lung disease on CT scans, in conjunction with clinical data in MPP may be utilized for the early identification of RMPP.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
MP is a prominent pathogen associated with community-acquired pneumonia (CAP) in children. MPP is responsible for 10–40% of lower respiratory tract infections in hospitalized children, with a pneumonia incidence rate of up to 50% in children over 5 years of age1. While MPP is usually considered a type of self-limiting disease, sometimes it can cause various pulmonary and extrapulmonary complications, including necrotizing pneumonia, bronchitis obliterans, encephalitis, arthritis, pericarditis, hemolytic anemia, and develop into serious life-threatening pneumonia2,3. Macrolides are considered the primary treatment option for Mycoplasma pneumoniae in pediatric patients. Despite treatment with macrolide antibiotics for 7 days or more, some patients may exhibit ongoing clinical and imaging progression, which is defined as RMPP4. The incidence of RMPP is on the rise due to the emergence of drug-resistant MP strains5. Consequently, early recognition and management of RMPP are crucial for optimizing patient outcomes. Traditionally, laboratory biomarkers serve as major models for the prediction of RMPP including CRP, LDH, ferritin, d-dimer, and IL-17A, and the AUC values derived from individual markers ranging from 0.7 to 0.86,7,8. However, the prognosis of a disease cannot be accurately predicted by relying solely on a single indicator, and the results remain unsatisfactory.
A previous study showed the incidence of lung consolidation was found to be higher in the RMPP group compared to the CMPP group, suggesting that this factor may also contribute to the prediction of RMPP9. The amalgamation of clinical parameters and radiological features by models has been documented in the literature10,11. CT imaging plays a vital role in diagnosing and monitoring disease progression in patients with MPP by offering detailed visualization of lung tissue and adjacent structures involved in the disease process12,13. Weihong Lu et al. elevated CT scores manually in identifying RMPP and obtained an AUC of 0.78114. By combining lung CT characteristics with clinical indicators, a hybrid model could be developed to enhance the accuracy of RMPP prediction beyond that of existing models. Currently, the interpretation of CT scans for the MPP is typically performed manually by radiologists, leading to susceptibility to intra- and inter-observer variability, particularly during periods of high reporting volume. In these circumstances, the implementation of an automated technique for quantifying lung inflammation may prove beneficial in alleviating the burden on radiologists while potentially enhancing the accuracy and uniformity of diagnostic reporting.
Deep learning-based artificial intelligence (AI) has recently made tremendous progress and has made great achievements in the field of vision and image processing. Law Kumar Singh et al. propose an enhanced customized R2-ATT U-Net deep learning network for retinal blood vessel extraction, aiding in the early detection of eye diseases15. Similarly, Charu Bhardwaj et al. reported a deep-learning ensemble approach for the grading of diabetic retinopathy severity16. Several research-based AI algorithms have emerged as useful tools in terms of quantitative evaluation of pulmonary diseases. A previous study proposed a hybrid model (LSTMCNN) achieved 98.66% accuracy and an ensemble model using deep CNN achieved 99.78% accuracy for COVID-19 detection from chest X-ray images and CT images11. These AI-assisted models exhibit high accuracy and have justified their performance in clinical practice.
However, to our knowledge, associations between AI-derived CT features quantifying pneumonia lesions and the risk of RMPP in patients with MP infection have not yet been reported. If AI-derived features from CT at an early stage of MPP can be used to predict RMPP, they can be particularly beneficial because of their non-invasiveness and efficiency. The purpose of this study was to develop a model based on automated detection and quantification of chest CT images in conjunction with clinical parameters and evaluate its efficacy in the early diagnosis of RMPP.
Methods
Patients and groups
A retrospective cohort study was conducted on children hospitalized at the Children’s Hospital of Nanjing Medical University with MMP from January 2019 to December 2020. Validation of the findings was carried out using a prospective cohort from January to December 2021. The diagnosis of MP infection was established through the identification of positive laboratory test findings. (serum MP antibody ≥ 1:320, or serum MP antibody ≥ 1:160 and the MP polymerase chain reaction (PCR) positive, or MP antibody titer of the recovery phase and acute phase increased or decreased by 4 times or more). Nasopharyngeal aspirate/swab specimens were obtained within 24 h of admission. Exclusion criteria included: (1) patients with congenital disorders, metabolic disease, immunodeficiency disease, blood tumor disease, bronchopulmonary dysplasia, nervous system dysplasia, and epilepsy; (2) patients infected with other pathogens; (3) patients undergoing MPP recovery; (4) patients older than 18 years of age. Informed consents were obtained from all the guardians of the pediatric patients and the study was approved by the institutional ethics committee of the Children’s Hospital of Nanjing Medical University. The research followed the Helsinki Declaration17.
Data collection and study variables
Diagnostic Criteria of MPP: (1) The history as well as findings on physical examination and a chest X-ray were in line with the diagnosis of CAP; (2) had positive results for MP. (3) hospitalized patients under 18 years old. RMPP is defined as a case with prolonged fever accompanied by deterioration of radiological findings despite appropriate management and treatment with a macrolide antibiotic for ≥ 7 days18. The demographics, clinical information, and laboratory data were collected from inpatient electronic medical records and analyzed. Peripheral blood samples were obtained within 24 h after admission for the determination of the C-reactive protein (CRP), white blood cells (WBC), hemoglobin (Hb), platelets (PLT), lactate dehydrogenase (LDH), neutrophil (NEUT), erythrocyte sedimentation rate (ESR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine phosphokinase isoenzyme (CK-MB), creatine kinase (CK), procalcitonin (PCT), Total Protein (TP) and albumin (ALB).
In addition, the following data were also recorded: the highest temperature on the first day of admission (T), heart rate (HR) and respiratory rate (R) on the day of admission, preadmission fever duration (PFD), preadmission cough duration (PCD), and preadmission macrolides therapy duration (PMTD).
CT acquisition parameters
All patients diagnosed with MPP underwent imaging w with a 64-CT scanner (Toshiba or Philips Healthcare) within 48 h of diagnosis. The imaging protocol utilized exposure parameters of 110 kV scanning tube voltage, 100 mAs reference tube current, layer thickness 2.5 mm, maximum scanning layer thickness 10 mm, interval 2.5 mm, pitch 0.9, thin layer reconstruction layer thickness after scanning was 1.5 mm. The scanning range extended from the thoracic entrance to the base of the lungs.
AI-based quantization of CT images and processing
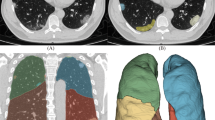
An AI-aided diagnosis system from Shenrui (Beijing, China) was used in this study. The segmentation of lung lobes, labeling of lung infections, and calculation of volumes were conducted using a commercially available deep-learning algorithm specifically designed for pulmonary pneumonia (Dr. Wise@Pneumonia, version 1.0, Beijing Deepwise & League of Ph.D. Technology Co., Ltd., China), which had been proved to be effective in the analysis of CT images from COVID-19 patients19. The system had three major modules. The first module was pneumonia lesion detection and segmentation, utilizing MVP-Net and 3D U-Net, with multi-view inputs and channel-wise attention mechanisms applied followed by multiple binary classifiers. The second module aimed to segment the pulmonary region into five lobes using an anatomical prior embedded network with a smooth margin loss. Finally, the Quantitative Evaluation module was able to calculate various metrics on top of the previously mentioned modules. Specific architecture details have been previously reported19,20. In general, the software is capable of automatically identifying the image characteristics of inflammatory lesions in chest CT images through the acquisition of a substantial dataset of CT images from pneumonia patients. It can then perform fully automated lesion delineation, measure lesion volume, and conduct quantitative analysis of volume proportions. Chest CT image analysis process: (1) Import the CT digital images from patients, and carry out 3D reconstruction of the whole lung, left and right lungs, lobes, and segments separately. (2) Segmentation of pulmonary lesions. (3) Count the total volume of the segmented lesions in the whole lung, left lung, and right lung separately. (4) The 3D reconstructed lung images underwent fine segmentation. 1 cubic centimeter (cm3) of lesion served as 1 minimum counting unit, the average density in this cubic centimeter was defined as ρ (ρ = HU + 1024, Hounsfield Unit, HU). (5) Finally, we define a constant Φ, Φ = ∑ (ρ1 + ρ2 + ρ3⋯ + ρ n-1 + ρn) (Fig. 1). (6) A logarithm of Φ to the base of 2 was used to analyze the data.
Pipeline for quantifying MP infection: The segmentation system based on deep learning is initially fed a chest CT scan. In the CT scan, quantifiable parameters are calculated to characterize infection areas, including but not limited to the following: infection volumes, and percentages of infection (POIs) in the whole lung, lung lobes, and bronchopulmonary segments. Finally, fine segment the 3D reconstructed lung image. 1 cubic centimeter (cm3) of the lesion was served as 1 minimum counting unit to calculate the CT value.
Statistical analysis
Statistical analysis was performed using the R 4.04 version (R Foundation for Statistical Computing, Vienna, Austria). The Chi-square test or Fisher’s exact test was applied to analyze the nominal variables, while the T-test was utilized for continuous variables. The decision curve was constructed using “rmda” package and corresponding calibration curves were created using the "rms" package. The variables for the model were selected through stepwise use of Akaike’s information criterion (AIC)15 and predictive models used multivariable logistic regression. Receiver operating characteristic curves (ROC) analysis was performed using the “pROC” package. DeLong test was used for statistical comparisons of ROC curves. P values less than 0.05 were considered statistically significant.
Results
Patient characteristics and laboratory findings
Between January 2019 and December 2021, a total of 3585 patients were hospitalized diagnosed with CAP at our hospital. Excluded from the study were patients with other pathogens infection (n = 2589) and those with other primary diseases in the study (n = 187). A further 629 were excluded because of the absence of complete clinical data or CT examination.
Ultimately, 126 patients from January 2019 to December 2020 were included in the retrospective cohort as a training set, comprising 41 cases of common Mycoplasma pneumoniae pneumonia (CMPP) and 85 cases of RMPP, and 54 patients from January to December 2021 were enrolled in the prospective cohort as a test set, consisting of 17 CMPP and 37 RMPP cases. Consequently,180 MPP patients were enrolled, as illustrated in Supplementary Fig. S1. Differences in clinical and imaging characteristics of patients between the two sets were not statistically significant (see Supplementary Table S1).
The characteristics of the patients in the training and test set on admission are summarized in Table 1. No significant difference was found in gender and age (P > 0.05). The clinical symptoms, biochemistry levels, and routine blood markers of RMPP and CMPP within 24 h of admission were compared. Compared with the CMPP group in the training set, differences in the highest temperature within 24 h of admission (P < 0.001), HR (P = 0.032), AST (P = 0.021), ALB (P = 0.010), CK (P = 0.049), PFD (P < 0.001), and PMTD (P < 0.001) were statistically significant and the other findings did not differ significantly (P > 0.05). In the test set, Hb (P = 0.028), LDH (P = 0.011), AST (P = 0.012), CK (P = 0.006) and PFD (P = 0.008) were statistically significant between CMPP and RMPP and the other results were not (P > 0.05).
Chest CT quantitative analysis
Quantitative lung features on CT are summarized in Table 2. The RMPP group had a larger volume of pulmonary lesions, and extent of lesions located in the lung of the right upper lobe and right lower lobe compared to the CMPP. The extent of lesions located in the lung of the left lower lobe of the RMPP group was larger than CMPP in the training set (P = 0.046), but it was not statistically significant in the test set (P > 0.05). The log2Φ of the RMPP group was higher than CMPP in two sets (P < 0.05).
Logistic regression and nomogram
Overfitting poses a notable challenge in modeling, resulting from the inclusion of an excessive amount of model parameters. To address this issue, a backward stepwise procedure was utilized, employing the AIC to select variables for the clinical models, penalizing those models with an abundance of parameters. The most influential variables were identified through a stepwise approach, systematically excluding variables until the optimal model fit was achieved. PFD, PMTD, temperature, and AST were included as predictors in the clinical model, Log2Φ was included in the imaging model and all these five predictors were included in the clinical-imaging model (Table 3).
Variables were presented in the model by using the following formula:
Clinical model:
Probability of RMPP × 100 = − 2272 + 54.7 T + 2.6AST + 14.9PFD + 34.2PMTD.
Imaging model:
Probability of RMPP × 100 = − 1315 + 85.3Log2Φ.
Clinical-imaging model:
Probability of RMPP × 100 = − 4709 + 142.1Log2Φ + 57.8 T + 1.7AST + 8.5PFD + 57.1PMTD.
The ROC of the training set was plotted in Fig. 2A with AUC values of 0.810, 0.764, and 0.897, and the test set was plotted in Fig. 2B with AUC values of 0.782, 0.769, and 0.895 respectively. The AUC value of the Clinical-imaging model (0.897, 95% CI 0.835, 0.957) was significantly higher than the Clinical model (0.810, 95% CI 0.734, 0.887, P = 0.012) and the imaging model (0.764, 95% CI 0.675, 0.854, P < 0.000). Independent verification conducted in the validation cohort demonstrated enhanced performance with clinical-imaging markers yielding a statistically significant AUC value of 0.895 (95% CI 0.789, 1.000). In the training set, the Clinical-imaging model exhibited a positive predictive value of 85.29% and a negative predictive value of 84.85%, while in the test set, these values were 78.57% and 87.18%, respectively.
Receiver operating characteristic (ROC) and areas under the ROC curve (AUC). (A) ROC analysis showed an AUC of 0.897 for the clinical-imaging prediction model, an AUC of 0.810 for the clinical prediction model, and an AUC of 0.764 for the imaging prediction model in the training set, suggesting that the combined predictor has a greater diagnostic value than clinical data or imaging data alone. (B) ROC curves of the clinical prediction model, the imaging prediction model, and the clinical-imaging prediction model in the test set showed the AUC were 0.895, 0.769 and 0.782 respectively.
A nomogram was constructed by incorporating the predictors in the clinical-imaging model (Fig. 3). The nomogram was created by giving each independent influencing element a weighted score. The highest score is 120 points, and the range of RMPP incidence is 0.1–0.9. A higher chance of occurrence is indicated by a larger score obtained by adding the distribution points of each high-risk factor in the nomogram.
Model evaluation
Figure 4A,B present graphical representations of calibration of the training set and the test set individually, the clinical-imaging prediction model (both apparent and bias-corrected) was close to ideal calibration.
Calibration curve of the clinical-imaging prediction model. The calibration curve was determined with bootstrap analysis to get bias-corrected estimation. The y-axis shows the actual result. The x-axis represents the predicted probability of refractory mycoplasma pneumoniae pneumonia. Ideal represents the ideal curve, apparent represents the uncalibrated prediction curve and Bias-corrected represents the calibration prediction curve. (A) The calibration curve of the training set. (B) The calibration curve of the test set.
We subsequently compared the clinical performance of the integrated model to the clinical model and imaging model separately using decision curve analysis, which is based on the idea of net benefit and considers the impact of potential benefit and potential harm from the use of predictive models. Figure 5A,B illustrate the decision curves for the three models to predict the correct diagnosis of RMPP in the training set and test set. The decision curve analysis graphically shows the clinical usefulness of each model based on a continuum of potential thresholds for diagnosis of RMPP risk (x-axis) and the net benefit of using the model to risk stratify patients (y-axis) relative to assuming CMPP. In this analysis, the clinical-imaging model provided a larger net benefit across the range of diagnosis of RMPP compared with the other two models.
The decision curves. The y-axis measures the net benefit. The blue solid line, grey solid line, and red solid line represent the net benefit of the clinical model, the imaging model, and the clinical-imaging model at different threshold levels, respectively. (A) DCA for three models in the training set. (B) DCA for three models in the test set.
Discussion
MP is a significant pathogen responsible for a notable percentage of CAP cases in children under the age of 1821,22. Although macrolide antibiotics are useful in treating MP infection, RMPP is on the rise due to increased macrolide use and the emergence of antibiotic-resistant bacteria10,23. RMPP typically induces a prolonged fever that may advance to severe, potentially fatal pneumonia9 and compared to CMPP, patients with RMPP usually require a longer course of antibiotics and higher doses of glucocorticoids24,25. In recent years, tetracyclines seem to be uplifting and promising in the treatment of RMPP26. A prediction model can help identify RMPP at an early stage and consider a change in antibiotic prescription.
Previous studies on predicting RMPP have predominantly concentrated on serological markers. Jun Wen et al. identified serum ferritin, d-dimer, and CRP as important predictors of RMPP7. Yaoyao et al. highlighted neutrophil/lymphocyte ratio, mean platelet volume/lymphocyte ratio as key predictors27. Currently, the visualized characters from CT scans have hardly been studied as potential markers for RMPP. The pathogenesis of RMPP is considered related to MP invasion and immune dysfunction28. MP infects the body and causes the expression of respiratory epithelial adhesion protein29. Simultaneously, excessive inflammation in the lungs and the whole body occurred following MP infection30. Compared with children with CMPP, RMPP is easier to complicate with pleural effusion and atelectasis Chest radiographs and CT scans can be particularly helpful in determining regional density distribution as well as the extent and form of lung injury. Lung lesion severity can assist doctors in differentiating RMPP. In contrast to the reporting with the visual quantitative estimation of lesions provided by a radiologist, AI analysis of radiologic images possesses good reproducibility and reduces the radiologist’s reading time. To the best of our knowledge, Weihong Lu et al. apply quantitative CT scoring to the chest imaging assessment of MPP, which was the latest study of radiological scoring tools for predicting RMPP14. However, they require radiologists or clinicians to determine CT scores on the image, which is time consuming. The quantitative assessment of CT features of MPP by AI software has not been reported extensively. In this study, we analyzed the CT scans of MPP patients using the AI Intelligent Assistant Analysis System. This system can precisely segment different anatomical features of the lungs to discover infection regions and calculate the proportion of infection in both lungs, as well as their lobes and segments. Studies have shown that the use of autonomous 3D AI software for quantitative assessment of radiological findings can predict clinical deterioration or mortality in patients with COVID-19 pneumonia31. Additionally, this AI-based software has been found to surpass the diagnostic accuracy of experienced human specialists in detecting pleural effusion and interstitial lung disease32.
The degree of the pulmonary lesion was a reliable feature for assessing RMPP33, Lobar pneumonia and large pleural effusion in MPP, suggest a more severe disease state possibly attributed to macrolide resistance, increased MP burden, intense host inflammatory responses, or other contributing factors. However, we have no specific indicator to quantify this correlation. In this study, we defined a constant Φ that was the summation of the CT value per unit volume of lesion quantified by AI automatically. Then, lesion volume and Log2Φ were compared between RMPP and CMPP in the training set. The analysis revealed that RMPP patients exhibited a higher lesion volume and Log2Φ value. We additionally confirmed this phenomenon in another test set. Log2Φ, a constant derived from the combination of lesion volume and CT value in each patient, could be identified as a prognostic indicator. In addition, four clinical prognostic factors were identified, including the highest temperature on the first day of admission, AST, PMTD, and PFD, to predict the occurrence of RMPP and reduce the incidence of complications and long-term pulmonary damage. Although the etiology of RMPP is unknown, it is largely assumed that inflammatory and immunological responses have a role in the progression of the disease. Some biomarkers linked to inflammatory reactions had been used to predict RMPP by previous studies33,34. The peak temperature in the RMPP group was significantly higher than that in the CMPP group on the first day of admission35. These markers were higher in the RMPP group than in the CMPP group, implying that children with refractory M. pneumoniae pneumonia have more pronounced inflammatory responses. After an MP infection, hepatic impairment is common extrapulmonary damage34. The study showed that the AST in the RMPP group was higher35. Macrolides have long been thought to be the best treatment for M. pneumoniae pneumonia (MP) in children36. Based on physicians ' clinical experience, most patients had already received macrolides therapy before admission, but they are still hospitalized because of poor response to macrolides. In this study, we found that the longer the macrolides therapy duration before admission they took, the greater the risk of RMPP.
Finally, we integrated the Log2Φ and clinical predictive factors to obtain a better comprehensive imaging-clinical prediction model. The sensitivity and specificity of the training set were 0.809 and 0.864, the AUC was 0.897 and the sensitivity and specificity of the test set were 0.892 and 0.875, the AUC was 0.895. This research is pioneering in its combination of clinical biomarkers and automated AI-driven CT quantification to predict RMPP. Our study demonstrated that the integration of Log2Φ with clinical biomarkers including temperature, AST, PMTD, and PFD could effectively predict the likelihood of MPP patients progressing to RMPP.
The novelty of our proposed method lies in the following aspects. First, quantitative CT analysis using automated quantification takes only a few seconds to achieve complete segmentation of lung lesions with nearly flawless reproducibility, whereas manual scoring is a more time-intensive process with only moderate reproducibility. This preliminary study confirms the suitability of available AI-based segmentation tools for analyzing MPP data, to support the diagnosis and quantification of MPP lung lesions. Second, our proposed constant, Φ, based AI-aided diagnosis system is a novel approach to quantitatively evaluate pulmonary lesions of MPP patients' CT scans. This is not seen in the literature up to this point. Third, to our knowledge, it is the first study to apply automated AI-driven CT quantification combined with clinical parameters that can diagnose RMPP earlier.
Inevitably there are assumptions and limitations in the models. First, initially, we analyzed CT scans obtained within 48 h of diagnosis. Due to the variability in radiological findings throughout the disease progression, our models were not trained on data representative of the intermediate and late stages of the disease. Second, because the data for the study was only taken from the baseline CT and one single-center CT, multi-center validation with a bigger sample size is needed to assess the possible benefit of AI software in MPP patient care.
In conclusion, this study presented an imaging-clinical prediction model that incorporates imaging and clinical risk indicators and may be utilized to quickly identify RMPP and CMPP, laying the groundwork for early clinical and accurate therapy for RMPP.
Data availability
The datasets used and/or analyzed during the current study can be obtained from the corresponding author on reasonable request.
References
Korppi, M., Heiskanen-Kosma, T. & Kleemola, M. Incidence of community-acquired pneumonia in children caused by Mycoplasma pneumoniae: Serological results of a prospective, population-based study in primary health care. Respirology 9, 109–114 (2004).
Poddighe & Dimitri. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: Recent insights into the pathogenesis. Curr. Opin. Rheumatol. 30, 380–387 (2018).
Cheng, Q. et al. Clinical features and risk factors analysis of bronchitis obliterans due to refractory Mycoplasma pneumoniae pneumonia in children: A nomogram prediction model. BMC Infect. Dis. 21, 1085 (2021).
Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (II). Zhonghua er ke za zhi=Chin. J. Pediatr. 51, 856 (2013).
Liu, L., Maharjan, S., Sun, J. L., Li, Y. C. & Cheng, H. J. Prevalence and clinical characteristics of septicemia in children with Mycoplasma pneumoniae pneumonia. J. Int. Med. Res. 49, 3000605211021733 (2021).
Zhao, J., Ji, X., Wang, Y. & Wang, X. Clinical role of serum interleukin-17A in the Prediction of refractory Mycoplasma pneumoniae pneumonia in children. Infect. Drug Resist. 13, 835–843 (2020).
Wen, J., Su, Y., Sun, H., Zhang, H. & Li, H. The combination of initial markers to predict refractory Mycoplasma pneumoniae pneumonia in Chinese children: A case control study. Respir. Res. 22, 89 (2021).
Huang, X. et al. Clinical significance of D-dimer levels in refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 21, 14 (2021).
Huang, W., Xu, X., Zhao, W. & Cheng, Q. Refractory Mycoplasma pneumonia in children: A systematic review and meta-analysis of laboratory features and predictors. J. Immunol. Res. 2022, 9227838 (2022).
Bi, Y. et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci. Rep. 11, 6595 (2021).
Khanna, M. et al. Radiologist-level two novel and robust automated computer-aided prediction models for early detection of COVID-19 infection from chest X-ray images. Arab. J. Sci. Eng. 48, 1–33 (2021).
Wang, D. et al. The value of CT radiomic in differentiating mycoplasma pneumoniae pneumonia from streptococcus pneumoniae pneumonia with similar consolidation in children under 5 years. Front. Pediatr. 10, 953399 (2022).
Choi, Y. J. et al. Clinical characteristics of macrolide-refractory Mycoplasma pneumoniae pneumonia in Korean children: A multicenter retrospective study. J. Clin. Med. 11, 306 (2022).
Lu, W. et al. Predictive value of bronchoscopy combined with CT score for refractory mycoplasma pneumoniae pneumonia in children. BMC Pulm. Med. 24, 251 (2024).
Singh, L. K., Khanna, M., Thawkar, S. & Singh, R. Deep-learning based system for effective and automatic blood vessel segmentation from Retinal fundus images. Multimed. Tools Appl. 83, 6005–6049 (2024).
Bhardwaj, C., Jain, S. & Sood, M. Deep learning-based diabetic retinopathy severity grading system employing quadrant ensemble model. J. Digit. Imaging 34, 440–457 (2021).
Bădărău, D. O. Declaration of Helsinki (Mental Health Practitioner's Guide to HIV/AIDS, 2013).
Tamura, A. et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J. Infect. 57, 223–228 (2008).
Yu, Q. et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics 10, 5641–5648 (2020).
Hu, Z. J. et al. Lower Circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Front. Immunol. 11, 585647 (2020).
Chiang, W. C. et al. Epidemiology, clinical characteristics and antimicrobial resistance patterns of community-acquired pneumonia in 1702 hospitalized children in Singapore. Respirology 12, 254–261 (2007).
Spadaro, S. et al. Biomarkers for acute respiratory distress syndrome and prospects for personalised medicine. J. Inflamm. Lond. 16, 1 (2019).
Okada, T. et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis. 55, 1642–1649 (2012).
Kim, H. S. et al. Efficacy of glucocorticoids for the treatment of macrolide refractory mycoplasma pneumonia in children: Meta-analysis of randomized controlled trials. BMC Pulm. Med. 19, 251 (2019).
Zhu, Z. et al. Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect. Dis. 21, 126 (2021).
Ha, S. G. et al. Therapeutic efficacy and safety of prolonged macrolide, corticosteroid, doxycycline, and levofloxacin against macrolide-unresponsive mycoplasma pneumoniae pneumonia in children. J. Korean Med. Sci. 33 (2018).
Ling, Y., Ning, J. & Xu, Y. Explore the predictive value of peripheral blood cell parameters in refractory Mycoplasma pneumoniae Pneumonia in children over 6 years old. Front. Pediatr. 9, 659677 (2021).
Lin, S. et al. Efficacy of Jiedu Pingsou decoction combined with azithromycin in the treatment of children with Mycoplasma pneumonia and its effects on inflammatory factors and immune function. J. Healthc. Eng. 2022, 9102727 (2022).
Huang, L. et al. Independent predictors for longer radiographic resolution in patients with refractory Mycoplasma pneumoniae pneumonia: A prospective cohort study. BMJ Open 8, e023719 (2018).
Lee, Y. C. et al. Altered chemokine profile in refractory Mycoplasma pneumoniae pneumonia infected children. J. Microbiol. Immunol. Infect. 54, 673–679 (2021).
Chabi, M. L. et al. Automated AI-driven CT quantification of lung disease predicts adverse outcomes in patients hospitalized for COVID-19 pneumonia. Diagnostics (Basel). 11 (2021).
Wang, C. et al. Development and validation of an abnormality-derived deep-learning diagnostic system for major respiratory diseases. NPJ Digit. Med. 5, 124 (2022).
Gong, H., Sun, B., Chen, Y. & Chen, H. The risk factors of children acquiring refractory mycoplasma pneumoniae pneumonia: A meta-analysis. Medicine (Baltimore) 100, e24894 (2021).
Bi, Y. et al. Risk of Mycoplasma pneumoniae-related hepatitis in MP pneumonia pediatric patients: A predictive model construction and assessment. BMC Pediatr. 21, 287 (2021).
Xu, J. J. & Shu, L. H. Clinical characteristics of refractory Mycoplasma pneumoniae pneumonia in children. Zhongguo dang dai er ke za zhi 20, 37–42 (2018).
Waites, K. B. & Talkington, D. F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17, 697–728 (2004).
Acknowledgements
We thank the patients and their families for their participation and contributions to this study. We would like to thank Zhen Zhou from Shenrui (Beijing, China) participated in this investigation by providing AI technology assistant.
Funding
This work was supported by the Project of Nanjing Science and technology development Foundation (YKK21150) to Xuhua Ge.
Author information
Authors and Affiliations
Contributions
Conceptualization, X.H.G. and H.M.; methodology, X.C.G.; software, Y.Q.; validation, Y.T.; formal analysis, L.W.; investigation, F.L. and C.Z.; resources, S.X.; data curation, Y.Q.; writing—original draft preparation, Y.T.; writing—review and editing, All authors. Visualization, X.H.G. supervision, X.C.G.; project administration, X.H.G. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, Y., Tao, Y., Wu, L. et al. Model based on the automated AI-driven CT quantification is effective for the diagnosis of refractory Mycoplasma pneumoniae pneumonia. Sci Rep 14, 16172 (2024). https://doi.org/10.1038/s41598-024-67255-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67255-8
- Springer Nature Limited