Abstract
Dietary antioxidants may have beneficial effects on bone health, but it remains uncertain in children and adolescents. This study investigates the association of composite dietary antioxidant index (CDAI) with bone mineral density (BMD) in children and adolescents aged 8–19 years from the National Health and Nutrition Examination Survey (NHANES) 2007–2010. The study assessed the relationship between CDAI and BMD in 2994 individuals aged 8–19 years (average age 13.48 ± 3.32 years) from the NHANES 2007–2010. Multivariate linear regression analyses were utilized to detect the association between CDAI and total spine, femur neck, and total femur BMD, adjusting for confounders including age, race/ethnicity, sex, poverty income ratio (PIR), body mass index (BMI), serum phosphorus and calcium. Stratified analyses and interaction tests were performed to examine the stability of the results. The weighted characteristics showed that subjects in the fourth CDAI quartile were more likely to be older, men, and Non-Hispanic White. They have higher values of serum total calcium and phosphorus. After adjusting all confounders, CDAI was positively associated with the total spine (β = 0.0031 95% CI 0.0021–0.0040), total femur (β = 0.0039 95% CI 0.0028–0.0049), and femur neck BMD (β = 0.0031 95% CI 0.0021–0.0040) in children and adolescents. Furthermore, we found no interaction effects between different race/ethnicity, age, and sex groups. Our findings suggest that dietary intake of multiple antioxidants was positively associated with BMD in children and adolescents. These findings provide valuable evidence for improving bone health in the early stages of life. However, more prospective studies are required to validate our findings and their causal relationship.
Similar content being viewed by others
Introduction
Osteoporosis is a disease characterized by poor bone quality that leads to an increased risk of fractures and higher mortality in the elderly1. Using the diagnostic criteria of the World Health Organization, the global prevalence of osteoporosis in people aged > 50 was 20.5%2. Peak bone mass (PBM) accumulated in late adolescence is an important determinant of fragility fractures and osteoporosis risk in older adults3. Previous studies showed that a 10% increase in PBM during adolescence may decrease 50% fracture risk in senior citizens4. The formulation of PBM can be affected by nutrition, genetics, physical activities, certain diseases, and other factors5,6,7.
Reactive oxygen species (ROS) play a critical role in degenerative diseases like liver diseases, cardiovascular disease, osteoarthritis, and osteoporosis8,9,10. ROS accumulation is closely related to osteoclast and osteoblast apoptosis, impairing osteogenesis and mineralization of bone11,12. Moreover, redox imbalance caused by the excessive production of ROS increases macrophage osteoclast differentiation and supports an increase in bone loss, leading to osteoporosis13. Therefore, antioxidants have become a potential therapy to attenuate bone loss and prevent osteoporosis induced by excess ROS11. Several studies have explored the effects of ingesting individual antioxidants on bone mineral density (BMD), but the results are controversial7,14,15,16. A study by Kim et al.17 analyzed 1196 postmenopausal females from the Korean National Health and Nutrition Examination Survey (KNHANES) over 50 years. The findings suggested that dietary vitamin C intake was positively related to BMD. However, another study with 17 years of follow-up showed no protective effect of vitamin C supplementation on hip fracture risk18. These studies examining the impact of single antioxidant intake on BMD or osteoporosis were not representative of the total antioxidant intake and may produce biased results. The composite dietary antioxidant index (CDAI) is a standardized combination of intakes of six major antioxidants: vitamins C, E, and A, carotenoids, zinc, and selenium. It has been widely used as a composite score in studies involving the total dietary antioxidant capacity19,20,21. The CDAI has been proven to be closely associated with oxidative stress and inflammatory biomarkers (TNF-α and IL-1β)22. Recently, Liu et al.23 found a positive relationship between CDAI and BMD in U.S. adults. However, no studies have examined the association between CDAI and BMD in children and adolescents.
Adolescence is the most critical period for PMB formation and may largely influence the occurrence of osteoporosis or fragility fractures during adulthood or ageing. Nevertheless, the association between CDAI and BMD in children and adolescents is still unclear. This study is the first to explore the association between CDAI and BMD in individuals aged 8–19, aiming to provide crucial information on bone health in the early stages of life.
Methods
Study design
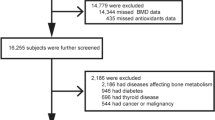
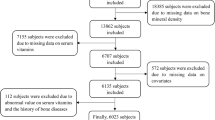
The NHANES is a national nutrition survey on the U.S. population that collects and publicly releases data biennially24. All participants or their guardians provided informed consent and the NHANES protocol was approved by the Research Ethics Review Board at NCHS. We combined data from the NHANES 2007–2008 and 2009–2010 because femoral BMD data of subjects less than 20 years only existed in these cycles. Then, children younger than 8 years were excluded since they did not receive the DXA examination in NHANES. Subjects with missing information on BMD (n = 625) and CDAI data (n = 645) were excluded. Finally, 2994 individuals aged 8–19 were enrolled in this study. The participant selection flowchart is displayed in Fig. 1.
Variables
Composite dietary antioxidant index
Dietary intake for each subject in the NHANES dataset was documented through two 24-h dietary recall interviews with children and adolescents. The initial dietary recall interview was performed at the Mobile Examination Center (MEC), followed by a second interview 3 to 10 days later via a return phone call. The U.S. Department of Agriculture's Dietary Research Food and Nutrient Database was used to calculate antioxidant, micronutrient, and total energy intakes25. To calculate the CDAI, we summed up the intake of six antioxidants by averaging the two interviews (vitamins C, E, and A carotenoids, zinc, and selenium). These summed antioxidants were calculated from diet alone and did not include additional supplements/medications. Vitamin E and A intake were defined as equal milligrams of alpha-tocopherol and retinol. The six standardized dietary antioxidant intakes were calculated by deducting the mean and dividing by the standard deviation. Then, they were summed to obtain the CDAI scores26. The calculation formula is as follows: CDAI = \({\sum }_{i=1}^{n=6}x=\frac{\text{Individual \, Intake}-\text{ Mean}} \, {\text{SD}}\).
Bone mineral density
Dependent variables included total spine, femur neck, and total femur BMD, which were measured by dual-energy X-ray absorptiometry (DXA) using Hologic densitometers. Professionals collected and standardized the information on total spine, femur neck, and total femur BMD. Detailed BMD data can be accessed in DXXLSA, DXXOFBMD, and DXXNKBMD datasets on NHANES website27.
Covariates
Covariates were chosen based on the published studies to eliminate potential effects on the final results: race/ethnicity, age, sex, poverty income ratio (PIR), body mass index (BMI), serum phosphorus, and serum calcium25,28,29. Detailed data on BMD, CDAI, and additional covariates could be seen at the NHANES website (http://www.cdc.gov/nchs/nhanes/).
Statistical analysis
All analyses were performed by R software (4.3.1) and EmpowerStats (4.1), using MEC weighted. A P value < 0.05 was regarded as significant. Percentages and mean ± standard deviation were used to represent categorical and continuous variables. To compare differences in continuous and categorical variables, weighted linear regression models and weighted χ2 tests were used, respectively. We employed weighted multivariate logistic regression analyses to evaluate the association of CDAI with total spine, femur neck, and total femur BMD. We first built an unadjusted model (Model 1). Then, Model 2 was created by adjusting race/ethnicity, age, and sex. Finally, Model 3 was created by adjusting the variables of race/ethnicity, age, sex, PIR, BMI serum phosphorus, and serum calcium. After dividing CDAI into quartiles, trend tests were utilized to analyze their linear association trend. Stratified analyses and interaction tests were performed by gender (male and female), age (8–13 and 14–19), and race/ethnicity (Mexican American, Non-Hispanic White, Other Hispanic, Non-Hispanic Black, and Other Race).
Results
A total of 2994 subjects aged 8–19 years were recruited in this study, of which 52.39% were males and 47.61% were females with a mean age of 13.48 ± 3.32 years. In addition, 13.77% of the participants were Mexican American, 59.28% were non-Hispanic white, 13.94% were non-Hispanic black, 7.16% were other races (including multiracial), and 5.85% were other Hispanic. The weighted participants' characteristics were analyzed based on CDAI quartiles (Q1–Q4), as listed in Table 1. In the fourth quartile of CDAI group, participants were older, men, and race of Non-Hispanic White (P < 0.05). They have higher values of serum phosphorus and calcium.
Association between CDAI and total spine BMD
The association between CDAI and total spine BMD can be found in Table 2 and Fig. 2A. The positive relationship between CDAI and total spine BMD was significant in both three models. In model 3, CDAI was positively associated with total spine BMD (β = 0.0031 95% CI 0.0021–0.0040). We divided the CDAI into quartiles and found that the lumbar BMD in subjects of the fourth CDAI quartile increases by 0.0325 g/cm2 than those in the first CDAI quartile (P for trend < 0.001). The results of stratified analyses are presented in Fig. 3A. After stratified by sex, we found no difference between males (β = 0.0024 95% CI 0.0013–0.0036) and females (β = 0.0027 95% CI 0.0012–0.0041) (P for interaction = 0.954). The positive association was significant in those aged 8–13 years (β = 0.0028 95% CI 0.0010–0.0045) and 14–19 years (β = 0.0031 95% CI 0.0017–0.0045) (P for interaction = 0.420). Then, we conduct a stratified analysis by race/ethnicity. Statistically significant correlation between CDAI and the lumbar BMD was found in non-Hispanic black (β = 0.0040 95% CI 0.0018–0.0063), non-Hispanic white (β = 0.0036 95% CI 0.0020–0.0052), and Mexican American (β = 0.0018 95% CI 0.0003–0.0033), but not in Other Hispanic (β = 0.0009 95% CI − 0.0018–0.0037) and other races (Including multi-racial) (β = 0.0010 95% CI − 0.0035–0.0055). However, the results showed no interaction effect between different race/ethnicity (P for interaction = 0.284).
Association between CDAI and total femur BMD
The association between CDAI and total femur BMD can be found in Table 2 and Fig. 2B. The positive relationship between CDAI and total femur BMD was significant in both three models. In model 3, CDAI was positively associated with total femur BMD (β = 0.0039 95% CI 0.0028–0.0049). We divided the CDAI into quartiles and found that the total femur BMD in subjects of the fourth CDAI quartile increases by 0.0434 g/cm2 than in the first CDAI quartile (P for trend < 0.001). The results of stratified analyses are presented in Fig. 3B. After stratified by sex, we found no difference between males (β = 0.0028 95% CI 0.0014–0.0041) and females (β = 0.0040 95% CI 0.0025–0.0056) (P for interaction = 0.284). The positive association was significant in those aged 8–13 years (β = 0.0033 95% CI 0.0017–0.0049) and 14–19 years (β = 0.0035 95% CI 0.0020–0.0056) (P for interaction = 0.482). Statistically significant correlation between CDAI and the total femur BMD was found in non-Hispanic black (β = 0.0028 95% CI 0.0003–0.0053), non-Hispanic white (β = 0.0040 95% CI 0.0022–0.0059), and Mexican American (β = 0.0030 95% CI 0.0014–0.0047), but not in Other Hispanic (β = 0.0023 95% CI − 0.0008–0.0055) and other races (Including multi-racial) (β = 0.0015 95% CI − 0.0042–0.0073). However, the results showed no interaction effect between different race/ethnicity (P for interaction = 0.722).
Association between CDAI and femur neck BMD
The association between CDAI and femur neck BMD can be found in Table 2 and Fig. 2C. In both three models, the positive relationship between CDAI and femur neck was significant. In model 3, CDAI was positively associated with femur neck BMD (β = 0.0031 95% CI 0.0021–0.0040). We divided the CDAI into quartiles and found that the total femur BMD in subjects of the fourth CDAI quartile increases by 0.0316 g/cm2 than in the first CDAI quartile (P for trend < 0.001). The results of stratified analyses are presented in Fig. 3C. After stratified by sex, we found no difference between males (β = 0.0025 95% CI 0.0012–0.0038) and females (β = 0.0030 95% CI 0.0015–0.0045, P < 0.001) (P for interaction = 0.689). The positive association was significant in those aged 8–13 years (β = 0.0024 95% CI 0.0010–0.0038) and 14–19 years (β = 0.0032 95% CI 0.0016–0.0047) (P for interaction = 0.272). Statistically significant correlation between CDAI and the femur neck BMD was found in non-Hispanic black (β = 0.0032 95% CI 0.0009–0.0055), non-Hispanic white (β = 0.0033 95% CI 0.0016–0.0050), and Mexican American (β = 0.0021 95% CI 0.0006–0.0037), but not in Other Hispanic (β = 0.0009 95% CI − 0.0021–0.0039) and other races (Including multi-racial) (β = 0.0022 95% CI − 0.0024–0.0068). However, the results showed no interaction effect between different race/ethnicity (P for interaction = 0.402).
Discussion
Our study is the first to explore and identify a positive association between CDAI and BMD in children and adolescents. This positive association remained stable across age, gender, and race/ethnicity. The results of this study provide evidence supporting the role of antioxidant intake in the early prevention of osteoporosis.
In recent years, dietary intake of antioxidants has been shown to be associated with a variety of health factors, including bone health30,31,32. In an early study in the United States, Morton et al.33 found that vitamin C supplements were beneficial for increasing BMD of the femur, femoral neck, and radius in postmenopausal women. In another four-year study, Sahni et al.34 found that carotenoid intake could protect bone health in an elderly population. Wu et al.35 found that a higher selenium status is independently related to an osteoporosis risk in subjects aged > 40 years. However, there are few studies on the effects of antioxidant intake on bone health in children and adolescents. Furthermore, these studies always focus on a single antioxidant and ignore the overall total dietary antioxidant capacity7,36,37,38. For example, in a cross-sectional study of 426 children, Zhang et al.36 found that vitamin A intake is positively related to BMD after adjusting confounders. However, in another study involving 888 subjects aged 15–19 years, Teigmo et al.39 found no association between vitamin A status and BMD. Selenium is believed to play a role in bone health because of its antioxidant ability. In a recent NHANES study, the dose–response analyses showed an inversed U-shaped association between selenium status and BMD in individuals aged 8–19 years. Appropriate selenium intake benefits children's bone health, while excessive selenium may exert adverse effects on children's bone health37. These studies involving a single antioxidant may ignore overall total dietary antioxidant capacity as some dietary antioxidants require interactions for synergistic effects40. A study by Turan et al.41 found that the concomitant use of selenium with vitamins E and C prevented osteoporosis in a rabbit model. The combination effect of these antioxidants was better than that of the single one41. The latest review indicated that separate antioxidants may help bone health, while multiple antioxidants from whole plant foods may have more overall benefits42. The CDAI considers dietary intake of various antioxidants such as minerals (zinc, selenium), vitamins (vitamin C, vitamin E), and phytochemicals (flavonoids, carotenoids) and is able to adequately respond to the antioxidant capacity of our diet. In a previous study, Liu et al.43 found a positive relationship between CDAI and femur BMD in American adults aged ≥ 20 years. Their results showed that every unit increase in dietary CDAI was associated with 0.003 and 0.001 g/cm2 increase in femoral neck and total spine BMD, respectively. Similar to adults, our study also confirmed the positive association between CDAI and BMD in children and adolescents. Our study showed that every unit increase in dietary CDAI was associated with 0.0039 and 0.0031 g/cm2 increase in femur neck and total spine BMD, which is larger than in adults. Thus, combined intake of dietary antioxidants may contribute to bone health in adolescents.
Although there is no direct evidence on the mechanism of CDAI on bone health in children and adolescents, several cellular or animal studies have explained the positive effects of antioxidants on bone health30,44. Oxidative stress is an imbalance of oxidative and antioxidant effects that tends to oxidize in our body and is an important contributor to ageing and disease, including osteoporosis45. Excess ROS generated by oxidative stress imbalance could inhibit the expression of osterix and Runx2, thereby reducing osteogenic activity46. The evidence suggested that osteoclastogenic markers like TRAP, NFATc1, and c-Fos were upgraded after ROS induction47. In addition, the researcher also found decreased cell viability, increased lipogenic differentiation, and increased osteogenic differentiation in H2O2-treated bone marrow mesenchymal stem cells (BM-MSCs)48. Moreover, NADPH oxidase 4 (NOX4) is an essential source of active enzymes that make up ROS. Goettsch et al.49 found that NOX4 was overexpressed in patients with increased osteoclast activity. Furthermore, their results suggested a reduced bone loss in Nox4 knockdown or pharmacologically suppressed ovariectomized mice.
In total, we first evaluated and found a positive association of dietary antioxidant exposure with bone health in children and adolescents. To ensure the stability of the findings, we performed subgroup analyses. The results showed that the positive association between CDAI and BMD remained stable across gender and age. Interestingly, though results showed no interaction effect between different race/ethnicity, we found that the positive correlation was significant only among non-Hispanic whites, Mexican Americans, and non-Hispanic blacks. Genetic differences between races may partly account for these differences, as studies suggest that 50% to 85% of PBM is genetically determined50. However, more investigations are required to validate our findings.
Our results have several advantages. First, this is a large sample analysis from NHANES survey. All analyses used MEC sampling weights that are representative of the general population in the United States. Second, we found that CDAI was positively associated with lumbar and femoral BMD. The use of CDAI as an exposure variable rather than a single antioxidant provides valuable evidence for the association between dietary antioxidants and bone health during adolescence. Third, we used dietary data that were the average of two 24-h dietary measurements, thereby increasing the reliability of the results. Our study also has some limitations. First, it is hard to infer causality because of the nature of the cross-sectional study. Second, our study population did not include individuals aged less than 8 years since their BMD data were not available in NHANES. Third, we tried to adjust for some of the confounders. This may only partially rule out the effect of confounders on the final results. Covariates such as children's physical activity and vitamin D status were not available in these NHANES cycles and thus may have affected the stability of the results. Fourth, CDAI may not be representative of the total intake of antioxidant ability. Future research may require a more representative indicator of the overall antioxidant activity of these compounds.
Conclusions
Our findings suggest that dietary intake of multiple antioxidants was positively associated with BMD in children and adolescents. These findings provide valuable evidence for improving bone health in the early stages of life. However, prospective studies are required to validate our findings and their causal relationship.
Data availability
The datasets generated during and/or analysed during the current study are available in the [NHANES] repository, [https://www.cdc.gov/nchs/nhanes/].
References
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733. https://doi.org/10.1007/s00198-006-0172-4 (2006).
Xiao, P. L. et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 33, 2137–2153. https://doi.org/10.1007/s00198-022-06454-3 (2022).
Matkovic, V. et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis: Inference from a cross-sectional model. J. Clin. Invest. 93, 799–808. https://doi.org/10.1172/JCI117034 (1994).
Rozenberg, S. et al. How to manage osteoporosis before the age of 50. Maturitas 138, 14–25. https://doi.org/10.1016/j.maturitas.2020.05.004 (2020).
Cui, A. et al. Blood lead level is negatively associated with bone mineral density in US children and adolescents aged 8–19 years. Front. Endocrinol. 13, 928752. https://doi.org/10.3389/fendo.2022.928752 (2022).
Karlsson, M. K. & Rosengren, B. E. Exercise and peak bone mass. Curr. Osteoporos. Rep. 18, 285–290. https://doi.org/10.1007/s11914-020-00588-1 (2020).
Cui, A. et al. Associations between vitamin E status and bone mineral density in children and adolescents aged 8–19 years: Evidence based on NHANES 2005–2006, 2017–2018. PLoS ONE 18, e0283127. https://doi.org/10.1371/journal.pone.0283127 (2023).
Murphy, E. & Liu, J. C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 119, 1105–1116. https://doi.org/10.1093/cvr/cvac134 (2023).
Che, Z. et al. ROS/RNS as molecular signatures of chronic liver diseases. Trends Mol. Med. 29, 951–967. https://doi.org/10.1016/j.molmed.2023.08.001 (2023).
Riegger, J., Schoppa, A., Ruths, L., Haffner-Luntzer, M. & Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell Mol. Biol. Lett. 28, 76. https://doi.org/10.1186/s11658-023-00489-y (2023).
Marcucci, G. et al. Oxidative stress and natural antioxidants in osteoporosis: Novel preventive and therapeutic approaches. Antioxidants https://doi.org/10.3390/antiox12020373 (2023).
Oliveira, M. C., Campos-Shimada, L. B., Marcal-Natali, M. R., Ishii-Iwamoto, E. L. & Salgueiro-Pagadigorria, C. L. A long-term estrogen deficiency in ovariectomized mice is associated with disturbances in fatty acid oxidation and oxidative stress. Rev. Bras. Ginecol. Obstet. 40, 251–259. https://doi.org/10.1055/s-0038-1666856 (2018).
Zhu, C. et al. Autophagy in bone remodeling: A regulator of oxidative stress. Front. Endocrinol. 13, 898634. https://doi.org/10.3389/fendo.2022.898634 (2022).
Shi, W. Q. et al. Association of dietary and serum vitamin E with bone mineral density in middle-aged and elderly Chinese adults: A cross-sectional study. Br. J. Nutr. 115, 113–120. https://doi.org/10.1017/S0007114515004134 (2016).
Malmir, H., Shab-Bidar, S. & Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 119, 847–858. https://doi.org/10.1017/S0007114518000430 (2018).
Sugiura, M. et al. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: Findings from post-menopausal Japanese female subjects. Osteoporos. Int. 22, 143–152. https://doi.org/10.1007/s00198-010-1239-9 (2011).
Kim, Y. A. et al. Favorable effect of dietary vitamin C on bone mineral density in postmenopausal women (KNHANES IV, 2009): Discrepancies regarding skeletal sites, age, and vitamin D status. Osteoporos. Int. 26, 2329–2337. https://doi.org/10.1007/s00198-015-3138-6 (2015).
Sahni, S. et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture: A 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos. Int. 20, 1853–1861. https://doi.org/10.1007/s00198-009-0897-y (2009).
Liu, C., Lai, W., Zhao, M., Zhang, Y. & Hu, Y. Association between the composite dietary antioxidant index and atherosclerotic cardiovascular disease in postmenopausal women: A cross-sectional study of NHANES data, 2013–2018. Antioxidants https://doi.org/10.3390/antiox12091740 (2023).
Wang, M., Huang, Z. H., Zhu, Y. H., He, P. & Fan, Q. L. Association between the composite dietary antioxidant index and chronic kidney disease: Evidence from NHANES 2011–2018. Food Funct. https://doi.org/10.1039/d3fo01157g (2023).
Maugeri, A. et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radic. Biol. Med. 131, 274–281. https://doi.org/10.1016/j.freeradbiomed.2018.12.018 (2019).
Luu, H. N. et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels?. Antioxid. Redox Signal 22, 951–959. https://doi.org/10.1089/ars.2014.6212 (2015).
Liu, J., Tang, Y., Peng, B., Tian, C. & Geng, B. Bone mineral density is associated with composite dietary antioxidant index among US adults: Results from NHANES. Osteoporos. Int. https://doi.org/10.1007/s00198-023-06901-9 (2023).
US CDC. National Center for Health Statistics NHANES Comprehensive Data List. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx.
Ahuja, J. K., Moshfegh, A. J., Holden, J. M. & Harris, E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J. Nutr. 143, 241S-S249. https://doi.org/10.3945/jn.112.170043 (2013).
Yu, Y. C. et al. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study. Int. J. Cancer 150, 1599–1608. https://doi.org/10.1002/ijc.33925 (2022).
Anderson, J. J. Calcium, phosphorus and human bone development. J. Nutr. 126, 1153S-S1158. https://doi.org/10.1093/jn/126.suppl_4.1153S (1996).
Li, T. et al. The association between lead exposure and bone mineral density in childhood and adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients https://doi.org/10.3390/nu14071523 (2022).
Tan, J. et al. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: A possible mechanism of age related osteoporosis. Bone Res. 3, 15003. https://doi.org/10.1038/boneres.2015.3 (2015).
Yamaguchi, M. Role of carotenoid beta-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 19, 36. https://doi.org/10.1186/1423-0127-19-36 (2012).
Yee, M. M. F., Chin, K. Y., Ima-Nirwana, S. & Wong, S. K. Vitamin A and bone health: A review on current evidence. Molecules https://doi.org/10.3390/molecules26061757 (2021).
Morton, D. J., Barrett-Connor, E. L. & Schneider, D. L. Vitamin C supplement use and bone mineral density in postmenopausal women. J. Bone Miner. Res. 16, 135–140. https://doi.org/10.1359/jbmr.2001.16.1.135 (2001).
Sahni, S. et al. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 89, 416–424. https://doi.org/10.3945/ajcn.2008.26388 (2009).
Wu, C. C., Wang, C. K., Yang, A. M., Lu, C. S. & Lin, C. Y. Selenium status is independently related to bone mineral density, FRAX score, and bone fracture history: NHANES, 2013 to 2014. Bone 143, 115631. https://doi.org/10.1016/j.bone.2020.115631 (2021).
Zhang, X. et al. Vitamin A nutritional status is a key determinant of bone mass in children. Nutrients https://doi.org/10.3390/nu14214694 (2022).
Cui, A. et al. Associations between serum selenium and bone mineral density in 8–19-year-old children and adolescents: NHANES 2013–2018. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-023-03808-8 (2023).
Gunnes, M. & Lehmann, E. H. Dietary calcium, saturated fat, fiber and vitamin C as predictors of forearm cortical and trabecular bone mineral density in healthy children and adolescents. Acta Paediatr. 84, 388–392. https://doi.org/10.1111/j.1651-2227.1995.tb13656.x (1995).
Teigmo, M. S. W., Gundersen, T. E., Emaus, N. & Grimnes, G. Distribution and determinants of retinol in Norwegian adolescents, and its relation to bone mineral density: the Tromso Study: Fit futures. Eur. J. Clin. Nutr. 72, 1373–1384. https://doi.org/10.1038/s41430-018-0193-z (2018).
Persson, T., Popescu, B. O. & Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail?. Oxid. Med. Cell Longev. 2014, 427318. https://doi.org/10.1155/2014/427318 (2014).
Turan, B., Can, B. & Delilbasi, E. Selenium combined with vitamin E and vitamin C restores structural alterations of bones in heparin-induced osteoporosis. Clin. Rheumatol. 22, 432–436. https://doi.org/10.1007/s10067-003-0809-z (2003).
Kimball, J. S., Johnson, J. P. & Carlson, D. A. Oxidative stress and osteoporosis. J. Bone Joint Surg. Am. 103, 1451–1461. https://doi.org/10.2106/JBJS.20.00989 (2021).
Liu, J., Tang, Y., Peng, B., Tian, C. & Geng, B. Bone mineral density is associated with composite dietary antioxidant index among US adults: Results from NHANES. Osteoporos. Int. 34, 2101–2110. https://doi.org/10.1007/s00198-023-06901-9 (2023).
Filaire, E. & Toumi, H. Reactive oxygen species and exercise on bone metabolism: Friend or enemy?. Joint Bone Spine 79, 341–346. https://doi.org/10.1016/j.jbspin.2012.03.007 (2012).
Zhang, C. et al. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 163, 114834. https://doi.org/10.1016/j.biopha.2023.114834 (2023).
Li, S. et al. Metallothionein 3 promotes osteoblast differentiation in C2C12 cells via reduction of oxidative stress. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22094312 (2021).
Wang, N., Hao, Y. & Fu, L. Trimethylamine-N-oxide promotes osteoclast differentiation and bone loss via activating ROS-dependent NF-kappaB signaling pathway. Nutrients https://doi.org/10.3390/nu14193955 (2022).
Zhang, Q. et al. Dissecting molecular mechanisms underlying H(2)O(2)-induced apoptosis of mouse bone marrow mesenchymal stem cell: Role of Mst1 inhibition. Stem Cell Res. Ther. 11, 526. https://doi.org/10.1186/s13287-020-02041-7 (2020).
Goettsch, C. et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 123, 4731–4738. https://doi.org/10.1172/JCI67603 (2013).
Zhai, G., Andrew, T., Kato, B. S., Blake, G. M. & Spector, T. D. Genetic and environmental determinants on bone loss in postmenopausal Caucasian women: A 14-year longitudinal twin study. Osteoporos. Int. 20, 949–953. https://doi.org/10.1007/s00198-008-0751-7 (2009).
Acknowledgements
We acknowledge the data from the National Health and Nutrition Examination Survey (NHANES).
Author information
Authors and Affiliations
Contributions
Conceptualization, Aiyong Cui, Juan Yan; Data curation, Aiyong Cui, Juan Yan; Formal analysis, Aiyong Cui, Juan Yan, Yuan Zeng, Baoqiang Shi, Long Cheng; Investigation, Baoqiang Shi, Hongli Deng, Xing Wei, and Yan Zhuang; Methodology, Aiyong Cui, Juan Yan, and Yan Zhuang; Project administration, Aiyong Cui, Juan Yan, and Yan Zhuang; Software, Aiyong Cui, Juan Yan, Yuan Zeng, and Baoqiang Shi Visualization, Aiyong Cui, Juan Yan; Writing—original draft, Aiyong Cui, Juan Yan, Yuan Zeng, Baoqiang Shi, Long Cheng,; Writing—review & editing, Hongli Deng, Xing Wei, and Yan Zhuang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, A., Yan, J., Zeng, Y. et al. Association between composite dietary antioxidant and bone mineral density in children and adolescents aged 8–19 years: findings from NHANES. Sci Rep 14, 15849 (2024). https://doi.org/10.1038/s41598-024-66859-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66859-4
- Springer Nature Limited