Abstract
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. It involves disturbances in carbohydrate, fat, and protein metabolism due to defects in insulin secretion, insulin action, or both. Novel therapeutic approaches are continuously being explored to enhance metabolic control and prevent complications associated with the disease. This study investigates the therapeutic potential of kaempherol-3-rhamnoside, a flavonoid, in managing diabetes by modulating the AMP-activated protein kinase (AMPK) pathway and improving metabolic enzyme activities in streptozotocin (STZ) -induced diabetic mice. Diabetic mice were treated with varying doses of kaempherol-3-rhamnoside and/or insulin over a 28-day period. Glycolytic and gluconeogenesis enzyme activities in the liver, fasting blood glucose levels, serum insulin levels, lipid profiles and oxidative stress markers were assessed. Treatment with kaempherol-3-rhamnoside significantly improved glycolytic enzyme activities, reduced fasting blood glucose, and enhanced insulin levels compared to diabetic controls. The compound also normalized lipid profiles and reduced oxidative stress in the liver, suggesting its potential in reversing diabetic dyslipidemia and oxidative damage. Furthermore, kaempherol-3-rhamnoside activated the AMPK pathway, indicating a mechanism through which it could exert its effects. Kaempherol-3-rhamnoside exhibits promising antidiabetic properties, potentially through AMPK pathway activation and metabolic enzyme modulation. These findings support its potential use as an adjunct therapy for diabetes management. Further clinical studies are warranted to validate these results in human subjects.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) refers to a cluster of metabolic disorders that cause elevated levels of glucose, lipids, amino acids, and lactate circulating in the body. The root causes of DM include inadequate insulin secretion from the pancreas or diminished sensitivity to insulin or both simultaneously. It affects both insulin secretion and action resulting in hyperinsulinemia1,2. According to the World Health Organisation (WHO), it is predicted that by 2030, there will be more than twice as many people worldwide who have DM compared to the numbers recorded in 2000. The estimated figure could surpass a staggering total of 300 million people affected by this condition3.
To effectively manage diabetes, the ultimate aim is to regulate blood sugar levels in both type 1 and type 2 cases4. Achieving this requires efficient glycolysis, which transforms glucose into energy that manages insulin and cell activity. Enzymes such as glucokinase and 6-phosphofructo-1-kinase control the glycolysis rate. Enzymes vary in their regulation between cell types. The 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase enzyme is also crucial in this process5. Enzymes respond to signals from nutrition and hormones, which affect their transcription, translation, and post-translational modifications. The intricate process of hepatic glucose production is essentially a result of the complex interplay between enzymes and metabolic pathways within hepatocytes, which work together to regulate glycolysis6. When there is too much, the latter can lead to high blood sugar levels in people with diabetes. Glycolysis results in glucose-stimulated insulin release in pancreatic beta cells. Hyperglycemia can occur when there is a deficiency of circulating insulin or when its function is impaired5. Adipocytes use glycolysis to produce metabolites that facilitate lipogenesis and redirect surplus fatty acids toward the synthesis of triglycerides. This mechanism curtails oxidative stress within cells. Adipocytes generate pro-hyperglycemic factors when the body is in a state of increased inflammation, leading to insulin resistance and elevated blood glucose levels7.
When insulin levels are inadequate or the body becomes resistant to it, there is a malfunctioning of glycolysis. This is caused by the metabolic and regulatory enzymes involved in glycolysis functioning inappropriately in terms of quantity and/or activity. Effective strategies to manage diabetes and its associated complications may include measures to improve glycolytic activity by modulating key metabolic and regulatory enzymes8,9. The association of enzymes affects glucose oxidation. The high association of enzymes favours glucose for fermentation and biosynthesis. In skeletal and cardiac muscles of diabetic patients, glycolysis undergoes down-regulation due to hexokinase and phosphofructokinase enzyme deficiency. Cancer conditions commonly show elevated glycolytic rates associated with this phenomenon6.
The appropriate utilization of glucose by various tissues in the body, including the liver, pancreas, and kidneys, is critical in diabetes to prevent the accumulation and toxicity of cellular glucose of cellular glucose that can lead to serious complications. This highlights the importance of regulating both under- and over-use of glucose. Insulin-dependent diabetes mellitus is characterised by metabolic dysfunction primarily resulting from hyperinsulinemia and hyperglycemia. The maintenance of carbohydrate and lipid homeostasis depends on the equilibrium between their production and usage in the main peripheral tissues, which is substantially altered in individuals with diabetes10,11,12. This mechanism is key to developing new antidiabetic drugs. The 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling pathway effectively regulates diabetes. Activation of the AMPK protein can increase glucose uptake and suppress intracellular glucose production. This promotes optimal cell function, as shown in several studies. Impaired AMPK activity is common among people with diabetes, making activation of this protein a persuasive solution. In particular, drugs used to treat this condition, including metformin, exert their therapeutic effects by regulating AMPK function. As a result, pharmacological substances with promising potential as therapeutic choices for the treatment of DM include those that can efficiently activate and modulate AMPK activity13,14. It is important to consider alternative treatments based on scientific research for patients who experience adverse effects from allopathic drugs, including hypoglycemia. Plant-based medications such as berberine, quercetin, and resveratrol can effectively regulate the AMPK pathway to manage diabetes mellitus without harmful side effects15.
Insulin therapy and oral drugs such sulfonylureas, biguanides, -glucosidase inhibitors, and glinides are often used to manage diabetes. However, in resource-limited settings where access to these drugs is limited due to high costs and unavailability; adopting alternative therapies or preventive measures becomes crucial. To provide optimal diabetes care, healthcare providers should explore lifestyle changes and natural remedies16. In the world of healthcare, there is a new wave of interest in herbal remedies. This can be attributed to the harmful side effects associated with taking oral hypoglycemic agents, which are often prescribed to treat diabetes mellitus. With more and more people looking for natural alternatives to traditional medications, it is crucial that we embrace this shift towards holistic healing methods. Herbal remedies have been used for centuries to treat various ailments and now offer a safe solution for those seeking relief from their diabetic symptoms without risking dangerous side effects. Traditional plant-based remedies are vital in treating DM. Based on scientific evidence, these traditional remedies have proven effective in controlling blood sugar levels and alleviating the harmful effects of this persistent medical condition17,18. Kaempferol-3-rhamnoside is a flavonoid glycoside that belongs to the class of phytochemicals known as flavonoids, recognized for their medicinal properties and health benefits. It is derived primarily from the sources of Ficus palmata and Nymphaea odorata19. The molecule kaempferol-3-rhamnoside is a flavonoid glycoside comprised of the flavonol kaempferol attached to a rhamnose sugar molecule20. The kaempferol core features three hydroxyl groups linked to its two benzene rings and a heterocyclic ring. This compound is glycosylated at the 3-position where the rhamnose is bonded through an oxygen atom21. The functional groups, particularly the hydroxyl groups, are key to the molecule's solubility, reactivity, and overall biological activity. The chemical structure of kaempferol-3-rhamnoside is given in Fig. 1.
Kaempherol-3-rhamnoside is an exceptional natural compound that acts as both a potent inhibitor of NOS and NADPH oxidase. Its impressive therapeutic potential is attributed to its remarkable multifunctional properties, which include powerful antibacterial, anti-inflammatory, antiapoptotic, and antitumour activities. It is no wonder why kaempherol-3-rhamnoside continues to be a promising candidate for future drug development efforts. kaempherol-3-rhamnoside might serve as an effective treatment against P. aeruginosa-related diseases22. While promising results have been observed in animal studies, further research is needed to determine the efficacy and safety of kaempherol-3-rhamnoside as a potential treatment for breast23. We investigated the effects of kaempherol-3-rhamnoside on metabolic and oxidative enzymes in diabetic mice. This study is significant to understanding the benefits of using kaempherol-3-rhamnoside to manage diabetes, which could lead to more effective treatments.
Materials and methods
Chemicals
Streptozotocin purchased from Sisco Research Laboratories Pvt. Ltd., India. Glucose kit (120200- Erba Mannheim, India), Insulin ELISA Kit (Millipore, Billerica, MA, USA) were used. Cholesterol, triglycerides, high-density lipoproteins and free fatty acids were assessed using assay kits purchased from Spin react, Spain. Kaempherol-3-rhamnoside was obtained as gift sample from Humed life sciences. Anti-AMPK and anti-p-AMPK from Abcam Inc., Cambridge, MA. The enzyme kits were purchased from: hexokinase type I enzyme—ab136957, Colorimetric assay kit from Abcam Inc.); phosphofructokinase- MAK093, and pyruvate kinase—MAK072 from Sigma Aldrich, India; lactate dehydrogenase, glucose-6-phosphatase and fructose-1,6-bisphosphatase from BT LAB, Bioassay Technology Laboratory, India. It is imperative that all reagents administered to animals must adhere to pharmacological and chemical grade standards, ensuring they are free from contamination.
Acute oral toxicity study
A study was conducted to evaluate the acute oral toxicity of the substance in accordance with guidelines provided by the OECD (Organisation for Economic Cooperation and Development) No. 423. In this study, albino mice with weight ranging from 18 to 28 g and male gender were used. To ensure consistency in drug administration and minimize variability between individuals, all mice underwent an overnight fast prior to receiving any drugs or treatments. Three mice received a robust single oral dose equivalent to 500 mg/kg of their body weight, courtesy of the powerful compound known as kaempherol-3-rhamnoside. After giving kaempherol-3-rhamnoside, food was not allowed for the next 4–5 h. The animals were carefully monitored as individuals within the first half hour of being dosed and then at various intervals throughout the initial 24 h (with a particular focus on the first four). Following this initial period, they were observed daily for 2 weeks. Overall, their well-being was closely monitored over an extended period of time. Daily, observations were made on the animal’s appearance and behaviour including skin, fur, eyes, nose, breathing rate, heart rate, piloerection (hair standing), involuntary urination or defecation along with changes in drowsiness, gait, tremors, and seizures. Any deaths that occurred within 14 days were recorded24.
Animals
This study was conducted using male albino mice, weighing 18–28 g and aged 7–8 weeks. The mice were housed in a temperature-controlled environment (22 ± 2 °C) with a 12-h light–dark cycle. Ad libitum access to commercially available rodent food from the local market of Saudi Arabia and tap water was provided. Food were replenished daily to ensure constant availability. All methods and animal care procedures were carried out in strict accordance with the ARRIVE guidelines (https://arriveguidelines.org). Additionally, this protocol was approved by the Internal Animal Ethics Committee under authorization number SCBR-24/2022, ensuring all ethical and welfare considerations were met.
Experimental design
Induction of diabetes
The development of DM was begun by administering a single ip dose of 65 mg/kg STZ that had been dissolved in citrate buffer (100 mM, pH 4.5) according to the meth-od described in the reference source provided25. All animals involved in the experiment were fed a high-fat diet, except the normal control group, who received citrate buffer only. After four days, mice that showed hyperglycemia (measured at ≥ 250 mg/dL) were classified as diabetic. The animals were divided into the following groups:
-
Group I: Normal control, received vehicle only.
-
Group II: Diabetic control, received streptozotocin (STZ) only.
-
Group III: Diabetic mice treated with regular human insulin at 2 U/kg daily for 28 days.
-
Group IV: Diabetic mice treated with kaempherol-3-rhamnoside at 2.5 mg/kg daily for 28 days.
-
Group V: Diabetic mice treated with kaempherol-3-rhamnoside at 5 mg/kg daily for 28 days.
-
Group VI: Diabetic mice treated with a combination of human insulin (1U/kg) and kaempherol-3-rhamnoside (2.5 mg/kg) daily for 28 days.
Mice underwent 28 days of treatment, after which they were fasted and weighed. Blood samples were then collected from the retroorbital area. Following anesthesia, the mice were euthanized by cervical decapitation26,27. Subsequently, the pancreas and liver tissue were isolated, weighed and stored in a deep freezer with a temperature of − 70 °C28,29.
Fasting blood glucose level
The fasting blood glucose level was recorded on days 0, 14, 21 and 28. Blood samples were taken from the retro-orbital area, and the findings were reported as milli-grams per deciliter (mg/dl)30. Blood glucose levels were measured using a glucometer (Accu-Chek).
Plasma insulin level
On the 28th day, plasma insulin levels were measured using a Rat/Mouse Insulin ELISA Kit (Millipore, Billerica, MA, USA) and reported as µIU/mL30.
Preparation of tissue extracts for enzyme assays
To prepare tissue extracts, animals from each group were starved overnight and then euthanized. The liver was washed in saline, weighed, minced, and homogenized in a cold isotonic sucrose buffer using a tissue homogenizer. The homogenization buffer contained 0.25 M sucrose, 0.02 M triethanolamine, and 0.12 mM dithiothreitol to enhance uniformity, with a pH of 7.4. All procedures were performed at 4 °C. The homogenate was then centrifuged for 10 min at 1000 g. The supernatant from this centrifugation was further centrifuged at 105,000g for 45 min at 4 °C. The clear fraction of the supernatant was collected for enzyme assays31.
Glycolytic/metabolic enzyme assays in liver
Coupled enzymatic reaction systems were utilised to estimate the activity of hexokinase isozymes in the supernatant fraction by spectrophotometric measurement, according to the Gumaa and McLean method32. Phosphofructokinase33, pyruvate kinase and lactate dehydrogenase34 was carried out as per paper published earlier. The gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase were tested using the methods of Baginsky et al.35 and Tashima and Yoshimura36,37, respectively. The NADP-linked lipogenic enzyme glucose6-phosphate dehydrogenase was essentially determined by the method of Baquer et al.38. An enzyme unit is equal to the formation of 1 µmole of NAD/NADH or NADP/NADPH per gram of fresh tissue weight each minute at a temperature of 25 °C. Similarly, for glucose-6-phosphatase and fructose-6-bisphosphatase, one unit refers to the liberation amount of Pi per gram fresh weight every minute while being subjected to a temperature equivalent to 37 °C.
Lipid profile
Serum levels of cholesterol (CHO), triglycerides (TG), high-density lipoproteins (HDL) and free fatty acids (FFA) were accurately assessed using Spin react assay kits, Spain. Comprehensive testing helped to gain a complete understanding of the metabolic health of the participants. These evaluations provided significant information on the lipid profile of each participant, which can be used to target specific aspects of their risk factors. Each milligram per deciliter (mg/dl) measurement was meticulously examined to ensure data collection accuracy and precision. In addition, the advanced features integrated with the assay kit streamlined the processes by allowing seamless execution and reducing processing time39.
Lipid peroxidation assay
The presence of malondialdehyde (MDA) in tissue was evaluated through the utilisation of a reactive substance of thiobarbituric acid (TBA) at an elevated temperature, producing a pigmented compound. The amount was quantified as nanomoles per milligram of tissue using a molar absorption coefficient set at 156,000 M−1 cm−1 with λ = 532 nm. The mixture included phosphate buffer, potassium permanganate (1 mM), and the sample. The pH was 7.4 at a concentration of 10 mM for the buffer. To start the reaction, ferrous sulphate (10 mM) was added twice. The reaction was stopped by adding trichloroacetic acid (20%). Malondialdehyde (MDA) reacted with TBA to produce a coloured product40.
Estimation of TNF- α
ELISA kits were used to estimate the levels of TNF-α, which is an inflammatory cytokine found in tissues. Manufacturer instructions were followed during this process.
Antioxidant enzymes assays
The evaluation of superoxide dismutase (SOD) was conducted using the technique described by Marklund and Marklund41, with results expressed in units per milligram of protein. Catalase activity (CAT) was measured following Sinha’s (1972) methodology42, and the results were presented as units per milligram of protein. Glutathione peroxidase (GPx) levels were assessed by measuring the activity, expressed as the amount of glutathione utilized per minute per milligram of protein43.
Total AMP-activated protein kinase (AMPK) and phospho-AMPK (p-AMPK) proteins
A liver supernatant homogenate, which contained 20 mg of protein, was subjected to SDS-PAGE and then transferred to a nitrocellulose membrane by electrophoresis. The NC membrane was blocked for 2 h at room temperature before being incu-bated with polyclonal antibodies against anti-AMPK and anti-p-AMPK from Abcam Inc., Cambridge, MA, overnight at 4 °C. Following this process, the membranes were treated with horseradish-peroxidase-conjugated antirabbit IgG. Ultimately, Nitrocellulose membranes received a thorough wash in solution for half an hour, while immunoreactive lanes detected by Band Scan software version 5.0 were enhanced using chemiluminescence method before digitalization took place44.
Statistical analysis of data
The results were presented as average ± standard error of the mean. A one-way analysis of variance was employed to establish the significance between two values, and statistical significance was defined at a level of p < 0.05. The evaluations were executed using Graphic Pad prism 7.0 software.
Institutional Review Board Statement
All procedures regarding animal care and treatment conformed to the Animal Care Guidelines of the Standing Committee on Bioethics of Prince Sattam Bin Abdulaziz University (SCBR) 24/2022), Al-Kharj, Ministry of Education, Kingdom of Saudi Arabia.
Results
Acute toxicity study
Based on the experiments conducted, it appears that the administration of kaempherol-3-rhamnoside to animals did not result in any harmful symptoms or death observed. These findings suggest that the LD50 value for kaempherol-3-rhamnoside is likely above 200 mg/kg body weight, indicating its safety and suggesting the potential use as a compound for further research in pharmacology. To proceed with this study, 2.5 mg/kg of 2.5 mg/kg p.o. and 5 mg/kg of weight were chosen for additional investigation based on their relevance to expected physiological effects within an animal model system under experimental conditions.
Effect of kaempherol-3-rhamnoside on fasting blood glucose (FBG) level
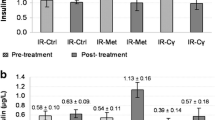
Figure 2a illustrates the impact of a 28-day study on FBG in various experimental groups. The presence of high fasting glucose levels confirmed diabetes induction in the mice, compared to the normal group. After treating diabetic mice with kaempherol-3-rhamnoside for four weeks, there was a significant reduction (p < 0.05) reduction in their blood sugar levels compared to those of the diabetic control group, indicating its effectiveness as a treatment option. Furthermore, the effect of kaempherol-3-rhamnoside appeared to be dose-dependent.
(a) Effect of kaempherol-3-rhamnoside and its combination on glucose level was analyzed. Statistical significance was determined by a p-value less than 0.05 (*p < 0.05). The following pairs were compared for statistical significance: a) normal control versus diabetic control corresponding to the same day, b) diabetic control versus treatment groups corresponding to the same day; (b) Effect of kaempherol-3-rhamnoside and its combination on insulin level was studied. The statistical significance threshold was set at a p-value of less than 0.05, denoted as *p < 0.05 in the results section. Comparison between treatment groups and diabetic control (b) as well as normal control (a) were analyzed for significant differences in insulin levels.
Plasma insulin level
The results of the study examining the impact of kaempherol-3-rhamnoside and insulin on serum insulin levels in mice are shown in Fig. 2b. Before treatment, normal control mice had an average insulin level of 7.81 µU/mL. During the course of 28 days, diabetic mice experienced a notable decrease in serum insulin levels. However, administering kaempherol-3-rhamnoside orally daily resulted in serum insulin levels (3.89 µU/mL), but a combination of insulin + kaempherol-3-rhamnoside resulted in a higher insulin level (5.91 µIU/mL) as compared to untreated diabetic controls.
Hepatic glycolytic/metabolic enzyme assays
After being treated with kaempherol-3-rhamnoside and kaempherol-3-rhamnoside + Insulin for 28 days, diabetic mice showed metabolic activities similar to those of the normal control group. In diabetic mice, there was a significant decrease in overall levels of insulin-sensitive type I hexokinase enzyme present in their liver. Although not significantly different from that of normal controls, the level of hexokinase type I enzyme decreased among diabetic control groups. However, the treatment groups showed a statistically significant increase primarily when receiving combination therapy. These concise findings are illustrated in Fig. 3a.
Effect of kaempherol-3-rhamnoside and its combination was studied on metabolic enzymes: (a) hexokinase type I enzyme; (b) phosphofructokinase; (c) pyruvate kinase; (d) lactate dehydrogenase; (e) glucose-6-phosphatase and; (f) fructose-1,6-bisphosphatase. Any p-value less than 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001). Here a represents normal control versus diabetic control while b represents diabetic control versus treatment groups comparison without plagiarism involved in the content.
Following 28 days of treatment, significant elevations were observed in the activity levels of three key enzymes involved in glucose metabolism-phosphofructokinase, pyruvate kinase, and lactate dehydrogenase-in the livers of diabetic mice treated with kaempferol-3-rhamnoside, compared to untreated diabetic controls. This enhancement in enzymatic activity was statistically substantiated by a p-value of less than 0.001 (p < 0.001), as illustrated in Fig. 3b–d. Diabetic mice administered either kaempferol-3-rhamnoside alone or in combination with insulin exhibited enzyme activities comparable to those observed in the nondiabetic control group. Collectively, these findings indicate that kaempferol-3-rhamnoside may serve as a promising therapeutic agent for addressing diabetes-associated metabolic dysfunctions in the liver.
In diabetic mice, there was a significant increase in the activity of glucose-6-phosphatase and fructose-1,6-bisphosphatase, enzymes critical for gluconeogenesis, within the cytosolic fractions of their livers45, evidenced by a p-value of less than 0.001. Treatment with kaempferol-3-rhamnoside alone or in combination with insulin for 28 days normalized the activities of these gluconeogenic enzymes as well as those of NADH-dependent lipogenic enzymes to levels observed in the livers of non-diabetic control mice, as demonstrated in Fig. 3e and f.
Lipid profile
The results shown in Fig. 4a indicate that the serum lipid profiles of the diabetic control group experienced significant pathological changes after the experimental period. Compared to the control groups, this group displayed significantly higher levels of total cholesterol and triglycerides (p < 0.001), along with markedly lower levels of HDL, which are key biomarkers for diabetes. These findings highlight the urgent need for interventions to improve the serum lipid profiles in the diabetic control group to enhance health outcomes. The study conclusively demonstrated that treatment with kaempferol-3-rhamnoside, both as a monotherapy and in conjunction with insulin, significantly attenuated elevated serum lipid concentrations, specifically total cholesterol and triglycerides, with a statistical significance of (p < 0.001). Administration of kaempferol-3-rhamnoside at a lower dosage of 2.5 mg/kg resulted in only a marginal improvement in total cholesterol levels (p < 0.05). However, the combination of kaempferol-3-rhamnoside with insulin was markedly more effective, leading to substantial reductions in both total cholesterol and triglyceride levels, and significantly enhancing serum HDL concentrations (p < 0.01) and (p < 0.001), respectively). These results indicate that the combined use of kaempferol-3-rhamnoside and insulin is superior to their individual applications for the management of hyperlipidemia.
(a) Effect of kaempherol-3-rhamnoside and its combination on lipid levels was investigated, and statistical significance was determined using a p-value threshold of 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001). The normal control group was compared to the diabetic control group (a), as well as to the treatment groups; (b) The impact of kaempherol-3-rhamnoside and its amalgamation on lipid peroxidation was analyzed, considering a p-value less than 0.05 as statistically significant. The significance values are expressed as *p < 0.05; **p < 0.01; ***p < 0.001 for the comparison between a-normal control versus diabetic control and b-diabetic control versus treatment groups.
Lipid peroxidation in pancreas
Diabetes is characterized by enhanced lipid peroxidation, contributing to tissue damage and playing a role in the pathogenesis of both Type I and Type II diabetes46. Our study demonstrated elevated lipid peroxidation levels in the diabetic control group. However, treatment with kaempferol-3-rhamnoside and insulin significantly mitigated these levels, restoring them to near-normal, as illustrated in Fig. 4b. While low concentrations of lipid peroxides might stimulate insulin secretion, excessive levels can induce uncontrolled lipid peroxidation, leading to cellular damage within the pancreas. This damage demonstrates as complications associated with diabetes, including cellular infiltration and destruction of islet cells. The effects of kaempferol-3-rhamnoside and its combinations on lipid peroxidation were assessed, with statistical significance set at a p-value less than 0.05. Significance levels are denoted as *p < 0.05, **p < 0.01, and ***p < 0.001 for comparisons: (a) between normal control and diabetic control groups, and (b) between diabetic control and treatment groups.
Antioxidant parameters
The results indicated a significant decrease in the levels of serum GSH, SOD, and CAT in the diabetic control group compared to the normoglycemic controls (p < 0.01). Conversely, treatment with kaempferol-3-rhamnoside, either alone or in conjunction with insulin, significantly elevated the serum levels of these antioxidants (p < 0.01). Notably, administration of kaempferol-3-rhamnoside at a dosage of 2.5 mg/kg did not enhance antioxidant levels when compared to diabetic controls. In contrast, the combination of kaempferol-3-rhamnoside with insulin elicited the most substantial increase in GSH, SOD, and CAT levels (p < 0.01), as depicted in Fig. 5a. These findings underscore the potential efficacy of this therapeutic approach in improving antioxidant status in individuals with diabetes, highlighting the importance of combination therapies in optimizing the therapeutic outcomes of such treatments.
(a) Effect of kaempherol-3-rhamnoside and its combination on antioxidant enzymes was studied while ensuring originality. The statistical significance level was set at a p-value of 0.05, represented as *p < 0.05; **p < 0.01; ***p < 0.001 where “a” indicates normal control versus diabetic control and “b” represents diabetic control versus treatment groups; (b) The impact of kaempherol-3-rhamnoside and its combination on glucose levels and TNF-α was assessed in this study. Statistical significance was determined using a p-value of 0.05, whereby ***p < 0.001 indicated a significant difference between the normal control group and diabetic control group, while b-diabetic control versus treatment groups also exhibited statistical significance.
TNF-alpha level
In comparison with non-diabetic control mice, mice with diabetes demonstrated elevated levels of tumor necrosis factor-alpha (TNF-α) in the pancreas. Administration of kaempferol-3-rhamnoside at doses of 2.5 mg/kg or 5 mg/kg orally over a period of 4 weeks resulted in a reduction of pancreatic TNF-α levels. This effect was particularly pronounced when kaempferol-3-rhamnoside was co-administered with insulin therapy in diabetic mice, as illustrated in Fig. 5b.
AMPK expression in liver
In Fig. 6, a comprehensive examination of AMPK and its phosphorylated form (p-AMPK) in liver tissue via Western blotting reveals a notable decrease in both p-AMPK and total AMPK protein expression levels in diabetic mice compared to normal controls (p < 0.01). However, following treatment with kaempferol-3-rhamnoside combined with insulin, these levels exhibited a significant increase relative to the diabetic control group (p < 0.05). These results indicate that diabetes substantially reduces AMPK activity in the liver, but the administration of kaempferol-3-rhamnoside and insulin substantially enhances the p-AMPK/AMPK ratio, thereby activating AMPK function in hepatic tissues.
(a) The expressions of AMPK and p-AMPK in the liver were analyzed using Western blot; (b) kaempherol-3-rhamnoside and its combination’s impact on AMPK and p-AMPK was examined; (c) The ratio of pAMPK/AMPK expressions was calculated. A significance level of < 0.05 was deemed statistically significant, represented as *p < 0.05; where a denotes normal control versus diabetic control, while b represents diabetic control versus treatment groups.
Discussion
The animal model for diabetes used in this study involves administering STZ via a single injection of i.p., resulting in impaired insulin secretion even at high levels of glycemia and a moderate level of insulin resistance. This mixed model disease shows characteristics similar to both Type I and Type II diabetes mellitus47. The fact that kaempherol-3-rhamnoside can promote hypoglycemic effects in diabetic models indicates its ability to bypass insulin resistance and lower glycemia. This advantageous property allows for the reduction of glycemia even when insulin resistance is present. However, it is unlikely that kaempherol-3-rhamnoside improves insulin responsiveness, since no decrease in insulin levels was observed alongside unchanged glycemia. These findings demonstrate that there are no noticeable effects on the insulin levels of the control and STZ groups due to the consumption of kaempherol-3-rhamnoside.
Kaempherol-3-rhamnoside, a flavonoid, has shown the ability to lower blood sugar levels when given orally to STZ-induced diabetic mice. The authors suggested that kaempherol-3-rhamnoside triggers insulin signaling pathways that lead to increased glucose absorption by tissues outside the pancreas48. To support this idea, we refer to the research conducted by Tzeng et al. Their study revealed that kaempherol-3-rhamnoside activates traditional insulin signaling pathways in 3T3-L1 cells. This is achieved through the phosphorylation of key proteins such as the insulin receptor substrate 1, the insulin receptor itself, and other regulatory molecules within the cell. As a result, there is an increase in GLUT-4 translocation, a glucose transporter located on cell membranes that facilitates glucose uptake into tissues sensitive to insulin49,50.
The occurrence of oxidative stress is associated with various diseases, such as diabetes and its related complications. The production of free radicals or mitochondrial superoxide leads to the development of this condition, which can be caused by different mechanisms including elevated glycolysis and polyol pathway activation, non-enzymatic protein glycation, auto-oxidation due to excessive glucose levels in tissues, and decreased antioxidant enzyme levels51. Our study showed that in diabetic mice induced by alloxan, there was a significant reduction in the levels of antioxidant enzymes. This depletion may be related to oxidative stress-related harm experienced by both the serum and liver. The free radical scavenging system comprises both enzymes (SOD, CAT, and GPx) and non-enzymes (GSH, vitamin C, vitamin E) antioxidants that are tightly controlled in normal circumstances52. Antioxidants decreased and higher levels of TBARS were observed with diabetes53. After kaempherol-3-rhamnoside administration, there was an improvement in enzymatic antioxidant potential, while non-enzymatic oxidative stress markers reduced tissue damage caused by oxidative stress. Furthermore, free radical oxidation due to oxidative stress causes the formation of lipid peroxide in the membranes that leads to membrane dysfunction that leads to membrane dysfunction54. The use of kaempherol-3-rhamnoside has shown significant potential in the treatment of both microvascular and macrovascular diabetic complications. This is evidenced by the reduction in accumulated peroxides after administration, indicating a persuasive improvement in overall health outcomes.
To this, how kaempherol-3-rhamnoside lowers blood sugar, we examined the activity of PFK in key tissues that regulate glycemia—specifically liver tissue from healthy mice and those with STZ-induced diabetes. The primary pathway for cellular glucose consumption is glycolysis, which is highly dependent on PFK as a rate-limiting enzyme50,55. The effectiveness of kaempherol-3-rhamnoside in reversing impaired enzymatic activity and increasing PFK activity in liver tissue from diabetic mice is indicative of its ability to promote glucose utilization. Although the stimulation of glycolysis may be one possible mechanism for its hypoglycemic effects, it should be noted that other mechanisms are likely involved to ensure a sustained reduction in glycemia. In general, this evidence strongly supports the persuasive argument for the use of kaempherol-3-rhamnoside as an effective treatment option for diabetes56. According to this study, kaempherol-3-rhamnoside has been found to stimulate not just PFK but also other crucial glycolytic enzymes such as hexokinase (HK) and pyruvate kinase (PK). These findings suggest that the compound can enhance glucose utilization by cells, thus improving both catabolic and anabolic pathways along with overall energy balance. Interestingly, while intracellular ATP levels were observed to increase at all concentrations used, only kaempherol-3-rhamnoside was able to augment glucose catabolism (such as glucose consumption, lactate production, and metabolizing enzymes). It should be noted that insulin also exhibits a similar trend in which a higher concentration of hormones of hormones negates its stimulatory effects on metabolism57,58.
To our knowledge, only one investigation has documented the activating impact of kaempherol-3-rhamnoside on PFK. However, we could not locate any research linking flavonoids to improving phosphofructokinase (PFK) function and diabetes development59. Several flavonoids possessing hypoglycemic properties have been found to exhibit efficacy in various glucose-metabolizing enzymes. For example, rutin and quercetin, which are glycosylated flavonols, demonstrated the ability to regulate HK activity, as well as fructose 1,6-bisphosphatase and glucose 6-phosphatase50,60. Similarly, fisetin, another glycosylated flavanols, has shown potential in modulating PK, LDH G6PDH glycogen synthase and glycogen phosphorylase alongside the aforementioned enzymes61. Numerous studies have supported the effectiveness of flavanones, such as naringenin and diosmin, in the regulation of essential enzymes involved in carbohydrate metabolism, leading to reduced glucose levels62,63. This emphasizes that the control of these key metabolic pathways is a recurring process attributed to the glucose-reducing properties of flavonoids. It is crucial to address the pressing issue of developing improved treatments to manage glucose homeostasis and increase insulin sensitivity. AMP-activated protein kinase (AMPK) emerges as a prime contender, being a well-preserved serine/threonine kinase that triggers favourable effects on insulin responsiveness. Therefore, it stands out as an ideal therapeutic focus to effectively tackle Type 2 Diabetes (TD2).
AMPK, an enzyme that monitors cellular energy levels, became active when depleted. It then sent signals to increase glucose absorption in skeletal muscles and promoted fatty acid oxidation in adipose tissue (as well as other tissues). Additionally, it curbed hepatic glucose production64. The evidence supporting AMPK dysregulation in both animals and humans with metabolic syndrome or type II diabetes is quite compelling. In addition, activating AMPK through physiological or pharmacological interventions has been found to lead to improvements in insulin sensitivity and overall metabolic health65. There is an abundance of pharmaceutical drugs, natural substances, and hormones that have the ability to activate AMPK through direct or indirect means. Some examples of these compounds include metformin and thiazolidinediones, which are already being used for the treatment of Type II diabetes66. This research also demonstrated that kaempherol-3-rhamnoside plays a role in activating AMPK, which then controls metabolic enzymes.
Kaempherol-3-rhamnoside can activate AMPK by increasing the cellular AMP: ATP ratio or by modulating the activity of upstream kinases. One of the primary pathways involved in Kaempherol-3-rhamnoside-mediated AMPK activation is through activation of the tumour suppressor kinase LKB1. kaempherol-3-rhamnoside can promote the phosphorylation and activation of LKB1, which in turn phosphorylates and activates AMPK. kaempherol-3-rhamnoside has also been reported to activate AMPK through the CaMKKβ pathway. kaempherol-3-rhamnoside can stimulate CaMKKβ, which phosphorylates and activates AMPK independently of changes in the AMP: ATP ratio.
Kaempherol-3-rhamnoside may also directly bind to AMPK and influence its activation. Studies have suggested that kaempherol-3-rhamnoside can bind to AMPK's γ subunit and promote conformational changes that enhance AMPK activation. Activation of AMPK by kaempherol-3-rhamnoside leads to various downstream effects that contribute to its potential therapeutic benefits. AMPK activation can increase glucose uptake, enhance fatty acid oxidation, inhibit gluconeogenesis (glucose production), promote mitochondrial biogenesis, and regulate gene expression involved in energy metabolism. It is important to note that the exact molecular mechanisms underlying kaempherol-3-rhamnoside activation of AMPK and p-AMPK may vary depending on the cell type, experimental conditions, and concentrations used. Further research is needed to elucidate the detailed mechanisms and signaling pathways involved in kaempherol-3-rhamnoside effects on AMPK activation. Additionally, it is worth mentioning that the activation of AMPK by kaempherol-3-rhamnoside is just one aspect of its overall biological activity, and its effects on other cellular processes and pathways may also contribute to its potential therapeutic effects.
Conclusions
The findings of this research suggest that kaempherol-3-rhamnoside could possess potential antidiabetic properties independently or in combination with insulin. The objective of the study was to assess how kaempherol-3-rhamnoside affected the AMPK pathway, metabolic enzymes, glycolytic enzymes, gluconeogenic enzymes, and NADP-linked lipogenic enzymes in liver tissues obtained from mice who had developed diabetes after STZ induction.
Upon analysis, the results showed that in diabetic mice, there was a decrease in glycolytic enzyme activity while gluconeogenic enzyme activities increased. This suggests an impaired glucose metabolism. In particular, these mice also exhibited reduced lipid-based enzyme activity. Interestingly, normalising the enzymatic activities associated with both glucose and lipid metabolism through treatment with kaempherol-3-rhamnoside led to therapeutic benefits. As such, it can be concluded that kaempherol-3-rhamnoside is effective against dysfunctional glucose metabolism found among diabetic subjects.
In addition, it was revealed that kaempherol-3-rhamnoside treatment triggered the activation of AMPK activity in liver tissues among diabetic mice. An increase in the p-AMPK/AMPK ratio indicated that this activation is vital. There had been a prior suppression of AMP kinase activity in these mice before its successful recovery by kaempherol-3-rhamnoside treatment. These findings point to the prospective role of kaempherol-3-rhamnoside as a regulator of metabolic processes related to diabetes by simplifying modulation throughout the AMPK pathway. Compelling evidence supporting the efficacy of kaempherol-3-rhamnoside in managing diabetes was obtained from a biochemical investigation, which demonstrated its ability to rejuvenate cellular activity on a molecular scale.
Overall, this study contributes to our understanding of the mechanisms underlying kaempherol-3-rhamnoside anti-diabetic effects and sheds light on its role in regulating glucose and lipid metabolism, as well as the AMPK pathway. To examine the complete therapeutic potential of kaempherol-3-rhamnoside in in the treatment of diabetes and its complications, additional research and clinical studies are required.
Data availability
All data used for this study are contained in this article.
References
Kainsa, S. & Singh, R. Chemical constituents, antihyperglycemic and antioxidant effects of Nepeta hindostana whole herb in alloxan and OGTT induced diabetes in rats. J. Chem. Pharm. Res. 7, 920–932 (2015).
Kanwar, T., Roy, A. & Prasad, P. Management of diabetes and complication: Herbal therapies. Pharm. Biosci. J. https://doi.org/10.20510/ukjpb/5/i5/166557 (2017).
Shashikala, E., Somnath, M., Raghawa, R. B. & Mohd, A. S. Study of lipid lowering effects of oral antidiabetic drugs in type 2 diabetes mellitus patients. Int. J. Basic Clin. Pharmacol. 7, 126 (2017).
Briscoe, V. J. & Davis, S. N. Hypoglycemia in type 1 and type 2 diabetes: Physiology, pathophysiology, and management. Clin. Diabetes 24, 115 (2006).
Guo, X. et al. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2, 358 (2012).
Kim, J. W. & Dang, C. V. Multifaceted roles of glycolytic enzymes. Trends Biochem. Sci. 30, 142 (2005).
Murao, N. et al. Increased glycolysis affects β-cell function and identity in aging and diabetes. Mol. Metab. 55, 101414. https://doi.org/10.1016/j.molmet.2021.101414 (2022).
Wu, C., Okar, D. A., Kang, J. & Lange, A. J. Reduction of hepatic glucose production as a therapeutic target in the treatment of diabetes. Curr. Drug Targets Immune Endocr. Metab. Disord. 5, 51 (2005).
Nishizawa, T. & Bornfeldt, K. E. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann. Med. 44, 555 (2012).
Scatena, R., Bottoni, P., Pontoglio, A., Mastrototaro, L. & Giardina, B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin. Investig. Drugs 17, 1533 (2008).
Akram, M. Mini-review on glycolysis and cancer. J. Cancer Educ. 28, 454 (2013).
Zhang, K. et al. The effect of diabetes on corneal endothelium: a meta-analysis. BMC Ophthalmol. 21, 1 (2021).
Kim, Y. & Park, C. W. Adenosine monophosphate-activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 35, 69 (2016).
Joshi, T. et al. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell. Physiol. 234, 17212 (2019).
Raju, J., Gupta, D., Rao, A. R., Yadava, P. K. & Baquer, N. Z. Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol. Cell. Biochem. 224, 45 (2001).
Pandeya, K. B. et al. A critical review on traditional herbal drugs: An emerging alternative drug for diabetes. Int. J. Org. Chem. 3, 1–22 (2013).
Dwivedi, C. & Daspaul, S. Antidiabetic herbal drugs and polyherbal formulation used for diabetes: A review. J. Phytopharm. 2, 44–51 (2013).
Rahul, G. & Nandhakumar, E. A systematic review of five herbal ingredients for the management of diabetes mellitus. Asian J. Pharm. Clin. Res. https://doi.org/10.22159/ajpcr.2022.v15i12.45427 (2022).
Akter, M., Parvin, M. S., Hasan, M. M., Rahman, M. A. A. & Islam, M. E. Anti-tumor and antioxidant activity of kaempferol-3-O-alpha-L-rhamnoside (Afzelin) isolated from Pithecellobium dulce leaves. BMC Complement. Med. Ther. 22, 169 (2022).
Diantini, A. et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. Inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 3, 1069 (2012).
Niñoles, R. et al. Kaempferol-3-rhamnoside overaccumulation in flavonoid 3′-hydroxylase tt7 mutants compromises seed coat outer integument differentiation and seed longevity. New Phytol. 238, 1461 (2023).
Lee, S. Y., So, Y. J., Shin, M. S., Cho, J. Y. & Lee, J. Antibacterial effects of Afzelin isolated from Cornus macrophylla on Pseudomonas aeruginosa, a leading cause of illness in immunocompromised individuals. Molecules 19, 3173 (2014).
Cao, M., Fan, B., Zhen, T. & Wang, J. A pre-clinical trial study on Afzelin: Anti-human lung cancer, anti-cholinesterase, and anti-glucosidase properties. Arch. Med. Sci. https://doi.org/10.5114/aoms/136283 (2021).
Bedi, O. & Krishan, P. Investigations on acute oral toxicity studies of purpurin by application of OECD guideline 423 in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 393, 565 (2020).
Alkholifi, F. K., Devi, S., Yusufoglu, H. S. & Alam, A. The cardioprotective effect of corosolic acid in the diabetic rats: A possible mechanism of the PPAR-γ pathway. Molecules 28, 929 (2023).
University-of-Pennsylvania. IACUC Guideline Mouse Anesthesia and Analgesia Recommendations. IACUC Guidelines, The University of Pennsylvania Institutional Animal Care and Use Committee (2007).
Al-Humadi, N. Pre-clinical toxicology considerations for vaccine development. Vaccine 35, 5762 (2017).
Nagy, C. & Einwallner, E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J. Vis. Exp. https://doi.org/10.3791/56672 (2018).
Devi, S., Kaur, N., Kumar, M. & Kumar, P. In vitro and in vivo evaluation of antidiabetic potential and drug-herb interactions of Euphorbia neriifolia in streptozotocin-induced diabetes in rats and it’s in vitro antioxidant studies. Food Chem. Adv. 2, 100199 (2023).
Zhu, Y., Devi, S., Kumar, M., Dahiya, R. S. & Tarkeshwer,. Evaluation of gamma amino butyric acid (GABA) and glibenclamide combination therapy in streptozotocin induced diabetes. Endocr. Metab. Immune Disord. Drug Targets 21, 2005 (2020).
Yadav, U. C. S., Moorthy, K. & Baquer, N. Z. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol. Cell. Biochem. 268, 111 (2005).
Muñoz, F., Fex, M., Moritz, T., Mulder, H. & Cataldo, L. R. Unique features of β‐cell metabolism are lost in type 2 diabetes. Acta Physiologica. 240(6), e14148 (2024).
Nikolaus, H., Ezzat, S., Vargas, D., Fischer, N. H. & Younathan, E. S. Inhibition of phosphofructokinase by Molluscicidal sesquiterpene lactones. Rev. de la Soc. Quím. de Méx. 45, 159–162 (2001).
Hankin, L. Enzymatic methods of analysis. J. AOAC Int. 53, 1306 (1970).
Baginski, E. S., Foà, P. P. & Zak, B. Glucose-6-phosphatase. In Methods of enzymatic analysis 2nd edn 876–880 (Academic Press, 1974).
Tashima, Y. & Yoshimura, N. Control of rabbit liver fructose-1, 6-diphosphatase activity by magnesium ions. J. Biochem. 78, 1161 (1975).
Amri, M. et al. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms 11, 783 (2023).
Baquer, N. Z., McLean, P. & Greenbaum, A. L. Enzymic differentiation in pathways of carbohydrate metabolism in developing brain. Biochem. Biophys. Res. Commun. 53, 1282 (1973).
Karthikesan, K., Pari, L. & Menon, V. P. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem. Biol. Interact. 188, 643 (2010).
Ghani, M. A., Barril, C., Bedgood, D. R. & Prenzler, P. D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 230, 195 (2017).
Marklund, S. & Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47, 469–474 (1974).
Sinha, A. K. Colorimetric assay of catalase. Anal. Biochem. 47, 389 (1972).
Rotruck, J. T., Pope, A. L. & Ganther, H. E. Selenium: Biochemical role as a component of glutathione peroxidase. Nutr. Rev. 38, 280 (1980).
Xiao, C. et al. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol. 196, 47 (2017).
Al-Ishaq, R. K., Abotaleb, M., Kubatka, P., Kajo, K. & Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 9, 430 (2019).
Shabalala, S. C. et al. Detrimental effects of lipid peroxidation in type 2 diabetes: Exploring the neutralizing influence of antioxidants. Antioxidants 11, 2071 (2022).
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C. L. & Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52, 313 (2005).
Hajiaghaalipour, F., Khalilpourfarshbafi, M., Arya, A. & Arya, A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 11, 508 (2015).
Tzeng, Y. M., Chen, K., Rao, Y. K. & Lee, M. J. Kaempferitrin activates the insulin signaling pathway and stimulates secretion of adiponectin in 3T3-L1 adipocytes. Eur. J. Pharmacol. 607, 27 (2009).
Da Silva, D. et al. Antidiabetic activity of Sedum dendroideum: Metabolic enzymes as putative targets for the bioactive flavonoid kaempferitrin. IUBMB Life 66, 361 (2014).
Naudi, A. et al. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp. Diabetes Res. 2012, 14 (2012).
Behl, T. et al. Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules 26, 6570 (2021).
Ramu, R. et al. Correction: Assessment of in vivo antidiabetic properties of umbelliferone and lupeol constituents of banana (Musa sp. var Nanjangud rasa bale) flower in hyperglycaemic rodent model. PLoS ONE 11, e0160048 (2016).
Behl, T. et al. Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 22, 7432 (2021).
Yang, T. et al. Modulation of gut microbiota and hypoglycemic/hypolipidemic activity of flavonoids from the fruits of Lycium barbarum on high-fat diet/streptozotocin-induced type 2 diabetic mice. Food Funct. 13, 11169 (2022).
Frenkel, Y. et al. Dietary supplementation with resveratrol attenuates serum melatonin level, pro-inflammatory response and metabolic disorder in rats fed high-fructose high-lipid diet under round-the-clock lighting. Pathophysiology 30, 37 (2023).
Zancan, P. & Sola-Penna, M. Calcium influx: A possible role for insulin modulation of intracellular distribution and activity of 6-phosphofructo-1-kinase in human erythrocytes. Mol. Genet. Metab. 86, 392 (2005).
Zancan, P. & Sola-Penna, M. Regulation of human erythrocyte metabolism by insulin: Cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function. Mol. Genet. Metab. 86, 401 (2005).
Mainzen Prince, P. S. & Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 20, 96 (2006).
Babujanarthanam, R., Kavitha, P. & Pandian, M. R. Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes. Fundam. Clin. Pharmacol. 24, 357 (2010).
Prasath, G. S. & Subramanian, S. P. Modulatory effects of fisetin, a bioflavonoid, on hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in hepatic and renal tissues in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 668, 492 (2011).
Jung, U. J., Lee, M. K., Jeong, K. S. & Choi, M. S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 134, 2499 (2004).
Pari, L. & Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 64, 477 (2010).
Carling, D., Thornton, C., Woods, A. & Sanders, M. J. AMP-activated protein kinase: New regulation, new roles?. Biochem. J. 445, 11 (2012).
Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15 (2005).
Saha, A. K. AMP-activated protein kinase: Its regulation by different sites. Curr. Res. Diabetes Obes. J. 2, 88 (2017).
Acknowledgements
This study was supported by funding from Prince Sattam Bin Abdulaziz University, project number PSAU/2024/R/1445.
Funding
This study was supported by funding from Prince Sattam bin Abdulaziz University, project number PSAU/2023/R/1444.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.H.A., and A.A.; methodology, F.K.A., and S.D.; formal analysis, S.D., and A.A..; investigation, F.K.A. and H.S.Y.; resources, F.K.A. and H.S.Y.; writing— original draft preparation, S.D., and A.A.; writing—review and editing, A.H.A.; A.I.F.; F.K.A.; visualization, A.I.F. and A.A.; supervision, A.H.A. and A.A.; project administration, S.D. and H.S.Y.; funding acquisition, A.H.A.; K.M.A and F.K.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aodah, A.H., Alkholifi, F.K., Alharthy, K.M. et al. Effects of kaempherol-3-rhamnoside on metabolic enzymes and AMPK in the liver tissue of STZ-induced diabetes in mice. Sci Rep 14, 16167 (2024). https://doi.org/10.1038/s41598-024-66426-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66426-x
- Springer Nature Limited