Abstract
Vitamin D deficiency (VDD) is associated with increased risk of type 2 diabetes mellitus (T2DM) and insulin resistance (IR). We aimed to investigate the association between the triglyceride-glucose (TyG) index that represents IR and VDD in elderly patients with T2DM. We enrolled 572 elderly participants with T2DM in this study. TyG index was calculated as ln [fasting triglyceride (TG, mg/dL) × fasting blood glucose (mg/dL)/2]. Serum 25-hydroxyvitamin D [25(OH)D] level below 50 nmol/L was defined as VDD. The association between the TyG index and the VDD risk was evaluated by multivariate logistic regression analysis. We observed a significant decreased 25(OH)D level with the increase of the TyG index in elderly diabetic patients, and a negative correlation between the TyG index and 25(OH)D level. The participants in the highest TyG quartile had a 2.40-fold higher risk of VDD than those in the lowest TyG index quartile [OR 2.40; 95% CI 1.47–3.92; P < 0.001]. The association persisted after adjustments for age, sex, smoking, obesity, insulin therapy, hypoglycemic agents’ medication, and some biochemical parameters. TyG index may be involved in the pathophysiology of VDD, which could be a predictor for VDD in elderly diabetic patients.
Similar content being viewed by others
Introduction
Vitamin D deficiency (VDD) has become a pressing issue globally, posing serious risks to health consequences. The global prevalence of VDD has been estimated to be 60–80%, and even higher in elderly population1. Vitamin D comes in two main forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 from the skin and vitamin D2 from the diet are hydroxylated into 25-hydroxyvitamin D [25(OH)D] in the liver firstly and then into 1, 25-dihydroxyvitamin D [1,25(OH)2D] in the kidney2. 25(OH)D is the main storage form in the body and can reflect vitamin D status.
It has been known that VDD usually lead to abnormalities in calcium, phosphorous and bone homeostasis, which resulted in secondary hyperparathyroidism, decreased bone mineral density, and osteoporosis2. Recently, emerging evidence from observational and longitudinal cohort studies has demonstrated that VDD was associated with insulin resistance (IR) and type 2 diabetes mellitus (T2DM)3,4,5,6,7. However, the underlying mechanisms responsible for the relationship between vitamin D and T2DM have not fully understood. One hypothesis was that vitamin D could improve IR through enhancing glucose utilization8,9.
The triglyceride-glucose (TyG) index, which is determined as ln [fasting triglyceride (TG, mg/dL) × fasting blood glucose (mg/dL)/2], is a simple and credible marker for identifying IR10. Du et al. reported that TyG index associated most significantly with homeostatic model assessment of insulin resistance (HOMA-IR) than some indicators (such as visceral adiposity index, lipid accumulation product, and some traditional lipid ratios), and is better for identifying IR individuals early11. Besides, Huang et al. found a significant positive correlation between TyG index and HOMA-IR, and demonstrated that the TyG index is accurate and applicable for the identification of IR in middle-aged people of China12. In addition, a growing number of studies revealed that the TyG index was positively related to the risk of newly diagnosed T2DM and diabetic complications13,14,15,16.
Several studies have evaluated the relationships between the TyG index and vitamin D status in different populations. Mustafa et al. reported an inverse association between the TyG index and 25(OH)D level in adolescents17. Besides, Dhas et al. described that 25(OH)D level had a negative association with the TyG index in middle-aged patients with T2DM3. In addition, Jia et al. demonstrated that 25(OH)D level was negatively correlated to TyG index in male patients with T2DM, but not in female patients18. Moreover, we found that the TyG index could be an efficient predictor for the risk of VDD in elderly diabetic patients than younger adults19. Hence, the present study aimed to further explore the association between the TyG index and vitamin D status in elderly patients with T2DM.
Method
Study design and participants
The participants were consecutively enrolled in the Second Xiangya Hospital of Central South University from September 2020 to October 2022, which has been described detailed previously19. Briefly, a total of 1034 participants diagnosed as T2DM were included in this cohort, and a sub-cohort of 572 elderly participants aged ≥ 60 years was selected in the present analyses. The detailed exclusion criteria were described previously19. VDD was defined as the serum level of 25(OH)D < 50 nmol/L. The flowchart presented the procedure for inclusion and exclusion of the study participants (Fig. 1).
The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University ([2022]085), and carried out conformed to the Declaration of Helsinki. Informed consent was obtained from each participant.
Data collection and biochemical detection
The demographic information, lifestyle, histories, previous medications, and some other basic clinical characteristics of the participants were collected at admission, and then experienced caregivers carried out the physical examination, including the body weight, height, and blood pressure. Blood samples were gained from all patients after an overnight fast, followed by biochemical detection. Fasting blood glucose (FBG), and blood lipids, including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured on a HITACHI 7170A analyzer (Instrument Hitachi Ltd., Tokyo, Japan). Serum levels of C-peptide and glycated hemoglobin (HbA1c) were measured by ARCHITECT c8000 System (Abbott Laboratories, Irving, TX, USA) and Hemoglobin Testing System (VARIANT-11, Bio-Rad, Hercules, USA), respectively. Serum level of 25(OH)D was detected using chemiluminescence assay (Siemens ADVIA Centaur XP, Germany)19. According to suggestions from the research of China and abroad, a serum level of 25(OH)D < 50 nmol/L was considered as VDD20,21.
Statistical analysis
The quantitative normally distributed data were expressed as the mean ± standard deviation (SD) and were compared using one-way ANOVA analysis among the four groups of the TyG index quartiles, while those with abnormally distributed data were presented as median and interquartile range, and were analyzed using Mann–Whitney U test between the two groups or Kruskal–Wallis test among the four groups. The qualitative data were described as numbers and percentages and were compared using Chi-square test for linear trend. The correlation between the TyG index and serum 25(OH)D level was performed by Spearman’s correlation analysis. The association of the TyG index with the risk of VDD was determined using multivariate logistic regression analyses: (1) model 1: unadjusted. Model 2: adjustments for age, sex, smoking, obesity, insulin therapy, and hypoglycemic agents’ medication. Model 3: adjustments for model 2 covariates plus body mass index (BMI), FBG, HbA1c, TC, LDL-C, and HDL-C. The predictive value of the TyG index for the risk of VDD was presented as odds ratio (OR) and 95% confidence interval (CI), and the lowest quartile of the TyG index was set as a reference. All statistical analyses were performed in SPSS software (version 25.0, Inc, Chicago, Illinois) or Graph Pad Prism software (version 7.0, Inc., LaJolla, CA). A two-tailed P value < 0.05 was considered as statistically significant difference.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University ([2022]085), and carried out conformed to the Declaration of Helsinki. Each participate provided informed written consent.
Results
Clinical characteristics of the participants
A total of 572 elderly participants with T2DM were included in this study. Of the whole cohort, 311 were males (54.4%) and 261 were females (45.6%), and the age range was 60–94 years (average 70.05 ± 7.00 years). Participants were stratified into four groups based on the quartiles of the TyG index [Q1 (first quartile): 7.38 ≤ TyG ≤ 8.51, Q2 (second quartile): 8.52 ≤ TyG ≤ 8.95, Q3 (third quartile): 8.96 ≤ TyG ≤ 9.42, Q4 (fourth quartile): 9.43 ≤ TyG ≤ 11.34)]. Participants in the higher quartiles of the TyG index showed increased levels of BMI, TC, TG, LDL-C, FBG, HbA1c, and C-peptide, while decreased 25(OH)D and HDL-C levels, when compared with those in the lowest TyG index quartile (all P for trend < 0.05). In addition, sex, smoking, obesity, and insulin therapy were significantly different among groups (all P for trend < 0.05). No significant differences were found in terms of age, hypertension, SBP, DBP, the usage of calcium channel blockers (CCB), angiotensin-converting enzyme inhibitors (ACEI), β blockers, oral hypoglycemia agents, and statin (all P for trend > 0.05) (Table 1).
Correlation between the TyG index and 25(OH)D
Compared with the participants in the lowest TyG index quartile, those in the higher quartiles of the TyG index had an increased trend of serum 25(OH)D level, with statistically significance were observed among Q3 and Q4 groups (all P < 0.05) (Table 1, Fig. 2A). Besides, a negative correlation was showed between the TyG index and serum 25(OH)D level (Spearman’s rho = − 0.158, P < 0.001, Fig. 2B).
Association between the TyG index and serum 25(OH)D level. (A) Comparisons of 25(OH)D level among the quartiles of the TyG index. (B) Spearman’s correlation between the TyG index and 25(OH)D level. *P < 0.05, ***P < 0.001, compared with the Q1 quartile of the TyG index. ns no significant, TyG index triglyceride-glucose index, 25(OH)D 25-hydroxyvitamin D.
Association between the TyG index and the risk of VDD
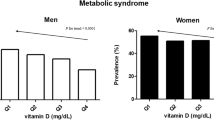
To explore the relationship between the TyG index and VDD, the percentages of VDD prevalence among the TyG index quartiles were compared. As shown in Fig. 2, an increased VDD prevalence was observed in line with the increase of the TyG index quartiles (51.7%, 55.9%, 60.8%, 72.0% in the Q1, Q2, Q3 and Q4 quartiles, P for trend = 0.003, Fig. 3).
To further explore the predictive value of the TyG index for the risk of VDD, multivariate logistic regression analyses were used. It was showed that when taking the lowest quartile of the TyG index as a reference, the risks of VDD were increased in another three groups, with the a 2.40-fold higher risk of VDD in the highest TyG quartile [OR 2.40; 95% CI 1.47–3.92; P < 0.001]. An increased trend of VDD prevalence in line with the increase of the TyG index quartiles was still observed after adjustments for clinical and laboratory parameters. Participants in the highest TyG index quartile showed statistically increased risk of VDD when compared with those in the lowest TyG index quartile after adjustments for age, sex, smoking, obesity, insulin therapy, hypoglycemic agents’ medication [OR 2.44; 95% CI 1.45–4.10; P < 0.001]. Additionally, a slightly higher risk was observed in the highest TyG index quartile after further adjustments for BMI, FBG, HbA1c, TC, LDL-C, and HDL-C [OR 2.89; 95% CI 1.33–6.24; P < 0.001] (Table 2).
Discussion
In this study, we observed a significant decreased level of 25(OH)D with the increase of the TyG index in elderly patients with T2DM, and a negative correlation between the TyG index and 25(OH)D level. Further exploration showed that elevated TyG index increased the risk of VDD in elderly diabetic patients. These results suggested that TyG index was involved in the development of VDD and could be a predictor for VDD.
Exposure to sunlight is the major source of vitamin D for most people. However, a variety of factors influence the amount of sunlight hitting to the skin and the cutaneous of production of vitamin D, such as age, skin color, sunscreen use, clothing, season, lifestyle, altitude, and latitude22. The generally accepted assessment of vitamin D status is measuring circulating 25(OH)D level, but the definition of VDD varied. It has been suggested that the values of 25(OH)D below 50 nmol/L (20 ng/mL) is considered as VDD in China21,23,24. The prevalence of VDD varies in different regions. It has been estimated that almost 60%–80% of the people had VDD or vitamin D insufficiency1,2. In China, the prevalence of VDD was 70–90% in northern cities, while almost 50% in southern cities21,23,24,25. The prevalence of VDD was higher in individuals with T2DM than those without T2DM7. Besides, the prevalence of VDD was higher in elderly population due to less sun exposure and reduced dietary vitamin D intake26. Elderly individuals with VDD are at high risk for falls and fractures27. In this study, the prevalence of VDD was 60.1% in elderly people with T2DM, which was close to that in our previous study19. This may be related to the relatively higher use of vitamin D supplements in elderly.
The main physiologic functions of vitamin D is to maintain the balance of bone metabolism through promoting the absorption of calcium and phosphorus and inhibiting the release of parathyroid hormone2. Recently, with the identification of vitamin D receptor in multiple tissues and cells, the biological functions of vitamin D has been recognized, such as stimulating insulin production and enhancing glucose utilization8,9. Emerging evidence from observational and longitudinal cohort studies demonstrated that vitamin D could alleviate IR and decrease the risk of T2DM3,6,7. However, conclusion from randomized controlled trials were controversial28. Results from some studies supported the benefits of vitamin D supplementation on pancreatic beta cell function29, while those from other studies suggested that vitamin D supplementation did not affect insulin secretion and T2DM development30,31. Cojic et al.32 reported that oral daily doses of vitamin D supplementation improved HbA1c levels and decreased the oxidation protein products levels when given higher doses of vitamin D. They also found that vitamin D supplementation improved the endothelial dysfunction in diabetic patients through reducing the production of reactive oxygen species and inflammation33. In this observational study, we found a decreased 25(OH)D level in diabetic patients with high TyG index than those with lower TyG index. This suggested that vitamin D status was negatively associated with IR, which was consistent with the findings of most observational studies.
Although numerous studies reported the role of vitamin D on the pathogenesis of IR or T2DM, few studies focused on the role of IR on VDD development. Adipose tissue is the main storage site for circulating 25(OH)D. It has been known that obese individuals were more likely to have lower 25(OH)D level in circulation due to its deposition in adipose tissue34. The mechanisms may be related to impaired metabolism35, decreased sequestration36, and declined release of vitamin D37 in obese IR state. Our previous and the present studies found that high TyG index is associated with increased risk of VDD19. Elderly diabetic individuals in the highest quartile of the TyG index showed a 2.40-fold higher risk of VDD when compared to those in the lowest TyG index quartile. Based on these evidences, it was speculated that high TyG index representing IR may be involved in the pathogenesis of VDD in T2DM. But this needs to be verified in large-scale cohort studies and randomized controlled trials, and the underlying mechanisms remain to be further clarified.
IR is a main feature as well as a key pathophysiological mechanism for T2DM. Euglycemic–hyperinsulinemic clamp test is the gold standard for the evaluation of IR, but it is relatively invasive and expensive, which is limited to clinical application38. HOMA-IR has been proposed to replace the euglycemic-hyperinsulinemic clamp test for assessing IR39. However, the strict requirements of HOMA-IR, such as no use drugs that affect the metabolism of intrinsic insulin, have also limited its application. TyG index has been confirmed to be a simple and accurate marker for the assessment of IR. Emerging evidence has demonstrated that TyG index associated significantly with HOMA-IR, and is an appropriate indicator for identifying IR11,12. Besides, it has been revealed that the TyG index was associated positively to the risk of T2DM and diabetic complications13,14,15,16. Hence, we used TyG index as a marker for IR in the present study.
The major findings of this study highlighted the association of elevated TyG index with increased risk of VDD in elderly individuals with T2DM from the central part of China. These findings provided a reference for future explorations of the association between IR and VDD. Still, there were several limitations in our study. Firstly, it was a retrospective study without investigation on any cause and effect relationship. Secondly, the study was carried out in a central part of China and the conclusions may not represent the situation elsewhere. Thirdly, the sample size was relatively small, which needs to be further studied in large cohort and prospective studies.
In summary, this study found that the TyG index was negatively associated with serum 25(OH)D level in elderly individuals with T2DM. Besides, with the increase of the TyG index, the prevalence of VDD showed an increasing trend. These results suggested that TyG index representing IR may be involved in the pathogenesis of VDD. Future studies are required to focus on the mechanisms of IR on VDD.
Data availability
Data will be made available from the corresponding author on request.
Abbreviations
- VDD:
-
Vitamin D deficiency
- 25(OH)D:
-
25-Hydroxyvitamin D
- 1,25(OH)2D:
-
1,25-Dihydroxyvitamin D
- IR:
-
Insulin resistance
- T2DM:
-
Type 2 diabetes mellitus
- TyG:
-
Triglyceride-glucose
- TG:
-
Triglyceride
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- BMI:
-
Body mass index
- FBG:
-
Fasting blood glucose
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- HbA1c:
-
Glycated hemoglobin
- SD:
-
Standard deviation
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- CCB:
-
Calcium channel blockers
- ACEI:
-
Angiotensin-converting enzyme inhibitors
References
van Schoor, N. & Lips, P. Global overview of vitamin D status. Endocrinol. Metab. Clin. N. Am. 46(4), 845–870 (2017).
Carlberg, C., Raczyk, M. & Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 62, 102695 (2023).
Dhas, Y., Banerjee, J., Damle, G. & Mishra, N. Association of vitamin D deficiency with insulin resistance in middle-aged type 2 diabetics. Clin. Chim. Acta 492, 95–101 (2019).
Akter, S. et al. Serum 25-hydroxyvitamin D3 and risk of type 2 diabetes among Japanese adults: the Hitachi Health Study. Clin. Nutr. 39(4), 1218–1224 (2020).
Pittas, A. G. et al. Vitamin D and risk for type 2 diabetes in people with prediabetes: A systematic review and meta-analysis of individual participant data from 3 randomized clinical trials. Ann Intern Med 176(3), 355–363 (2023).
Afzal, S., Bojesen, S. E. & Nordestgaard, B. G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 59(2), 381–391 (2013).
Gagnon, C. et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 34(5), 1133–1138 (2011).
Abbas, M. A. Physiological functions of vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 165(Pt B), 369–381 (2017).
Fan, Y. et al. Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 23(1), 56 (2016).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 21(1), 68 (2022).
Du, T. et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 13, 146 (2014).
Huang, R. et al. Usefulness of four surrogate indexes of insulin resistance in middle-aged population in Hefei, China. Ann. Med. 54(1), 622–632 (2022).
Zhang, M. et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc. Diabetol. 16(1), 30 (2017).
Xuan, X. et al. U-shaped association between the triglyceride-glucose index and the risk of incident diabetes in people with normal glycemic level: A population-base longitudinal cohort study. Clin. Nutr. 40(4), 1555–1561 (2021).
Zou, S. et al. Association between the triglyceride-glucose index and the incidence of diabetes in people with different phenotypes of obesity: A retrospective study. Front. Endocrinol. (Lausanne) 12, 784616 (2021).
Pan, Y. et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with type 2 diabetes. J. Diabetes Res. 2021, 8757996 (2021).
Mustafa, A. & Shekhar, C. Association between serum 25-hydroxyvitamin-D and triglycerides-glucose index among Indian adolescents. BMC Nutr. 8(1), 69 (2022).
Jia, Y., Song, T., Li, Z., Zhou, L. & Chen, S. The relationship between triglyceride glucose index and vitamin D in type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 15, 511–525 (2022).
Xiang, Q. et al. Association between the triglyceride-glucose index and vitamin D status in type 2 diabetes mellitus. Nutrients 15(3), 639 (2023).
Bouillon, R. et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 40(4), 1109–1151 (2019).
Ning, Z. et al. High prevalence of vitamin D deficiency in urban health checkup population. Clin. Nutr. 35(4), 859–863 (2016).
Dominguez, L. J., Farruggia, M., Veronese, N. & Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 11(4), 255 (2021).
Lu, H. K. et al. High prevalence of vitamin D insufficiency in China: Relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS One 7(11), e47264 (2012).
Zhen, D., Liu, L., Guan, C., Zhao, N. & Tang, X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone 71, 1–6 (2015).
Yu, S. et al. The high prevalence of hypovitaminosis D in China: A multicenter vitamin D status survey. Medicine (Baltimore) 94(8), e585 (2015).
Hossein-nezhad, A. & Holick, M. F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 88(7), 720–755 (2013).
Chakhtoura, M. et al. Vitamin D supplementation and fractures in adults: A systematic umbrella review of meta-analyses of controlled trials. J. Clin. Endocrinol. Metab. 107(3), 882–898 (2022).
Pittas, A. G., Jorde, R., Kawahara, T. & Dawson-Hughes, B. Vitamin D supplementation for prevention of type 2 diabetes mellitus: To D or not to D?. J. Clin. Endocrinol. Metab. 105(12), 3721–3733 (2020).
Mitri, J., Dawson-Hughes, B., Hu, F. B. & Pittas, A. G. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 94(2), 486–494 (2011).
Davidson, M. B., Duran, P., Lee, M. L. & Friedman, T. C. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 36(2), 260–266 (2013).
Gulseth, H. L., Wium, C., Angel, K., Eriksen, E. F. & Birkeland, K. I. Effects of vitamin D supplementation on insulin sensitivity and insulin secretion in subjects with type 2 diabetes and vitamin D deficiency: A randomized controlled trial. Diabetes Care 40(7), 872–878 (2017).
Cojic, M., Kocic, R., Klisic, A. & Kocic, G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: A 6-month follow up randomized controlled study. Front. Endocrinol. (Lausanne) 12, 610893 (2021).
Cojic, M. et al. A novel mechanism of vitamin D anti-inflammatory/antioxidative potential in type 2 diabetic patients on metformin therapy. Arch. Med. Sci. 16(5), 1004–1012 (2020).
Pramono, A., Jocken, J. W. E. & Blaak, E. E. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab. Res. Rev. 35(5), e3146 (2019).
Wamberg, L. et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. (Lond.) 37(5), 651–657 (2013).
Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z. & Holick, M. F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 72(3), 690–693 (2000).
Di Nisio, A. et al. Impaired release of vitamin D in dysfunctional adipose tissue: New cues on vitamin D supplementation in obesity. J. Clin. Endocrinol. Metab. 102(7), 2564–2574 (2017).
Gastaldelli, A. Measuring and estimating insulin resistance in clinical and research settings. Obesity (Silver Spring) 30(8), 1549–1563 (2022).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985).
Acknowledgements
We thank all the participants in this study for their contributions.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82071593).
Author information
Authors and Affiliations
Contributions
Q.X.: formulated the research, performed the analysis and drafted the manuscript. H.X.: collected the data and supervised the analysis. Y.L.: supervised, revised and reviewed the manuscript. W.H.: formulated the research, revised and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiang, Q., Xu, H., Liu, Y. et al. Elevated TyG index is associated with increased risk of vitamin D deficiency among elderly patients with type 2 diabetes. Sci Rep 14, 16098 (2024). https://doi.org/10.1038/s41598-024-67127-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67127-1
- Springer Nature Limited