Abstract
Mucus stasis is a pathologic hallmark of muco-obstructive diseases, including cystic fibrosis (CF). Mucins, the principal component of mucus, are extensively modified with hydroxyl (O)-linked glycans, which are largely terminated by sialic acid. Sialic acid is a negatively charged monosaccharide and contributes to the biochemical/biophysical properties of mucins. Reports suggest that mucin sialylation may be altered in CF; however, the consequences of reduced sialylation on mucus clearance have not been fully determined. Here, we investigated the consequences of reduced sialylation on the charge state and conformation of the most prominent airway mucin, MUC5B, and defined the functional consequences of reduced sialylation on mucociliary transport (MCT). Reduced sialylation contributed to a lower charged MUC5B form and decreased polymer expansion. The inhibition of total mucin sialylation de novo impaired MCT in primary human bronchial epithelial cells and rat airways, and specific α-2,3 sialylation blockade was sufficient to recapitulate these findings. Finally, we show that ST3 beta-galactoside alpha-2,3-sialyltransferase (ST3Gal1) expression is downregulated in CF and partially restored by correcting CFTR via Elexacaftor/Tezacaftor/Ivacaftor treatment. Overall, this study demonstrates the importance of mucin sialylation in mucus clearance and identifies decreased sialylation by ST3Gal1 as a possible therapeutic target in CF and potentially other muco-obstructive diseases.
Similar content being viewed by others
Introduction
Cystic fibrosis (CF) is a genetic disease hallmarked by viscous and adhesive airway mucus in several organs1,2,3. In the respiratory tract, CF mucus becomes static and leads to chronic infection, progressive organ decline, and early mortality1,4. The rheological characteristics of the mucus gel are largely governed by the gel-forming mucins MUC5B and MUC5AC, the chief structural component of mucus2,5. In CF, electrostatic driven abnormalities, stemming from impaired anion transport, alter mucin conformation and contribute to increased mucus viscosity and impaired mucociliary clearance (MCC)4,6,7,8,9.
Gel-forming mucins are extensively modified with hydroxyl (O)-linked glycans that determine biophysical properties including normal mucin expansion and rheological characteristics5,10,11. Mucin glycans are largely terminated by sialic acid attached in either an α-2,3 or α-2,6 linkage, facilitated by either ST3 beta-galactoside alpha-2,3-sialyltransferase (ST3Gal) or ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase (ST6GalNAc), respectively12,13. Sialic acid is a negatively charged monosaccharide and exerts key electrostatic properties to mucins via its negative charge14. The high anionic density of mucins is postulated to help stiffen the mucin polymer through charge repulsion and mediate interactions with cations to facilitate mucin granular packaging and post-secretory expansion10,15,16,17. Within intracellular granules, calcium (Ca2+) shields these negative charges to mitigate electrostatic repulsion and promote mucin condensation11,18. Upon secretion, divalent sodium (Na+) is exchanged with Ca2+ to facilitate mucin expansion and hydration5,6. Although anionic density is central to the mechanisms of mucin maturation and hydration, studies have historically centered around the altered ionic environment as the driver of aberrant mucin in CF, and the role of intrinsic mucin sialylation/charge in mucin biogenesis and MCC has remained understudied.
Previous work has implicated lower charged mucin in several muco-obstructive diseases. The predominant gel-forming mucin of the airway, MUC5B, has been characterized to exist in two forms:1 a more negative “high” charged form, and2 a less negative “low” charged form (based on its migration following agarose gel electrophoresis)19. Increased levels of the low charged form have been reported in CF, asthma, and chronic obstructive pulmonary disease (COPD)5,20,21. In asthmatics, the low charged form was enriched within a viscous mucus plug and exhibited a compact and entangled conformation, linking decreased MUC5B charge with conformational and rheological defects22. However, the etiology of this MUC5B form and the functional consequences of reduced/low mucin charge on polymer expansion and MCC have not been elucidated. Interestingly, changes in mucin sialylation have been documented in CF. Specifically, evidence supports that sialylation may be reduced as a direct consequence of defective cystic fibrosis transmembrane conductance regulator (CFTR), although the role of CFTR in regulating mucin sialylation/charge and its impact on CF mucus pathology remain unclear23,24,25,26.
In this study, we aimed to determine the role of mucin sialylation on the biophysical properties of mucin and overall mucus function in terms of charge state, compaction, and mucus transport. Furthermore, we aimed to determine the clinical relevance of aberrant mucin sialylation in CF. Here, we show that reduced sialylation of mucin contributed to a low charged form of MUC5B, increased MUC5B compaction, and ultimately impaired mucociliary transport (MCT) in vitro and in vivo. Additionally, we provide evidence for a link between the defective CFTR and reduced sialylation of mucin in CF mucus stasis. Overall, this study demonstrates the importance of sialylation in mucus function and provides impetus to investigate the molecular mechanisms of mucin sialylation for treatment of mucociliary dysfunction in CF, as well as potentially other muco-obstructive diseases.
Results
Reducing the sialylation of contributes to a low charge form of MUC5B
Since sialic acid contributes to the overall charge of MUC5B, we hypothesized that reducing sialylation would result in a lower charged mucin, similar to the predominant forms in COPD and asthma5,22,27. To test this, we collected and partially purified mucin from non-CF primary human bronchial epithelial cells (HBECs) secretions, then treated this mucin with increasing concentrations of sialidase. Using an agarose polyacrylamide gel electrophoresis (Ag-PAGE) gel mobility assay to characterize mucin charge state28, sialidase treated mucins were separated followed by sialylation analysis with wheat germ agglutinin (WGA) lectin blotting (Fig. 1A). As sialylation was reduced by sialidase treatment, the faster migrating mucin forms were dose-dependently shifted to a slower mobility in the gel (Fig. 1A) indicative of charge reduction. In parallel experiments, an incremental shift in gel mobility of MUC5B was observed, reflecting the dose-dependent loss of sialic acid following sialidase treatment (Fig. 1B). We extended this study to salivary MUC5B which exhibited a broader separation range of charged forms. Following isolation via cesium chloride (CsCl) density centrifugation, as previously described, control and sialidase treated MUC5B was separated via Ag-PAGE and evaluated for sialic acid using a SIAFIND™ Pan Lectenz probe and MUC5B29. Consistent with HBEC derived MUC5B, sialic acid strongly overlapped with the faster migrating/ more charged MUC5B (SI Fig. 1A-B). Following sialidase treatment, the bands demonstrating the most charge were lost and MUC5B mobility was incrementally shifted (SI Fig. 1B). Together, these results provide evidence that sialylation is important in the charge state of mucins and its reduction may contribute to the low charged MUC5B form previously implicated in muco-obstructive disease.
Reducing the sialylation of secreted mucin contributes to a low charge form of MUC5B. Agarose-PAGE western blots of partially purified mucin from non-CF HBEC secretions. Mucin was treated with increasing concentrations of neuraminidase, ranging from 0 to 25 mu/mL, to remove sialic acid and separated by gel electrophoresis before being probed for (A) sialic acid (WGA) and (B) MUC5B. The faster migrating/highest charged species (red bracket) disappears as sialic acid is increasingly removed. The gel mobility of MUC5B is decreased as sialic acid is removed indicating a decrease in charge. Blots were cropped to improve clarity and conciseness; the original, uncropped blots are presented in SI Fig. 4A.
Reducing sialylation of salivary MUC5B impairs mucin linearization
To determine the consequences of reduced sialylation on MUC5B conformation, we natively purified salivary MUC5B via CsCl gradient centrifugation as previously described, incubated it with either sialidase or vehicle, and subsequently evaluated the macromolecular conformations of MUC5B polymers via transmission electron microscopy (TEM)29. In parallel, we incubated untreated MUC5B with 10 mM Ca2+ in pH 5 to induce MUC5B condensation (Fig. 2A)8. We observed mostly linearized polymer chains under vehicle conditions, and condensed polymers that formed highly overlapping networks under high Ca2+/ low pH (Fig. 2A). MUC5B treated with sialidase primarily presented as highly entangled molecules, where the polymers frequently overlapped with themselves and did not take on a fully extended form, indicative of less electrostatic stiffening of the mucin (Fig. 2A). A minimum of 72 polymers per condition were categorized into either linear, entangled, or condensed morphologies based on a previously established scoring method (Fig. 2B)6,8. Sialidase treated MUC5B was enriched with highly entangled molecules (43.0% linear, 44.4% entangled, 12.5% condensed; P < 0.0001) compared to vehicle treated MUC5B, which primarily exhibited linearized polymer chains (93.1% linear, 6.8% entangled, 0.0% condensed). MUC5B exposed to high Ca2+/low pH exhibited substantially more condensed polymers s (34.1% linear, 31.8% entangled, 34.1% condensed; P < 0.0001) (Fig. 2A,B). These data suggest that reduced sialylation contributes to compaction of secreted MUC5B.
Reducing Sialylation of MUC5B Impairs Mucin Linearization. (A,B) Natively purified MUC5B was treated with either vehicle or sialidase at pH 7.4 or 10 mM CaCl2 at a pH of 5. 10 mM EGTA at pH 7.4 was added to vehicle and sialidase groups prior to ON incubation at 4 °C. MUC5B polymers were subsequently visualized by negative stain TEM. (A) representative TEM images of MUC5B polymers treated with either vehicle, sialidase, or calcium CaCl2/pH 5. Imaging was performed to capture 72–85 polymers per group (vehicle, N = 73; sialidase, N = 72; and 10 mM CaCl2/pH 5, N = 85). After blinding, polymers were counted and categorized into 3 groups for each condition: linear, entangled, or condensed. White arrows indicate a linearized polymer in the vehicle group, an entangled polymer in the sialidase group, and a condensed polymer in the CaCl2/pH 5 group. (B) Quantification showing the percentage total polymers that were linear, entangled, or condensed for each group. N = 72–85 per group. **P < 0.01, ****P < 0.0001 by Chi Square. Scale bars, 200 nm. (C,D) Untreated MUC5B was separated on 10–35% Sucrose gradient by rate zonal centrifugation to separate MUC5B by shape; more compact mucins sediment faster. Gradient fractions were slot blotted and probed for MUC5B and sialic acid content (SIAFIND™ Pan Lectenz). (C) Intensities were quantified as a percentage of whole for both MUC5B and sialic acid. (D) Representative slot blots of MUC5B and sialic acid, showing a higher percentage of sialic acid content compared to MUC5B in the early (more linear) fractions. (E) Fractions were pooled to represent 4 different sedimentation rates across the gradient and imaged via TEM. Scale bars, 200 nm.
To further define the relationship between mucin conformation and sialylation, we performed rate zonal centrifugation on purified salivary MUC5B using a linear 10–35% sucrose gradient to separate mucin by size and shape. Mucins that sediment faster during rate zonal centrifugation have been described as more compact and pathologic, while mucins that sediment slower exhibit an expanded (linear) conformation6,9. We therefore hypothesized that slower sedimenting MUC5B would have a higher degree of sialylation, while faster sedimentation would be associated with reduced sialylation/charge. After rate zonal centrifugation, we collected fractions from the top of the gradient and subjected them to slot blotting for MUC5B and sialic acid. While MUC5B sedimented over fractions 3 to 14, the majority of sialylated MUC5B glycoforms were observed in the less dense (slower) sedimenting fractions (3 through 5) (Fig. 2C,D). TEM images of pooled fractions from different sedimentation rates across the gradient show that the slowest sedimenting, highest sialylated MUC5B (Fractions 3–5) represent primarily linearized polymers, while faster sedimenting, less sialylated MUC5B (Fractions 11–14) represent more condensed and less expanded polymers (Fig. 2E). To directly evaluate the relationship between MUC5B sedimentation and charge form, we combined MUC5B containing fractions into two new pools: 1) Fractions 3–6 representing the slower sedimenting (more linear) half of MUC5B, and 2) fractions 7–14 representing the faster sedimenting (more compact) half of MUC5B. We separated these two pools out by Ag-PAGE and blotted them for sialic acid and MUC5B. Consistent with our slot blotting of individual fractions (Fig. 2C,D), fractions 3–6 contained the majority of the sialic acid, which colocalized with the high charged MUC5B forms (SI Fig. 2). The high charged MUC5B forms were almost completely absent in fractions 7–14. Overall, these data indicate that increased charge conferred by sialylation plays a major role in mucin linearization.
Sialyltransferase inhibition impairs mucociliary transport in primary HBECs
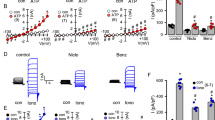
To determine the consequences of reduced mucin sialylation on mucus transport, we treated non-CF HBECs with the sialyltransferase inhibitor 3Fax-Peracetyl Neu5Ac (STI) or dimethyl sulfoxide (DMSO) vehicle for 24 h and subsequently imaged them via micro Optical Coherence Tomography (µOCT)13,30,31. To ensure we studied only mucus biosynthesized under sialyltransferase inhibited conditions, we stimulated release of pre-existing mucin granules by purinergic stimulation with uridine triphosphate (UTP) prior to sialyltransferase treatment32. HBECs treated with STI had significantly slower MCT rates (0.29 ± 0.05 mm/min; P < 0.001; Fig. 3B,E, S1 and S2 Videos) compared to vehicle treated cells (1.0 ± 0.16 mm/min). In contrast to effects on MCT rate, treatment with STI had no effect on the hydration state of the mucus layer as indicated by airway surface liquid layer (ASL) and periciliary liquid layer (PCL) depths (Fig. 3A–D) or ciliary beating frequency (CBF) (SI Fig. 3A). Together, these data provide preliminary evidence that adequate sialylation of secreted mucin is vital for normal mucus transport, and this phenomenon occurs independently from the hydration state of the mucus.
Sialyltransferase inhibition impairs mucociliary transport in primary HBECs. (A) Representative μOCT images of HBECs treated with either vehicle or 200 μM STI demonstrate no differences in ASL (yellow bar) or PCL (red bar). (B) Reprocessed M-mode (e.g., x vs time) µOCT images show tracks of mucus particles above the epithelial surface of HBECs treated with vehicle or 200 μM STI; the more horizontal direction of particle streaks (yellow arrow) indicates more rapid transport. Summary data shows the effect of STI on (C) ASL and (D) PCL depths and (E) MCT. Regions of interest were measured and averaged for each HBEC filter. N = 18–19/condition, representing 3 donors. Measurements were normalized to vehicle for each donor. ns P > 0.05, ***P < 0.001 by unpaired T-test or Mann–Whitney. Scale bars, 20 μm.
Sialyltransferase inhibition impairs mucociliary transport in rat tracheae
To better understand the consequences of inhibiting sialylation of mucins in vivo and in the context of mucin-rich gland secretions, we administered STI (500 μM) to wild-type (WT) rat trachea by intratracheal instillation daily for 7 days33,34. The day after the last treatment, tracheae were excised and imaged via µOCT to evaluate the airway microanatomy. Consistent with the effects of STI on HBECs, tracheae treated with STI had significantly slower MCT (0.16 mm/min ± 0.05; P < 0.01; Fig. 4B,E, S3 and S4 Videos) compared to vehicle treated tracheae (0.49 ± 0.12 mm/min). Additionally, there was no difference in ASL or PCL depth (Fig. 4A,C,D), indicating that STI had no effect on airway hydration similar to our findings in HBE cells. CBF was also unaffected (SI Fig. 3B). These data support that reduced sialylation impairs mucus transport independent from hydration in an in vivo mucus model and in the presence of mucus glands.
Sialyltransferase inhibition impairs mucociliary transport in rat tracheae. (A) Representative μOCT images of excised WT rat tracheae from rats, treated with either PBS vehicle or 500 μM STI by intratracheal instillation daily for 7 days, demonstrate no differences in ASL (yellow bar) or PCL (red bar). (B) Reprocessed M-mode (e.g., x vs time) µOCT images show tracks of mucus particles above the epithelial surface of rat tracheae treated with vehicle or 500 μM STI; the more horizontal direction of particle streaks (yellow arrow) indicates more rapid transport. Summary data shows the effect of STI on (C) ASL and (D) PCL depths and (E) MCT. Regions of interest were measured and averaged for each trachea. N = 9/condition. ns P > 0.05, **P < 0.01 by unpaired T-test or Mann–Whitney. Scale bars, 20 μm.
α-2,3 sialyltransferase inhibition alone is sufficient to impair mucociliary transport
Since mucins contain mostly α-2,3 linked sialylated O-glycans35,36,37 and evidence suggests that α-2,3 linked sialylation is selectively expressed in mucus producing goblet cells38 we hypothesized that inhibition of α-2,3 sialylation alone would be sufficient to impair mucus transport. We treated non-CF HBECs with 120 μM glycolithocholic acid (GA), an α-2,3 specific sialylation inhibitor, or vehicle for 24 h and assessed for mucus physiology via µOCT13,39,40. Similar to our findings with STI, cells treated with GA had significantly impaired MCT (0.35 ± 0.09 mm/min; P < 0.01; Fig. 5B,E, S5 and S6 Videos) compared to vehicle treated cells (1.0 ± 0.18 mm/min), and GA had no effect on ASL or PCL (Fig. 5A,C,D) or CBF (SI Fig. 3C). Consistent with this, rat tracheae instilled with 300 µM GA in WT daily for 7 days exhibited a significant impairment in MCT (0.18 ± 0.06 mm/min; P < 0.05; Fig. 6B,E, S7 and S8 Videos) compared to those treated with vehicle (0.34 ± 0.07 mm/min). Furthermore, tracheae treated with GA showed no differences in ASL, PCL, or CBF (Fig. 6A,C,D; SI Fig. 3D). Altogether, these findings provide increasing evidence that normal sialylation, and specifically α-2,3 sialylation alone, may be vital for healthy mucus transport in vitro and in vivo, and that this mechanism is occurring independent from airway hydration.
α-2,3 sialyltransferase inhibition alone is sufficient to impair mucociliary transport in HBECs. (A) Representative μOCT images of HBECs treated with either vehicle or 120 μM GA demonstrate no differences in ASL (yellow bar) or PCL (red bar). (B) Reprocessed M-mode (e.g., x vs time) µOCT images show tracks of mucus particles above the epithelial surface of HBECs treated with vehicle or 120 μM GA; the more horizontal direction of particle streaks (yellow arrow) indicates more rapid transport. Summary data shows the effect of GA on (C) ASL and (D) PCL depths and (E) MCT. Regions of interest were measured and averaged for each HBEC filter. N = 17/condition, representing 3 donors. Measurements were normalized to vehicle for each donor. ns P > 0.05, **P < 0.01 by unpaired T-test or Mann–Whitney. Scale bars, 20 μm.
α-2,3 sialyltransferase inhibition alone is sufficient to impair mucociliary transport in trachea. (A) Representative μOCT images of excised WT rat tracheae from rats, treated with either PBS vehicle or 300 μM GA by intratracheal instillation daily for 7 days, demonstrate no differences in ASL (yellow bar) or PCL (red bar). (B) Reprocessed M-mode (e.g., x vs time) µOCT images show tracks of mucus particles above the epithelial surface of rat tracheae treated with vehicle or 300 μM GA; the more horizontal direction of particle streaks (yellow arrow) indicates more rapid transport. Summary data shows the effect of GA on (C) ASL and (D) PCL depths and (E) MCT. Regions of interest were measured and averaged for each trachea. N = 15–17/condition. ns P > 0.05, *P < 0.05 by unpaired T-test or Mann–Whitney. Scale bars, 20 μm.
ST3Gal1 expression is decreased in CF and increased with CFTR modulation in HBECs
Changes in terminal mucin sialylation have been documented in CF, but the clinical implications of this remain poorly understood23,24,41. To determine a potential impact of mucin sialylation in CF airway disease, we next evaluated sialyltransferase expression of the predominant ST3Gal and ST6GalNAC isoforms, ST3Gal1 and ST6GalNAC1, expressed in mucus secreting epithelial cells42,43. We treated CF HBECs with the triple modulator combination, elexacaftor/tezacaftor/ivacaftor (ETI), for 72 h to restore CFTR function44,45. Non-CF cells and paired CF cells were each treated with vehicle control. Prior to sialyltransferase evaluation, we measured mucus physiology via μOCT. As expected, CF HBECs had significantly depleted ASL and PCL depths (ASL 12.4 ± 1.0 µm; P < 0.01; PCL 6.2 ± 0.2 µm; P < 0.001; Fig. 7A,C,D) compared to non-CF HBECs (ASL 56.2 ± 12.0 µm; PCL 7.4 ± 0.1 µm). CF HBECS also showed impaired MCT (0.02 ± 0.003 mm/min; P < 0.05; Fig. 7B,E, S9 and S10 Videos) compared to non-CF HBECs (0.80 ± 0.3 mm/min). Furthermore, ETI treatment of CF HBECs significantly restored ASL and PCL depths (ASL 46.9 ± 8.1 µm; P < 0.01; PCL 7.4 ± 0.14 µm; P < 0.0001; Fig. 7A,F,G), and improved MCT (0.75 ± 0.25 mm/min; P < 0.05; Fig. 7B,H, S11 Video). These data showed expected phenotypic differences in mucus transport in the presence and absence of CFTR function, allowing us to interrogate how this relates to the forms of MUC5B present.
MCT and ST3Gal1 expression are decreased in CF and increased with CFTR modulation in HBECs. (A) Representative μOCT images of non-CF HBECs treated with vehicle and CF HBECs treated with either vehicle or ETI triple modulators for 72 h demonstrate decreased ASL (yellow bar) and PCL (red bar) in CF HBECs that were increased with ETI treatment. (B) Reprocessed M-mode (e.g., x vs time) µOCT images show tracks of mucus particles above the epithelial surface of non-CF HBECs treated with vehicle and CF HBECs treated with vehicle or ETI modulators; the more horizontal direction of particle streaks (yellow arrow) indicates more rapid transport. Summary data shows the differences in (C) ASL and (D) PCL depths and (E) MCT between non-CF and CF and (F–H) CF after ETI correction. Regions of interest from 4 different filters were measured and averaged for each HBEC donor. (I) Representative western blot of cell lysates probed for ST3Gal1 from non-CF HBECs treated with vehicle and CF HBECs treated with either vehicle or ETI triple modulators for 72 h, demonstrate decreased expression of ST3Gal1 in CF HBECs that was partially restored after ETI treatment. (J) Representative western blot of cell lysates probed for ST6GalNAC1 from non-CF HBECs treated with vehicle and CF HBECs treated with either vehicle or ETI triple modulators for 72 h, demonstrate no differences in expression of ST6GalNAC1. Quantification by densitometry of ST3Gal1 expression showing (K) a significant decrease in ST3Gal1 expression in CF HBECs compared to non-CF and (L) an increase in paired ETI treated CF HBECs. Quantification by densitometry of ST6GalNAC1 expression showing no differences in ST6GalNAC1 expression between (M) non-CF and CF or (N) paired ETI treated CF HBECs. (O) Slot blot and accompanying densitometry of secreted, isolated MUC5B probed for sialic acid shows decreased MUC5B sialylation in CF and an increase in sialylation after ETI treatment. N = 5–6 donors/ condition. ns P > 0.05, *P < 0.05, **P < 0.01 by unpaired or paired T-test as appropriate. Scale bars, 20 μm. Western blot densitometry is presented normalized to βactin. Slot blots were normalized to MUC5B prior to loading and represent pooled mucin from 3 HBEC filters per 5 separate donors for each group. Blots were cropped to improve clarity and conciseness; the original, uncropped blots are presented in SI Fig. 4D-E.
Following μOCT evaluation, HBECs were collected and cell lysates immunoblotted for ST3Gal1 and ST6GalNAC1, the two primary siaylatransferases responsible for O-linked α-2,3 and α-2,6 sialylation, respectively12,46. ST3Gal1 expression was significantly lower in CF HBECs (0.45 ± 0.1 ST3Gal1/βactin; P < 0.05; Fig. 7I,K) than that of non-CF HBECs (0.76 ± 0.1 ST3Gal1/βactin). CF HBECs treated with ETI showed significantly increased expression of ST3Gal1 (0.56 ± 0.13 ST3Gal1/βactin; P < 0.05;Fig. 7I,L) when compared to their paired, vehicle treated CF HBECs. There were no notable differences in ST6GalNAC1 expression when comparing non-CF, CF, or CF HBECs post ETI treatment (Fig. 7J,M,N). To evaluate the sialylation state of secreted mucin, mucus secretions were pooled for each condition following treatment, and MUC5B was isolated from these pools via two step CsCl gradient centrifugation as described previously47. Equal amounts of MUC5B were slot blotted and evaluated for sialic acid. MUC5B sialylation was decreased in CF (0.71 relative intensity/ non-CF; Fig. 7O) compared to non-CF (1.0 relative intensity/non-CF). The sialylation of MUC5B was increased in CF HBECs after ETI treatment (1.02 relative intensity/non-CF) compared to vehicle treated CF HBECs. In summary, these data provide evidence that sialylation is dysregulated in CF muco-obstructive disease, and that this finding is linked to CFTR function.
Discussion
In muco-obstructive diseases, such as CF, mucus stasis has largely been attributed to airway dehydration and mucus hyper-concentration3,48, but recent evidence shows that electrostatic abnormalities of gel-forming mucins, MUC5B and MUC5AC, also contribute to aberrant mucus physiology8,9,49. Although sialic acid highly contributes to mucin charge and electrostatics, the exact role of sialylation on the physiological and biophysical properties of mucin remains vastly understudied. Here, for the first time, we demonstrate the consequences of reduced mucin sialylation on the biophysical properties of MUC5B and the functional consequences of sialylation inhibition on mucus transport in vitro and in vivo. Furthermore, we identify decreased expression of ST3Gal1 in CF HBECs. Overall, our findings indicate that aberrant MUC5B sialyation occurs in CF lung disease, resulting in compact mucin forms that contribute to abnormally delayed MCT.
The glycosylation profiles of mucins can be heterogeneous, resulting in multiple glycoforms. Previous reports evaluating the charge states of MUC5B and MUC5AC demonstrated that MUC5AC exists as a single major charge form, while MUC5B exists in two major charge forms, denoted as “high” and “low” charge forms19. The high charged form predominates in healthy airway secretions, while the low charged form is more abundant in several muco-obstructive diseases including CF, COPD, and asthma. Furthermore, studies have demonstrated increased levels of the low charged MUC5B form within viscous mucus plugs20,21,22,27. This suggests that decreased mucin charge may bear pathological significance in muco-obstructive diseases through increased mucus compaction and transport impairment. Here, using the same technique that initially identified the two charge variants of MUC5B, we show that the highest charged species of MUC5B from HBEC and salivary secretions also has the strongest sialic acid detection by WGA lectin and SIAFIND™ Pan Lectenz blotting, respectively (Fig. 1A, SI Fig. 1A-B)). Furthermore, we show that sialylation reduction decreased the charge state of MUC5B and produced a low charged MUC5B similar to that observed in pathologic mucus (Fig. 1B, SI Fig. 1B). Although other modifications, such as sulfation, are likely to also play a role in determining the charge state of MUC5B, reducing sialylation alone was sufficient to obtain the lower MUC5B charge form that is consistent with previously published reports in other muco-obstructive diseases20,21,27. MUC5B was utilized as the model mucin for our biophysical studies because of its lower charged form and well described conformation abnormalities implicated in muco-obstructive diseases. MUC5AC may exhibit similar biophysical consequences to reduced sialylation. Future studies on MUC5AC are required to fully describe the consequences of reduced sialylation on this and other secreted airway mucins.
High negative charge density on mucins has been shown to promote stiffening of the mucin backbone through repulsion of neighboring charges, which is an important feature in the maturation of mucin polymers and the formation of the mucus gel10,50,51. During packaging and prior to secretion, these anionic charges are stabilized by divalent Ca2+, allowing the mucin to condense for packaging and transport6,18,52. Upon secretion into the airway, Ca2+ is chelated by bicarbonate, freeing these charges to repel and extend the mucin backbone7,16,53. Therefore, loss of these charges would be expected to weaken these repulsive forces and hinder mucin expansion after secretion. In support of this, we show a significantly increased occurrence of entangled polymers after sialidase treatment of salivary MUC5B (Fig. 2A,B), suggesting that decreased MUC5B sialylation increases mucin compaction. Interestingly, the conformation of sialidase treated MUC5B resembles the morphology of the previously reported low charged MUC5B from a mucus plug, which was also composed of mostly entangled and non-linear polymers (similar to our observations)22. The low charge or reduced sialylated form of MUC5B may contribute to more compaction of the mucin and impaired expansion. Evidence for this was also demonstrated through rate zonal centrifugation, where the degree of sialylation strongly correlated with the sedimentation of MUC5B (Fig. 2). Here, we show that the slower sedimenting MUC5B forms that are mostly linear and mature contain higher amounts of sialic acid and a more charged form of MUC5B (SI Fig. 2). Several studies are congruent with this finding and show slower sedimenting MUC5B during rate zonal centrifugation exhibits a more expanded conformation that is more fully extended and mature6,8. Interestingly, previous work shows that MUC5B secreted from primary CF HBECs has an increased sedimentation rate during rate zonal centrifugation compared to non-CF MUC5B9. Since we observed a positive correlation between reduced sialylation and increased sedimentation rate (Fig. 2) and aberrant sialylation in CF HBECs (Fig. 7), we believe that reduced sialylation may contribute to the faster sedimentation of CF MUC5B as described previously. Future studies are required to more precisely determine the relationship between sialylation defects in CF and CF mucin compaction and charge. Overall, these data provide evidence that higher levels of mucin sialylation facilitate linearization of secreted mucin, a feature imperative for clearance of mucus.
We utilized sialyltransferase inhibitors to reduce the sialylation of secreted mucins de novo, which allowed us to evaluate the functional consequences of reduced mucin sialylation on MCT, where both MUC5B and MUC5AC are present and contribute to mucus clearance13,30,40,54. We show that decreased sialylation of secreted mucin by STI significantly impaired MCT and had no effects on ASL, PCL, or CBF in both non-CF HBECs and WT rat tracheae (Figs. 3, 4). In particular, inhibition of 2,3 sialyltransferase alone was sufficient to recapitulate this phenotype of impaired MCT without affecting hydration (Figs. 5, 6). In addition to secreted mucins, ciliated epithelia are lined with membrane bound (tethered) mucins that are important for PCL hydration and ciliary beating55,56. It is possible that reduced sialylation of tethered mucins may have contributed to the impaired MCT observed; however, it is more likely that the impaired MCT was due to reduced sialylation of secreted mucins, since we observed no changes in ASL or PCL depth or CBF. Additionally, previous work shows that ciliated epithelium primarily express α-2,6 linked sialylation while mucin secreting goblet cells selectively express α-2,3 linked38. Our findings are likely consequence to reduced sialyation of secreted mucin, since we observed a decrease in MCT after α-2,3 specific inhibition. These findings not only underscore the importance of normal sialylation for mucociliary clearance but also show that the relationship between mucin sialylation state and MCT is most likely attributed to the abundance of 2,3 sialylation on O-linked glycans37,38,39.
CFTR has been suggested to regulate terminal glycosylation of mucins23,24,41, but whether this is due to CFTR dependent anion transport is unknown. Some studies document changes in mucin sialylation as a result of infection in CF57,58, while others have reported altered mucin sialylation in CFTR−/− newborn piglets before the onset of inflammation or infection59. Interestingly, there is also evidence that sialylation may be altered as a direct consequence of the defective CFTR due to its role in organelle acidification or protein turnover both of which could affect glycosyltransferases including the sialyltransferases25,60,61. Here, we measured sialyltransferase protein expression in non-CF, CF, and CF-ETI corrected HBECs. We found that ST3Gal1 protein was significantly lower in CF HBECs compared to non-CF (Fig. 7). Additionally, ST3Gal1 expression was significantly increased after 72 h ETI treatment suggesting that CFTR correction may augment ST3GAL1 expression. Future studies are required to determine the relationship between CFTR and ST3Gal1 expression. Notably, examination of the effects of altering ST3Gal1 expression among cell types in the epithelium may be useful in future studies, given the prevalence of goblet cell hyperplasia characterized in CF and that sialyltransferase expression may be differentially regulated between goblet and ciliated cells38,42,43,62,63. Nevertheless, our findings suggest that dysregulated sialylation of mucin may be a contributing factor and therapeutic target in CF muco-obstructive disease.
In summary, we demonstrate the consequences of reduced mucin sialylation on mucin charge state, mucin conformation, and mucus transport. Our data supports a novel model in which sialylation promotes normal MUC5B linearization and MCC by increasing mucin charge state (Fig. 8A). Conversely, when sialylation/charge is reduced as observed in CF HBECs, MUC5B becomes entangled and MCC is impaired (Fig. 8B). In addition, we posit that reduced expression of ST3GAL1 in CF, which can be corrected by ETI, may contribute to reduced mucin charge, expansion, and mucus clearance. These findings provide impetus for evaluating mucin sialylation and cognate transferases as therapeutic targets to combat mucus stasis in a plethora of muco-obstructive diseases.
The consequences of reduced sialylation on mucus physiology. (A) Under normal conditions, gel-forming mucins are heavily sialylated on the terminus of their glycan chains. This sialylation produces a high negative charge density across the mucin backbone. These negative charges promote mucin expansion and linearization through charge repulsion of neighboring sialic acids within and across mucin polymers. This promotes the formation of a normal mucin network and facilitates mucus transport. (B) When the level of sialylation is reduced, the net charge across the mucin backbone is also reduced. The charge deficit ameliorates the repulsive forces within and between mucin polymers and results in less linear, more entangled mucins. This results in an aberrant mucin network and ultimately impairs mucus transport.
Methods
Sex as a biological variable
Our study examined male and female animals, and similar findings are reported for both sexes.
Primary HBE cell culturing
PrimaryHBECs harvested from lung explants of previously healthy (Non-CF) or F508del-CFTR homozygous (CF) donors. First or second-passage cells, were seeded onto 6.5-mm-diameter permeable supports (Corning Inc., Corning, NY) coated with NIH 3T3 fibroblast conditioned media at a density of 0.5 × 106 cells per filter. Cells were grown in in PneumaCult™-ALI Medium (STEMCELL Technologies, Canada) to induce terminal differentiation at air liquid interface for at least 4 weeks64,65. To ensure integrity of ALI monolayers, transepithelial resistance is routinely measured for a resistance of ≥ 200 Ohm*cm2 before experimentation. Prior to all studies, primary HBECs were treated apically with 100uM UTP in phosphate buffered saline (PBS) for 40 min to induce granular mucin secretion to remove pre-existing intracellular mucin produced before experimental conditions32.
Rat model
All animal experiments at UAB were conducted in accordance with the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC) approved protocols. All animal experiments used WT Sprague–Dawley rats. Animals were bred and housed in standard cages with a 12-h light/dark cycle with ad libitum access to food and water and were routinely monitored. Rats of the same sex were co-housed from time of weaning to study conclusion. Weaned rats were maintained on a standard rodent diet. Animals were euthanized by intraperitoneal injection of 500μL pentobarbital sodium (390 mg/mL) followed by exsanguination of the hepatic portal vein. Animals used in this study were ≥ 6 months to allow maturation of submucosal glands33. Male and Female rats were used and all experimental groups were matched by age and sex.
Sialyltransferase inhibition
Terminal differentiated HBECs at ALI were treated with 200 μM STI to block sialylation, or 120 μM GA to specifically inhibit α-2,3 sialylation, in the basolateral compartment for 24 h and then imaged via μOCT13. Control cells were treated with DMSO vehicle. To inhibit sialylation in rat tracheae, 100μL of 500 μM STI or 300 μM GA diluted in PBS, was intratracheally instilled in WT rats daily for seven days following established methods66. Control groups were treated with DMSO vehicle diluted in PBS.
μOCT imaging and analysis
Measurements of the functional microanatomy of primary HBECs or freshly excised rat tracheae were performed using μOCT, a high-speed, high-resolution microscopic imaging modality as previously described67. The μOCT instrument provides cross-sectional images of the epithelium with a sub-cellular resolution sufficient to directly visualize and quantify micro-anatomic parameters including ASL depth, PCL depth, MCT rates, and CBF. Images were acquired at a rate of 40 frames per second and at 512 A-lines per frame. ASL and PCL depths were quantified by direct geometric measurement of the respective layers with a correction factor based the estimated refractive index of n = 1.33, using ImageJ (NIH) software65. MCT rate was determined using time elapsed and distance traveled of native particles in the mucus layer over multiple frames. CBF and was investigated by Fourier analysis of the time-varying reflectance due to beating cilia using MATLAB. For consistency, HBECs were measured at four standardized locations for each culture. Tracheae were placed in the same proximal to distal orientation and the imaging beam was placed at six standardized locations along the ventral surface of the trachea as previously described33,65.
Native MUC5B isolation
Whole human saliva from a healthy donor was collected by chewing on Parafilm to stimulate secretion and collected into a 50-ml falcon tube. Saliva was centrifuged at 3000 × g for 25 min at 4 °C to remove cells and debris. Clarified saliva was solubilized overnight in 0.1 M NaCl 20 mM/Tris pH 7.4 at 4 °C with rotation. Following solubilization, CsCl was added to a starting density of 1.45 g/mL, and saliva was fractionated by isopycnic density centrifugation in a Beckman SW41 Ti swinging bucket rotor at 118 k x g for 72 h at 15C8,29. Following centrifugation, MUC5B containing fractions were pooled and buffer exchanged into 10 mM NaCl/10 mM Tris, pH 7.4.
MUC5B treatments
To evaluate the role of sialylation on charge state of MUC5B, secreted mucus from non-CF HBECs was collected and solubilized in 4 M guanidinium hydrochloride (GuHCl). Following solubilization, mucins were partially purified and subjected to a neuraminidase buffer exchange containing 50 mM sodium acetate, 4 mM CaCl2 pH 6 using a 100 kDa Amicon Ultra centrifugation column (Millipore Sigma, Burlington, MA). Partially purified mucin was split into equal (5 µg) aliquots and treated with increasing amounts of neuraminidase from Vibrio Cholerae (Roche, Bavaria, Germany), ranging from 0 mU/mL to 25 mU/mL, for 2 h at 37 °C. Neuraminidase vehicle was added to equalize volumes. After incubation, a denaturing buffer containing 6 M urea and 25 mM dithioreitol (DTT) was added to inactivate sialidase before electrophoresis.Purified salivary MUC5B was exchanged into neuraminidase buffer and treated with neuraminidase concentrations ranging from 0mu/mL to 100 mU/mL for 2 h at 37 °C. Vehicle was added to equalize volumes.
To evaluate the consequences of reduced sialylation on MUC5B conformation via TEM, 50 µg/mL of MUC5B purified by CsCl gradient centrifugation was treated with either neuraminidase vehicle or 12.5 mU/mL neuraminidase for 2 h at 37 °C. CaCl2 was added to a final of 1 mM to enable neuraminidase activity. After treatment, 10 mM ethyleneglycol- bis(β-aminoethyl)-N,N,Nʹ,Nʹ-tetraacetic Acid (EGTA), pH7.4 was added to vehicle and neuraminidase treated mucin to remove CaCl2. As a positive control, 10 mM CaCl2 was added to vehicle treated mucin and adjusted to pH 5. Samples were incubated overnight at 4 °C prior to negative staining.
Agarose-polyacrylamide gel electrophoresis
Following partial purification and neuraminidase treatment, secreted mucus from non-CF HBECs was buffer exchanged into gel-loading buffer containing 6 M Urea, 25 mM DTT, and 0.1% SDS and heated at 95 °C for 10 min. Mucins were separated by their inherent charge on an agarose-polyacrylamide-urea gel as previously described68. Briefly, gels were prepared with 1% agarose, 1.5% polyacrylamide, and 4 M urea. Sample (5 µg) was loaded and electrophoresed at 80 V for 2 h, followed by transfer at 40 V for 6 h onto a 0.45 μm nitrocellulose membrane. Separate, identical blots were incubated with either MUC5B primary antibody (4A10-H2, Novus Biologicals, Centennial, CO) at 1:500 dilution followed by mouse anti-IgG horse radish peroxidase (HRP) conjugated secondary (31,340, Invitrogen, Waltham, MA) at 1:3000 dilution or 1 µg/mL biotinylated WGA (B-1025–5, Vector Labs, Newark, CA) followed by Vectastain ABC-HRP reagent at 1:2000 dilution. HRP activity was detected using enhanced chemiluminescence detection solution (Bio-Rad, Hercules, CA) and imaged on a Bio-Rad Gel Doc XR Gel Documentation System.
Following isolation via CsCl gradient centrifugation, salivary MUC5B was reduced in 10 mM DTT for 5 h at 37 °C in 6 M urea and alkylated with 25 mM iodoacetamide ON. SDS was added to final concentration of 0.1% prior to electrophoresis on a 1% agarose, 1.5% polyacrylamide gel containing 4 M urea. Sample (15 µg) was loaded and electrophoresed at 80 V for 2 h, followed by transfer at 40 V for 6 h onto a 0.45 μm low fluorescence PVDF membrane (IPFL00010, Millipore Sigma, Burlington, MA). MUC5B content was evaluated using anti-human MUC5B primary antibody (4A10-H2, Novus Biologicals, Centennial, CO) at 1:500 dilution followed by IRDye® 680RD Goat anti-Mouse IgG Secondary Antibody (Li-COR, Lincoln, NE) at a 1:10,000 dilution. Sialic acid content was evaluated using SIAFIND™ biotinylated Pan-Specific Lectenz (Lectenz Bio, Athens, GA) at 20 μg/mL followed by IRDye® 800CW Streptavidin (Li-COR) at a 1:5000 dilution. Secondary antibodies were imaged using a LI- COR Odyssey® CLx Infrared Imaging System (Li-COR Biosciences, Lincoln, USA).
Transmission electron microscopy and evaluation
Samples were adjusted to 5 μg/mL and incubated for 30 s on carbon coated CF400-Cu grids (EMS, Hatfield, PA) that had been glow discharged at 30 Volts for 30 s. Grids were washed in ddH2O for 10 s and then negative stained with 2% (w/v) uranyl acetate for 1 min8. TEM data were recorded using a JEOL JEM-1400Flash microscope (JEOL USA, Peabody, MA) at 120 kV in a magnification range between 30,000 to 50,000x. MUC5B polymers from vehicle, sialidase, and 10 mM calcium pH 5 conditions were counted and categorized, based on their appearance, into three groups: condensed, entangled, or linearized8. A total of 30–35 images were collected for each condition, representing 72–85 polymers per condition. Images were blinded prior to polymer scoring.
Rate zonal centrifugation
Rate zonal centrifugation was performed on 11 mL linear 10–35% sucrose gradients in PBS, pH 7.4. Gradients were prepared as 4-step discontinuous gradients and thawed at 4 °C for 20 h prior to centrifugation to form linear gradients69. 500 μl of sample was layered onto the top of the gradient and centrifuged in a SW41 Ti swinging bucket rotor at 210 k x g for 90 min at 15 °C8. Following centrifugation, the gradient was fractioned from the top in 500 µL increments, giving 23 total fractions. The fractions were evaluated for mucin and sialic acid content.
Slot blotting and detection
Equal volumes of samples were diluted in buffer containing 4 M GuHCl, 25 mM DTT, 0.1 M Tris pH 7.4 and heated at 95 °C prior to loading. Samples (200uL) were transferred onto a 0.45 μm nitrocellulose membrane under gentle vacuum using Bio-Rad Bio-Dot SF microfiltration apparatus (Bio-Rad, Life Sciences, USA). MUC5B content was evaluated using anti-human MUC5B primary antibody (4A10-H2, Novus Biologicals, Centennial, CO) at 1:500 dilution followed by IRDye® 680RD Goat anti-Mouse IgG Secondary Antibody (Li-COR, Lincoln, NE) at a 1:10,000 dilution. Sialic acid content was evaluated using SIAFIND™ biotinylated Pan-Specific Lectenz (Lectenz Bio, Athens, GA) at 20 μg/mL followed by IRDye® 800CW Streptavidin (Li-COR) at a 1:5000 dilution. Secondary antibodies were visualized, and densitometry performed on band intensities using a LI- COR Odyssey® CLx Infrared Imaging System (Li-COR Biosciences, Lincoln, USA).
Elexacaftor tezacaftor ivacaftor (ETI) treatment on CF HBECs
Well differentiated CF HBECs, homozygous for F508del-CFTR, at ALI were treated with the three drug CFTR modulator combination of 3 μM Elexacaftor (E, VX-445), 3 μM Tezacaftor (T, VX-661), and 1 μM Ivacaftor (I, VX-770, Selleck Chemicals LLC, Houston, TX), or DMSO vehicle in the basolateral compartment for 72 h. Drugs were refreshed every 24 h. Non-CF HBECs were treated with DMSO vehicle. After 72 h, cells were imaged via μOCT followed by subsequent collection of apical mucus and cells for sialyltransferase protein evaluation.
Following treatments, secreted mucus was collected after simulation with 100 μM UTP for 40 min. Mucus was pooled from N = 3 filters per 5 different non-CF donors and 5 different paired CF or CF ETI treated donors. Mucin was solubilized in 6 M GuHCl and MUC5B was isolated via two-step CsCl gradient centrifugation as previously described47. Equal amounts of MUC5B from non-CF, CF, and CF ETI were slot blotted for sialic acid using SIAFIND™ biotinylated Pan-Specific Lectenz at 20 μg/mL followed by IRDye® 800CW Streptavidin at a 1:5000 dilution. Secondary antibodies were visualized, and densitometry performed on band intensities using a LI- COR Odyssey® CLx Infrared Imaging System.
Sialyltransferase western blotting
Following treatments, HBECs were lysed in RIPA buffer (RPI Corp, Mt. Prospect, Illinois) with Halt protease and phosphatase inhibitor cocktail (Thermo Scientific, Waltham, MA). Three filters per donor/condition were pooled and total protein concentration was determined by Bradford protein assay (Thermo Scientific, Waltham, MA). 15 µg of protein per condition were loaded and separated by SDS-PAGE. Separate, identical blots were incubated with ST3Gal1 (PA5-21721, Invitrogen) or ST6GalNAc1 (PA5-31,200, Invitrogen) primary antibody at 1:1000 dilution followed by rabbit anti-IgG horseradish peroxidase conjugated secondary antibody (31,466, Invitrogen). HRP activity was detected using enhanced chemiluminescence detection solution (Bio-Rad, Hercules, CA) and imaged on a Bio-Rad Gel Doc XR Gel Documentation System for quantification. ImageJ (NIH) software was used to perform densitometry measurements70.
Statistics
Statistical analysis was performed in GraphPad Prism version 9 or greater. Data were tested for normality using Shapiro–Wilk’s test followed by non-parametric or parametric analysis when appropriate. All µOCT data comparing two groups (Figs. 3, 4, 5, 6) and immunoblotting data were subjected to either a two-tailed, unpaired T-test when parametric or Mann–Whitney test comparing mean ranks when non-parametric. µOCT data comparing three groups were analyzed by Kruskal–Wallis test with Dunn’s post hoc to compare groups. The categorical scoring of TEM imaged mucin polymers was analyzed by Chi-Square to test occurrence. A P-value of less than 0.05 was considered statistically significant. Statistics are presented as mean ± SEM.
Study approval
Use of human bronchial epithelial cells was approved by the UAB Institutional Review Board (IRB) 300,001,383. Use of human saliva was approved by the UAB IRB 120,523,006. Written informed consent was received from all participants who provided sputum samples and for acquisition of airway tissues to procure primary human airway cells. All experiments were performed in accordance with relevant guidelines and regulations. Use of WT rats was approved by the UAB Institutional Animal Care and Use Committee (IACUC) IACUC-21806. All animal studies were reported in accordance with ARRIVE guidelines.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Rowe, S. M., Miller, S. & Sorscher, E. J. Cystic fibrosis. N. Engl. J. Med. 352(19), 1992–2001 (2005).
Fahy, J. V. & Dickey, B. F. Airway mucus function and dysfunction. N. Engl. J. Med. 363(23), 2233–2247 (2010).
Boucher, R. C. Muco-obstructive lung diseases. N. Engl. J. Med. 380(20), 1941–1953 (2019).
Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatr Pulmonol. 2019;54 Suppl 3(Suppl 3):S84-S96.
Ridley, C. & Thornton, D. J. Mucins: the frontline defence of the lung. Biochem Soc Trans. 46(5), 1099–1106 (2018).
Kesimer, M., Makhov, A. M., Griffith, J. D., Verdugo, P. & Sheehan, J. K. Unpacking a gel-forming mucin: A view of MUC5B organization after granular release. Am. J. Physiol. Lung. Cell. Mol. Physiol. 298(1), L15-22 (2010).
Quinton, P. M. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet. 372(9636), 415–417 (2008).
Hughes, G. W. et al. The MUC5B mucin polymer is dominated by repeating structural motifs and its topology is regulated by calcium and pH. Sci. Rep. 9(1), 17350 (2019).
Abdullah, L. H. et al. Defective postsecretory maturation of MUC5B mucin in cystic fibrosis airways. JCI Insight 2(6), e89752 (2017).
Gerken, T. A. Biophysical approaches to salivary mucin structure, conformation and dynamics. Crit. Rev. Oral Biol. Med. 4(3–4), 261–270 (1993).
Thornton, D. J., Rousseau, K. & McGuckin, M. A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 (2008).
Karlsson, N. G. & McGuckin, M. A. O-Linked glycome and proteome of high-molecular-mass proteins in human ovarian cancer ascites: Identification of sulfation, disialic acid and O-linked fucose. Glycobiology. 22(7), 918–929 (2012).
Wang, L., Liu, Y., Wu, L. & Sun, X. L. Sialyltransferase inhibition and recent advances. Biochim. Biophys. Acta 1864(1), 143–153 (2016).
Amanda, L., Lewis, X. C., Ronald, L., Schnaar, & Ajit, V. Essentials of Glycobiology [Internet]. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press (2022).
Verdugo, P. Mucin exocytosis. Am. Rev. Respir. Dis. 144(3 Pt 2), S33–S37 (1991).
Quinton, P. M. Role of epithelial HCO3(-) transport in mucin secretion: Lessons from cystic fibrosis. Am. J. Physiol. Cell Physiol. 299(6), C1222–C1233 (2010).
Weston, A. et al. The influence of ions on the lubricative abilities of mucin and the role of sialic acids. Colloids Surf. B Biointerfaces 227, 113327 (2023).
Verdugo, P., Deyrup-Olsen, I., Aitken, M., Villalon, M. & Johnson, D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J. Dent. Res. 66(2), 506–508 (1987).
Thornton, D. J., Howard, M., Khan, N. & Sheehan, J. K. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J. Biol. Chem. 272(14), 9561–9566 (1997).
Kirkham, S., Sheehan, J. K., Knight, D., Richardson, P. S. & Thornton, D. J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 361(Pt 3), 537–546 (2002).
Kirkham, S. et al. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178(10), 1033–1039 (2008).
Sheehan, J. K., Howard, M., Richardson, P. S., Longwill, T. & Thornton, D. J. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem. J. 338(Pt 2), 507–513 (1999).
Rhim, A. D., Stoykova, L. I., Trindade, A. J., Glick, M. C. & Scanlin, T. F. Altered terminal glycosylation and the pathophysiology of CF lung disease. J. Cyst Fibros. 3(Suppl 2), 95–96 (2004).
Kube, D., Adams, L., Perez, A. & Davis, P. B. Terminal sialylation is altered in airway cells with impaired CFTR-mediated chloride transport. Am. J. Physiol. Lung Cell. Mol. Physiol. 280(3), L482–L492 (2001).
Barasch, J. et al. Defective acidification of intracellular organelles in cystic fibrosis. Nature 352(6330), 70–73 (1991).
Dosanjh, A. & Muchmore, E. A. Expression of DeltaF508 cystic fibrosis transmembrane regulator (CFTR) decreases membrane sialylation. Open Respir. Med. J. 3, 79–84 (2009).
Welsh, K. G. et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 152(4), 771–779 (2017).
Thornton, D. J., Howard, M., Devine, P. L. & Sheehan, J. K. Methods for separation and deglycosylation of mucin subunits. Anal. Biochem. 227(1), 162–167 (1995).
Raynal, B. D., Hardingham, T. E., Sheehan, J. K. & Thornton, D. J. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J. Biol. Chem. 278(31), 28703–28710 (2003).
Rillahan, C. D. et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8(7), 661–668 (2012).
Macauley, M. S. et al. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 289(51), 35149–35158 (2014).
Abdullah, L. H., Wolber, C., Kesimer, M., Sheehan, J. K. & Davis, C. W. Studying mucin secretion from human bronchial epithelial cell primary cultures. Methods Mol. Biol. 842, 259–277 (2012).
Birket, S. E. et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight. 3(1), 97199 (2018).
Widdicombe, J. H. et al. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 198(Pt 2), 207–221 (2001).
Lo-Guidice, J. M. et al. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J. Biol. Chem. 269(29), 18794–18813 (1994).
Baos, S. C., Phillips, D. B., Wildling, L., McMaster, T. J. & Berry, M. Distribution of sialic acids on mucins and gels: A defense mechanism. Biophys. J. 102(1), 176–184 (2012).
Brockhausen ISH, Stanley P. Essentials of Glycobiology: 2nd Edition: Cold Spring Harbor Laboratory Press (2009).
Gagneux, P. et al. Human-specific regulation of alpha 2-6-linked sialic acids. J. Biol. Chem. 278(48), 48245–48250 (2003).
Chen, X., Liu, H., Li, A., Ji, S. & Fei, H. Hydrophobicity-tuned anion responsiveness underlies endosomolytic cargo delivery mediated by amphipathic vehicle peptides. J. Biol. Chem. 297(6), 101364 (2021).
Chang, K. H., Lee, L., Chen, J. & Li, W. S. Lithocholic acid analogues, new and potent alpha-2,3-sialyltransferase inhibitors. Chem. Commun. (Camb). 6, 629–631 (2006).
Scanlin, T. F. & Glick, M. C. Terminal glycosylation in cystic fibrosis. Biochim. Biophys. Acta 1455(2–3), 241–253 (1999).
Sjostedt, E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 367(6482), eaay5947 (2020).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7(31), eabh2169 (2021).
Morrison, C. B. et al. Treatment of cystic fibrosis airway cells with CFTR modulators reverses aberrant mucus properties via hydration. Eur. Respir. J. 59(2), 2100185 (2022).
Bacalhau, M. et al. Elexacaftor-tezacaftor-ivacaftor: A life-changing triple combination of CFTR modulator drugs for cystic fibrosis. Pharmaceuticals. 16(3), 410 (2023).
Dall’Olio, F., Malagolini, N., Trinchera, M. & Chiricolo, M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta 1840(9), 2752–2764 (2014).
Thornton, D. J., Sheehan, J. K., Lindgren, H. & Carlstedt, I. Mucus glycoproteins from cystic fibrotic sputum. Macromolecular properties and structural “architecture”. Biochem J. 276(3), 667–675 (1991).
Boucher, R. C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 (2007).
Fernandez-Petty, C. M. et al. A glycopolymer improves vascoelasticity and mucociliary transport of abnormal cystic fibrosis mucus. JCI Insight. 4(8), 125954 (2019).
Shogren, R., Gerken, T. A. & Jentoft, N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: Light-scattering studies of ovine submaxillary mucin. Biochemistry. 28(13), 5525–5536 (1989).
Lutz, T. M., Marczynski, M., Grill, M. J., Wall, W. A. & Lieleg, O. Repulsive backbone–backbone interactions modulate access to specific and unspecific binding sites on surface-bound mucins. Langmuir. 36(43), 12973–12982 (2020).
Thornton, D. J., Sharpe, C. & Ridley, C. Intracellular processing of human secreted polymeric airway mucins. Ann. Am. Thorac. Soc. 15(Suppl 3), S154–S158 (2018).
Chen, E. Y., Yang, N., Quinton, P. M. & Chin, W. C. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 299(4), L542–L549 (2010).
Bos, M. F., Ermund, A., Hansson, G. C. & de Graaf, J. Goblet cell interactions reorient bundled mucus strands for efficient airway clearance. PNAS Nexus. 2(11), 388 (2023).
Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457 (2008).
Button, B. et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 337(6097), 937–941 (2012).
Schulz, B. L. et al. Glycosylation of sputum mucins is altered in cystic fibrosis patients. Glycobiology. 17(7), 698–712 (2007).
Davril, M. et al. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology. 9(3), 311–321 (1999).
Caballero, I. et al. Evidence of early increased sialylation of airway mucins and defective mucociliary clearance in CFTR-deficient piglets. J. Cyst Fibros. 20(1), 173–182 (2021).
Wei, X. et al. Turnover of the cystic fibrosis transmembrane conductance regulator (CFTR): slow degradation of wild-type and delta F508 CFTR in surface membrane preparations of immortalized airway epithelial cells. J. Cell. Physiol. 168(2), 373–384 (1996).
Liu, J. et al. Cystic fibrosis transmembrane conductance regulator contributes to reacidification of alkalinized lysosomes in RPE cells. Am. J. Physiol. Cell. Physiol. 303(2), C160–C169 (2012).
Burgel, P. R., Montani, D., Danel, C., Dusser, D. J. & Nadel, J. A. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 62(2), 153–161 (2007).
Henderson, A. G. et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Investig. 124(7), 3047–3060 (2014).
Birket, S. E. et al. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am. J. Physiol. Lung Cell. Mol. Physiol. 310(10), L928–L939 (2016).
Birket, S. E. et al. A functional anatomic defect of the cystic fibrosis airway. Am. J. Respir. Crit. Care Med. 190(4), 421–432 (2014).
Ortiz-Munoz, G. & Looney, M. R. Non-invasive Intratracheal Instillation in Mice. Bio Protoc. 5(12) (2015).
Liu, L. et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS ONE 8(1), e54473 (2013).
Issa, S. M., Schulz, B. L., Packer, N. H. & Karlsson, N. G. Analysis of mucosal mucins separated by SDS-urea agarose polyacrylamide composite gel electrophoresis. Electrophoresis. 32(24), 3554–3563 (2011).
Luthe, D. S. A simple technique for the preparation and storage of sucrose gradients. Anal. Biochem. 135(1), 230–232 (1983).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012).
Acknowledgements
We would like to thank Dr. Susan E Birket, PharmD, PhD and the CFTR Rat Models Core for providing all rats used in these studies. We would also like to thank Melissa Chimento and Ed Phillips of the UAB High Resolution Imaging Facility and James Kizziah, PhD of the UAB Cryo-EM Facility for their assistance and guidance with TEM preparation and imaging.
Funding
This work was supported by the NIH (www.nih.gov) F31HL164005-02 to ESH, 1R35HL135816-01 and P30DK072482 to SMR, R01HL152246 to JWB, and CF Foundation Grant (www.cff.org) ROWE19RO to SMR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.S.H., H.J.M., S.K., S.M.R. and J.W.B. conceived the experiments; E.S.H. and H.J.M. conducted the research; E.S.H., H.J.M., S.M.R., and J.W.B. analyzed the data; E.S.H., S.M.R., and J.W.B. wrote the manuscript; E.S.H., H.J.M., M.M., H.S.H., H.M.L., G.J.T., S.K., S.M.R., and J.W.B. edited the manuscript. M.M., H.S.H., H.M.L., and G.J.T. provided resources. S.M.R. and JWB supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video S1.
Supplementary Video S2.
Supplementary Video S3.
Supplementary Video S4.
Supplementary Video S5.
Supplementary Video S6.
Supplementary Video S7.
Supplementary Video S8.
Supplementary Video S9.
Supplementary Video S10.
Supplementary Video S11.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Harris, E.S., McIntire, H.J., Mazur, M. et al. Reduced sialylation of airway mucin impairs mucus transport by altering the biophysical properties of mucin. Sci Rep 14, 16568 (2024). https://doi.org/10.1038/s41598-024-66510-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66510-2
- Springer Nature Limited